Introduction
Matrix metalloproteinases (MMPs) are a family of
zinc- and carcium-dependent proteolytic enzymes (1). These enzymes are normally involved in
the breakdown of the extracellular matrix within the context of
physiological tissue remodeling and angiogenesis (2). MMP9 is normally associated with bone
remodeling (2) and dysregulated
states, such as rheumatoid arthritis and osteosarcoma (3). It also has a strong influence on many
phases of cancer progression, including angiogenesis, invasiveness
and metastasis. An excessive production of MMP9 has been recognized
to be an important factor in cancer invasion and metastasis
(4).
Decorin is a member of the extracellular matrix
small leucine-rich proteoglycan (SLRP) family of proteins that
exists and functions mainly in the stroma and epithelial cells
(5). It also has been found to play
an important role in tumor development and progression,
angiogenesis and metastasis (6).
Several studies have shown that decorin inhibits primary tumor
development and progression by antagonistically targeting multiple
tyrosine kinase receptors, such as epidermal growth factor receptor
(EGFR) (7).
The data in the pertinent literature show that
members of the MMP family are able to cleave some SLRPs (8–11)
including decorin (9). We hypothesize
that cervical cancer cells display enhanced MMP9 expression, which
results in infiltration to the extracellular matrix and uterine
stroma destroying the basement membranes. The resolution of decorin
by MMP9 from cancer cells promotes further infiltration, which
results in further destruction of the extracellular matrix and
stroma, where decorin is produced. The aim of the present study was
to investigate the role of MMP9 and decorin in cervical cancer.
Materials and methods
Participants
The present study included 100 randomly selected
patients who underwent cervical conization for squamous cell
neoplasia at Osaka Medical College between 2010 and 2012. The
Institutional Review Board approved this study (Ethics Committee of
Osaka Medical College, 0277), and informed consent was obtained
from all patients for the use of their tissue samples.
Expression of MMP9 and decorin by
immunohistochemistry
The specimens were fixed in 10% formalin and
embedded in paraffin. Serial sections cut out from
paraffin-embedded blocks were used for routine histopathology. A
4-µm section was cut from a paraffin embedded block and
immunohistochemically analyzed for the expression of MMP9 and
decorin. Deparaffinized and rehydrated sections (4 µm) were
autoclaved in 0.01 mol/l citrate buffer Ph 6.0 for 15 min at 121°C
for antigen retrieval. Endogenous peroxidase activity was blocked
with 0.3% solution hydrogen peroxide in methanol for 30 min. Tumor
sections were incubated at 4°C for 12 h with anti-MMP9 rabbit
polyclonal antibodies (AB13458; 1:50 dilutio; EMD Millipore,
Billerica, MA, USA) and anti-decorin rabbit polyclonal antibodies
(ab151988, 1:100 dilution; Abcam, Cambridge, MA, USA). The sections
were washed with 1X phosphate-buffered saline (PBS) and incubated
with Histofine simple stain MAX PO (multi; Nichirei, Tokyo, Japan)
for 30 min at room temperature. Finally, the sections were washed
with 1X PBS, signals and then visualized by incubation with
H2O2/diaminobenzidine substrate solution for 5 min. The sections
were counterstained with hematoxylin prior to dehydration and
mounting.
The evaluation of the immunohistochemical data was
performed by two independent observers who were blinded to the
clinicopathological data. The expression of MMP9 for each sample
was defined as detectable immunoreactions in cancer cells, as
described previously (12). The
evaluation of MMP9 expression was performed for stroma under the
basement membrane or epithelial cells with no invasive neoplasia.
In cases of invasive carcinoma, malignant cells or the stroma
adjacent to malignant cells were evaluated. Briefly, the MMP9
expression was considered to be negative (no more than 10% of cells
positive), low (more than 10% and up to 50% of cells positive) or
high (more than 50% of cells positive). Based on these data, two
groups were established: Negative MMP9 (no/low expression) and
positive MMP9 expression (high expression).
The evaluation of the stromal decorin expression was
also performed under the basement membrane with no invasive
neoplasia. In cases of invasive carcinoma, the stroma adjacent to
the malignant cells was evaluated. The expression of decorin was
assessed using a semiquantitative system. Briefly, the decorin
expression was scored as 0 (no stain), 1+ (weak immunoreactivity in
more than 10% of stromal cells), 2+ (moderate immunoreactivity in
more than 10% of stromal cells), or 3+ (strong immunoreactivity in
more than 10% of stromal cells). Based on these data, two groups
were established: Including a low decorin expression group (0 and
1+) and a high decorin expression group (2+ and 3+).
