Introduction
Nasopharyngeal cancer (NPC) is a relatively rare
form of squamous cell carcinoma with vast differences in incidence
globally (1). Prior infection with
Epstein-Barr virus (EBV) is considered an important etiological
factor in regions with endemic NPC, along with genetic
susceptibility, and dietary and social factors (1). According to a large study conducted in
Hong Kong (a region with endemic NPC) between 1996 and 2000, the
5-year disease-specific survival rates range from 65% to >92%
depending on the cancer stage (2).
The side effects of current primary treatments (radiotherapy with
or without chemotherapy) are marked (3). There is therefore a requirement for
novel treatment approaches.
The association between NPC and EBV provides an
opening for antigen-specific treatment for NPC. Adoptive
immunotherapy comprising of allogeneic or autologous cytotoxic T
lymphocyte (CTL) therapy, bypassing the antigen-presenting
procedure, has been investigated (4–7). For
instance, Smith et al (7)
demonstrated antitumor effects in patients with metastatic NPC
treated with autologous antigen-specific T-cell lines generated
in vitro using an adenoviral vector-based vaccine
(AdE1-LMPpoly) encoding for multiple EBV antigens. However, active
immunotherapy, to the best of our knowledge, has not been utilized
in the treatment of NPC.
Identification of a tumor-specific EBV antigen in
NPC suggests the possibility that the condition is amendable to
specific dendritic cell (DC)-targeting immunotherapy. DCs are
professional antigen-presenters and key regulators of T-cell
polarization (8). Blood DCs form a
heterogeneous population, including CD123+ plasmacytoid
DCs (pDCs) as well as CD1c+ and