Cell lines
We used the human cervical cancer CaSki and the
CRL-4003 immortalized human endometrial stromal cells, both
purchased from the American Type Cultured Collection (Manassas, VA,
USA). We also performed an STR polymorphism profiling analysis
(Wakennyaku Co., Ibaraki, Japan) to confirm the cell line identity.
These cells were cultured in growth media [DMEM/F12 10% FBS, 1% BD
Insulin, Transferrin, Selenous (ITS) +Premix Universal Culture
Supplement (catalog no: 354352; BD Biosciences, Franklin Lakes, NJ,
USA)], in a humidified atmosphere of 5% CO2 with 95% air
at 37°C.
Measurement of the concentration of
decorin with an enzyme-linked immunosorbent (ELISA)
We used the cervical cancer cell line CaSki and
endometrial stromal cell line CRL4003. DMEM was used as cell
culture medium. A total of 106 CaSki cells and CRL4003
cells each were cultured at 37°C in a humidified, 5% CO2
atmosphere in DMEM for 96 h. CaSki culture medium was then obtained
as medium containing MMP9. CRL4003 culture medium was also obtained
as medium containing decorin. The concentration of decorin in the
CRL4003 culture medium with or without CaSki culture medium or MMP
inhibitor (MMP-9 inhibitor, ab142180; Abcam) was investigated by an
ELISA. The experiment was carried out five times, and the
concentration of decorin was expressed as the mean ± standard
deviation (SD).
Influence of MMP9 and decorin on the
invasion ability using an invasion assay
The invasive potential was assessed using an
invasion assay. We examined the effects of MMP9 and decorin on the
invasive potential of CaSki cells with or without CRL4003 cells and
MMP inhibitor using this assay. A total of 5×105 CaSki
cells were cultured at 37°C in a humidified, 5% CO2
atmosphere in serum-free DMEM for 24 h. These cells with or without
MMP inhibitor were then seeded into upper wells coated with a thin
layer of Matrigel. The lower chamber contained 600 µl of DMEM with
or without 5×105 CRL4003 cells; we established four
groups: CaSki, CaSki with CRL4003, CaSki with MMP inhibitor, CaSki
with CRL4003 and MMP inhibitor. Following 24-h incubation at 37°C,
noninvading cells on the surface of the Matrigel-coated membrane
were removed by scraping with a cotton swab. Cells that migrated
through the Matrigel were stained with hematoxylin. Following
several washes with PBS, the stained cells were manually counted
for two independent experiments. Each point represents the mean ±
SD of four replicates.
Statistical analyses
All statistical analyses were performed using the
JMP software package (v11.1.1; SAS Institute Inc, Tokyo, Japan).
Continuous variables are expressed as the mean ± SD. The
Mann-Whitney U-test was used to compare continuous variables
between the two groups. When making multiple comparisons in
datasets with continuous variables containing more than two groups,
the Tukey honestly significant difference (HSD) was used. Fisher's
exact test with Bonferroni's correction was used to compare
frequencies between the three groups. Pearson's correlation
coefficient was calculated to determine the correlation between two
variables. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of MMP9 and decorin in
surgical specimens
The immunochemical staining of MMP9 and decorin is
shown in Fig. 1. In patients with
cervical intraepithelial neoplasia (CIN) 1 (Fig. 1A), the expression of MMP9 was not seen
under the basement membrane (Fig.
1B); however, decorin was expressed abundantly in the stroma
(Fig. 1C). In contrast, in patients
with microinvasive carcinoma (Fig.
1D), the expression of MMP9 was abundant (Fig. 1E); however, decorin was not seen in
the stroma adjacent to malignant cells (Fig. 1F). The results of the
immunohistochemical analyses are shown in Table I. The patients were categorized into
three groups: those with normal to CIN2 were categorized as having
low-grade neoplasia, those with CIN3 were categorized as being
premalignant, and those with microinvasive carcinoma (FIGO stage
IA1 squamous cervical cancer) were categorized as being malignant.
Five patients had no neoplasia, 14 had CIN 1 and 14 had CIN 2; a
total of 33 patients had normal to CIN2 (low-grade neoplasia
group), 31 had CIN3 (premalignant group), and 36 had microinvasive
carcinoma (malignant group). The mean ± SD age in the low-grade
neoplasia, premalignant and malignant groups was 37.2±10.0,
40.9±11.2 and 38.6±10.4 years old, respectively. No significant
difference was found between the groups. The rate of MMP9
positivity was 0% in the low-grade neoplasia group, 41.9% in the
premalignant group and 100% in the malignant group. There were
significant differences between each group (P<0.01). In
contrast, the rate of high decorin expression was 100% in the
low-grade neoplasia group, 74.2% in the premalignant group and 9.1%
in the malignant group. There were significant differences between
each group (P<0.01).
 | Table I.Expression of MMP9 and decorin in
uterine cervical neoplasm. |
Table I.
Expression of MMP9 and decorin in
uterine cervical neoplasm.
| Variables | Low-grade neoplasia
(n=33) | Premalignant
(n=31) | Malignant
(n=36) | P-value |
|---|
| Agea, years | 37.2±10.0 | 40.9±11.2 | 38.6±10.4 | N.S. |
| Positive for MMP9
(%) | 0/33 (0) | 13/31 (41.9) | 36/36 (100) | P<0.01 |
| High decorin
expression (%) | 33/33 (100) | 23/31 (74.2) | 3/36 (9.1) | P<0.01 |
Relationship between MMP9 and
decorin
The concentration of decorin in CRL4003 culture
medium with varying amounts of CaSki culture medium by an ELISA
(Fig. 2A). When the ratio of CaSki
culture medium to CRL4003 culture medium was 100, 50, 25, 10, 5, 3
and 1%, the concentration of decorin was 26.4±1.2, 138±6.4, 189±28,
368±61, 529±18, 582±81, 690±21 and 705±19 pg/ml, respectively.
However, the concentration of decorin in CRL4003 culture medium
with the same volume of CaSki culture medium and MMP inhibitor was
705±19 pg/ml. This value was not significantly different in
comparison to the concentration of decorin in the CRL4003 cultured
medium with 1% CaSki cultured medium (P=0.82). Pearson's
correlation coefficient showed a strong correlation between the
concentration of decorin and the amount of CaSki cultured medium
(r=−0.84, P<0.05) (Fig. 2B). These
findings indicate that the decorin released from CRL4003 cells was
resolved by MMP9 released from CaSki cells.
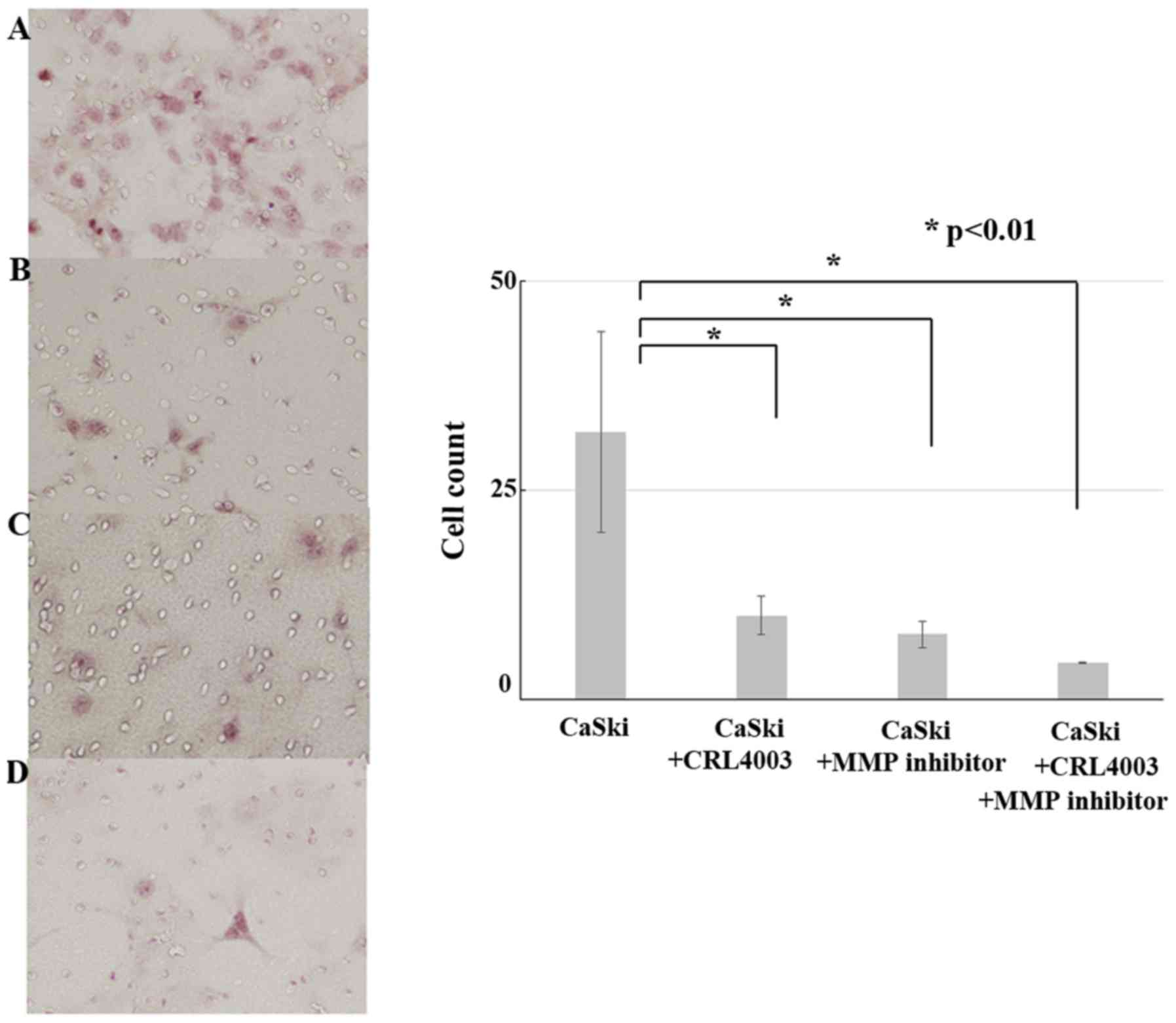
Migration and invasion in the cervical
invasion model
The results of migration and invasion assays is show
in Fig. 3. The cell counts of CaSki,
CaSki with CRL4003, CaSki with MMP inhibitor and CaSki with CRL4003
and MMP inhibitor were 32±12, 10±2.3, 7.8±1.6 and 4.4±1.1,
respectively. CRL4003 culture medium and MMP inhibitor decreased
the CaSki cell migration and invasion (P<0.01). CRL4003 culture
medium with MMP inhibitor suppressed the CaSki cell migration and
invasion the most effectively (P<0.01).
Discussion
In the present study, immunohistochemical analyses
in patients with cervical cancer showed an increased MMP9
expression and a decreased decorin expression in the stroma
adjacent to malignant cells. In vitro, the endometrial
stromal cell line CRL4003 produced decorin, which suppressed cell
invasion. Furthermore, the cervical cancer cell line CaSki produced
MMP9, which promoted cell invasion and decomposed decorin.
MMPs are a family of metalloendopeptidases that
break down the protein components of the extracellular matrix; they
play an important role in tissue remodeling and degradation
(1). Angiogenesis is one of the most
important process of tumor growth and requires the degeneration and
remodeling of the extracellular matrix, cell migration and
proliferation, and tube formation (13); the role of MMP in tumor growth and
progression is associated with angiogenesis. MMP9, which is
over-expressed in cancer cells, is a potent gelatinase that has
been shown to be associated with the process of tumor cell invasion
and metastasis (14,15). The overexpression of MMP9 has been
observed in pre-cancer and cancer lesion of the uterine cervix. The
published literature states that MMP9 is overexpressed in more than
90% of squamous cell carcinomas and 83–100% of high-grade squamous
intraepithelial lesions (HSILs) but is less strongly expressed in
low-grade squamous intraepithelial lesions (LSILs) and normal
squamous epithelium (13%) (16–28). Most
reports have shown that the activity of MMP9 tends to increase from
normal cervix to HSIL and SCC, being more strongly expressed in
more advanced stages (27,28). MMP9 is expressed in stromal cells and
inflammatory cells around tumors (29). These findings suggest the importance
of MMP9 in the pathogenesis of uterine cervical cancer; MMP9
affects various inflammatory cells infiltrating the tumor area, the
extracellular matrix and stromal cells and proteins released from
those cells.
Decorin, which is a member of the extracellular
matrix SLPR, has been thought to act exclusively as a structural
component (5). It is expressed in a
wide range of connective tissues, including skin, bone,
cartilaginous tissue and stroma and is secreted into the
extracellular matrix (30,31). Fibrillar collagen, which is the main
structural constituent of tendons, ligaments, skin and other
connective tissues, is bound and cross-linked by decorin (32–36).
Decorin knockout mice show skin fragility and abnormal collagen
fibril morphology (37), and a lack
of decorin in these mice causes a significant delay in the healing
of the excisional and incisional dermal wound compared to decorin
wild-type mice (38). Furthermore,
crossing decorin-null mice with P53-null mice caused early
lethality of double-mutant animals because of massive organ
infiltration by T-cell lymphoma (39). These findings indicate that decorin
thus plays a role in tissue development.
As mentioned below, decorin has biological functions
as a degenerator of endocytosis and inhibitor of tumor cell growth,
migration, angiogenesis, endothelial cell proliferation and
motility. Decorin affects signal transduction via erbB family
receptors characterized by the stimulation of mitogen-activated
protein kinases, mobilization of intracellular calcium and
up-regulation of P21WAF1/CIP, a potent inhibitor of
cyclin-dependent kinases, ultimately leading to growth suppression
(31,40–43). These
signal transductions are associated with modulating cell
proliferation, cell cycle progression and apoptosis. Decorin is
known to be a promoter of matrix organization (44), which constitutes a physical barrier
against the migration/motility of cancerous cells; decorin-collagen
interactions prevent metastasis (45). Decorin is also known to be a negative
regulator of signal transduction by interfering with growth factor
binding including TGF-β (46,47); the synthesis of matrix molecules and
the growth factor-dependent modulation of cell proliferation and
migration is inhibited by decorin. For these reasons, decorin has
recently emerged as a potential natural anticancer agent produced
by normal host cells against cancer cells. There have been a number
of reports about the anti-tumor effects of decorin on various
malignant tumors, including uterine cervical carcinoma cells
(48), ovarian cancer cells (49), breast cancer cells (50), colon carcinoma cells (51) and pancreatic cancer cells (52).
The published literature shows that MMPs digest
decorin into fragments by cleaving within the leucine-rich region
at multiple site (9). Those previous
authors examined the susceptibility of decorin to five different
MMPs. MMP2, MMP3 and MMP7 exhibited digestive activity for decorin,
with MMP1 and MMP9 showing lower activity. In the present study,
concentration of decorin in CRL4003 cultured medium was increased
by MMP9 inhibitor. These data suggest that MMP9 not only directly
digests decorin but also suppresses the production of decorin by
impairing the tissue.
In conclusion, MMP9 was found to be overexpressed in
uterine cervical cancer, but it was less strongly expressed in
normal or pre-malignant squamous epithelium. In contrast, the
activity of decorin in stroma adjacent to neoplastic cells was
lower in microinvasive carcinoma than in normal or pre-malignant
lesions. Decorin prevents the invasion of malignant cells, but MMP9
promotes cell invasion by destroying decorin, the extracellular
matrix and stroma.
Acknowledgements
The authors would like to thank Dr. Yoshihiro Joshua
Ono (Department of Obstetrics and Gynecology, Osaka Medical
College, Osaka, Japan) for advice on the experimental design, and
Ms. Junko Hayashi and Ms. Kumiko Satoh (Department of Obstetrics
and Gynecology, Osaka Medical College) for their secretarial
assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
TT and MO designed the study and performed the
experimental data collection and analysis. TT and YT provided the
diagnosis based on immunohistochemical analyses, and TT and MO
wrote the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Osaka Medical College, and written informed consent to
participate in the study was obtained from all patients
involved.
Patient consent for publication
No identifying patient information is included in
the published manuscript.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MMP9
|
matrix metalloproteinase-9
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
MMPs
|
matrix metalloproteinases
|
|
SLRP
|
small leucine-rich proteoglycan
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
Stamenkovic I: Extracellular matrix
remodelling: The role of matrix metalloproteinases. J Pathol.
200:448–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van den Steen PE, Dubois B, Nelissen I,
Rudd PM, Dwek RA and Opdenakker G: Biochemistry and molecular
biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit
Rev Biochem Mol Biol. 37:375–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moss Shuman LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: Changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaefer L and Iozzo RV: Biological
functions of the small leucine-rich proteoglycans: From genetics to
signal transduction. J Biol Chem. 283:21305–21309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bi XL and Yang W: Biological functions of
decorin in cancer. Chin J Cancer. 32:266–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldoni S, Humphries A, Nyström A, Sattar
S, Owens RT, McQuillan DJ, Ireton K and Iozzo RV: Decorin is a
novel antagonistic ligand of the Met receptor. J Cell Biol.
185:743–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monfort J, Tardif G, Reboul P, Mineau F,
Roughley P, Pelletier JP and Martel-Pelletier J: Degradation of
small leucine-rich repeat proteoglycans by matrix
metalloprotease-13: Identification of a new biglycan cleavage site.
Arthritis Res Ther. 8:R262006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imai K, Hiramatsu A, Fukushima D,
Pierschbacher MD and Okada Y: Degradation of decorin by matrix
metalloproteinases: Identification of the cleavage sites, kinetic
analyses and transforming growth factor-beta1 release. Biochem J.
322:809–814. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heathfield TF, Onnerfjord P, Dahlberg L
and Heinegård D: Cleavage of fibromodulin in cartilage explants
involves removal of the N-terminal tyrosine sulfate-rich region by
proteolysis at a site that is sensitive to matrix
metalloproteinase-13. J Biol Chem. 279:6286–6295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Aoki T, Mori Y, Ahmad M, Miyamori H,
Takino T and Sato H: Cleavage of lumican by membrane-type matrix
metalloproteinase-1 abrogates this proteoglycan-mediated
suppression of tumor cell colony formation in soft agar. Cancer
Res. 64:7058–7064. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shirabe K, Shimada M, Kajiyama K, Hasegawa
H, Gion T, Ikeda Y, Takenaka K and Sugimachi K: Expression of
matrix metalloproteinase-9 in surgically resected intrahepatic
cholangiocarcinoma. Surgery. 126:842–846. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giannelli G, Falk-Marzillier J, Schiraldi
O, Stetler-Stevenson WG and Quaranta V: Induction of cell migration
by matrix metalloprotease-2 cleavage of laminin-5. Science.
277:225–228. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheu BC, Lien HC, Ho HN, Lin HH, Chow SN,
Huang SC and Hsu SM: Increased expression and activation of
gelatinolytic matrix metalloproteinases is associated with the
progression and recurrence of human cervical cancer. Cancer Res.
63:6537–6542. 2003.PubMed/NCBI
|
|
17
|
Wang PH, Ko JL, Tsai HT, Yang SF, Han CP,
Lin LY and Chen GD: Clinical significance of matrix
metalloproteinase-2 in cancer of uterine cervix: A semiquantitative
study of immunoreactivities using tissue array. Gynecol Oncol.
108:533–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gaiotto MA, Focchi J, Ribalta JL, Stavale
JN, Baracat EC, Lima GR and da Silva Guerreiro ID: Comparative
study of MMP-2 (matrix metalloproteinase 2) immune expression in
normal uterine cervix, intraepithelial neoplasias and squamous
cells cervical carcinoma. Am J Obstet Gynecol. 190:1278–1282. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Argüello-Ramírez J, Pérez-Cárdenas E,
Delgado-Chávez R, Solorza-Luna G, Villa-Treviño S and
Arenas-Huertero F: Matrix metalloproteinases-2, −a3 and −9 secreted
by explants of benign and malignant lesions of the uterine cervix.
Int J Gynecol Cancer. 14:333–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato T, Sakai T, Noguchi Y, Takita M,
Hirakawa S and Ito A: Tumor-stromal cell contact promotes invasion
of human uterine cervical carcinoma cells by augmenting the
expression and activation of stromal matrix metalloproteinases.
Gynecol Oncol. 92:47–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmed N, Riley C, Oliva K, Barker G, Quinn
MA and Rice GE: Expression and localization of alphavbeta6 integrin
in extraplacental fetal membranes: Possible role in human
parturition. Mol Hum Reprod. 10:173–179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang SF, Wang PH, Lin LY, Ko JL, Chen GD,
Yang JS, Lee HS and Hsieh YS: A significant elevation of plasma
level of matrix metalloproteinase-9 in patients with high-grade
intraepithelial neoplasia and early squamous cell carcinoma of the
uterine cervix. Reprod Sci. 14:710–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baltazar-Rodriguez LM, Anaya-Ventura A,
Andrade-Soto M, Monrroy-Guizar EA, Bautista-Lam JR,
Jonguitud-Olguin G, Cepeda-Lopez FR, Centeno-Aguilar VA,
Gonzalez-Hernandez NA, Soriano-Hernández AD, et al: Polymorphism in
the matrix metalloproteinase-2 gene promoter is associated with
cervical neoplasm risk in Mexican women. Biochem Genet. 46:137–144.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rauvala M, Aglund K, Puistola U,
Turpeenniemi-Hujanen T, Horvath G, Willén R and Stendahl U: Matrix
metalloproteinases-2 and −9 in cervical cancer: Different roles in
tumor progression. Int J Gynecol Cancer. 16:1297–1302. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nasr M, Ayyad SB, El-Lamie IK and Mikhail
MY: Expression of matrix metalloproteinase-2 in preinvasive and
invasive carcinoma of the uterine cervix. Eur J Gynaecol Oncol.
26:199–202. 2005.PubMed/NCBI
|
|
26
|
Yoshida H, Sumi T, Hyun Y, Nakagawa E,
Hattori K, Yasui T, Morimura M, Honda K, Nakatani T and Ishiko O:
Expression of survivin and matrix metalloproteinases in
adenocarcinoma and squamous cell carcinoma of the uterine cervix.
Oncol Rep. 10:45–49. 2003.PubMed/NCBI
|
|
27
|
Talvensaari-Mattila A,
Turpeenniemi-Hujanen T and Puistola U: Matrix metalloproteinase 9
and relapse in patients with early stage squamous cervical
carcinoma. Int J Gynaecol Obstet. 91:75–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Talvensaari-Mattila A and
Turpeenniemi-Hujanen T: Matrix metalloproteinase 9 in the uterine
cervix during tumor progression. Int J Gynaecol Obstet. 92:83–84.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tellier E, Nègre-Salvayre A, Bocquet B,
Itohara S, Hannun YA, Salvayre R and Augé N: Role for furin in
tumor necrosis factor alpha-induced activation of the matrix
metalloproteinase/sphingolipid mitogenic pathway. Mol Cell Biol.
27:2997–3007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Corsi A, Xu T, Chen XD, Boyde A, Liang J,
Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, et al:
Phenotypic effects of biglycan deficiency are linked to collagen
fibril abnormalities, are synergized by decorin deficiency, and
mimic Ehlers-Danlos-like changes in bone and other connective
tissues. J Bone Miner Res. 17:1180–1189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iozzo RV: The biology of the small
leucine-rich proteoglycans. Functional network of interactive
proteins. J Biol Chem. 274:18843–18846. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Keene DR, Antonio San JD, Mayne R,
McQuillan DJ, Sarris G, Santoro SA and Iozzo RV: Decorin binds near
the C terminus of type I collagen. J Biol Chem. 275:21801–21804.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bidanset DJ, Guidry C, Rosenberg LC, Choi
HU, Timpl R and Hook M: Binding of the proteoglycan decorin to
collagen type VI. J Biol Chem. 267:5250–5256. 1992.PubMed/NCBI
|
|
34
|
Schonherr E, Hausser H, Beavan L and
Kresse H: Decorin-type I collagen interaction. Presence of separate
core protein-binding domains. J Biol Chem. 270:8877–8883. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kinsella MG, Bressler SL and Wight TN: The
regulated synthesis of versican, decorin, and biglycan:
Extracellular matrix proteoglycans that influence cellular
phenotype. Crit Rev Eukaryot Gene Expr. 14:203–234. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nareyeck G, Seidler DG, Troyer D,
Rauterberg J, Kresse H and Schönherr E: Differential interactions
of decorin and decorin mutants with type I and type VI collagens.
Eur J Biochem. 271:3389–3398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Danielson KG, Baribault H, Holmes DF,
Graham H, Kadler KE and Iozzo RV: Targeted disruption of decorin
leads to abnormal collagen fibril morphology and skin fragility. J
Cell Biol. 136:729–743. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Järveläinen H, Puolakkainen P, Pakkanen S,
Brown EL, Höök M, Iozzo RV, Sage EH and Wight TN: A role for
decorin in cutaneous wound healing and angiogenesis. Wound Repair
Regen. 14:443–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iozzo RV, Chakrani F, Perrotti D,
McQuillan DJ, Skorski T, Calabretta B and Eichstetter I:
Cooperative action of germ-line mutations in decorin and p53
accelerates lymphoma tumorigenesis. Proc Natl Acad Sci USA.
96:3092–3097. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Csordás G, Santra M, Reed CC, Eichstetter
I, McQuillan DJ, Gross D, Nugent MA, Hajnoczky G and Iozzo RV:
Sustained down-regulation of the epidermal growth factor receptor
by decorin. A mechanism for controlling tumor growth in vivo. J
Biol Chem. 275:32879–32887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu JX, Goldoni S, Bix G, Owens RT,
McQuillan DJ, Reed CC and Iozzo RV: Decorin evokes protracted
internalization and degradation of the epidermal growth factor
receptor via caveolar endocytosis. J Biol Chem. 280:32468–32479.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seidler DG, Faiyaz-Ul-Haque M, Hansen U,
Yip GW, Zaidi SH, Teebi AS, Kiesel L and Götte M: Defective
glycosylation of decorin and biglycan, altered collagen structure,
and abnormal phenotype of the skin fibroblasts of an Ehlers-Danlos
syndrome patient carrying the novel Arg270Cys substitution in
galactosyltransferase I (beta4GalT-7). J Mol Med (Berl).
84:583–594. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Seidler DG, Goldoni S, Agnew C, Cardi C,
Thakur ML, Owens RT, McQuillan DJ and Iozzo RV: Decorin protein
core inhibits in vivo cancer growth and metabolism by hindering
epidermal growth factor receptor function and triggering apoptosis
via caspase-3 activation. J Biol Chem. 281:26408–26418. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Koninger J, Giese T, di Mola FF, Wente MN,
Esposito I, Bachem MG, Giese NA, Büchler MW and Friess H:
Pancreatic tumor cells influence the composition of the
extracellular matrix. Biochem Biophys Res Commun. 322:943–949.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Skandalis SS, Kletsas D, Kyriakopoulou D,
Stavropoulos M and Theocharis DA: The greatly increased amounts of
accumulated versican and decorin with specific post-translational
modifications may be closely associated with the malignant
phenotype of pancreatic cancer. Biochim Biophys Acta.
1760:1217–1225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Border WA, Noble NA, Yamamoto T, Harper
JR, Yamaguchi YU, Pierschbacher MD and Ruoslahti E: Natural
inhibitor of transforming growth factor-beta protects against
scarring in experimental kidney disease. Nature. 360:361–364. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hildebrand A, Romaris M, Rasmussen LM,
Heinegård D, Twardzik DR, Border WA and Ruoslahti E: Interaction of
the small interstitial proteoglycans biglycan, decorin and
fibromodulin with transforming growth factor beta. Biochem J.
302:527–534. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grant DS, Yenisey C, Rose RW, Tootell M,
Santra M and Iozzo RV: Decorin suppresses tumor cell-mediated
angiogenesis. Oncogene. 21:4765–4777. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nash MA, Loercher AE and Freedman RS: In
vitro growth inhibition of ovarian cancer cells by decorin:
Synergism of action between decorin and carboplatin. Cancer Res.
59:6192–6196. 1999.PubMed/NCBI
|
|
50
|
Reed CC, Waterhouse A, Kirby S, Kay P,
Owens RT, McQuillan DJ and Iozzo RV: Decorin prevents metastatic
spreading of breast cancer. Oncogene. 24:1104–1110. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Santra M, Skorski T, Calabretta B, Lattime
EC and Iozzo RV: De novo decorin gene expression suppresses the
malignant phenotype in human colon cancer cells. Proc Natl Acad Sci
USA. 92:7016–7020. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Köninger J, Giese NA, di Mola FF, Berberat
P, Giese T, Esposito I, Bachem MG, Buchler MW and Friess H:
Overexpressed decorin in pancreatic cancer: Potential tumor growth
inhibition and attenuation of chemotherapeutic action. Clin Cancer
Res. 10:4776–4783. 2004. View Article : Google Scholar : PubMed/NCBI
|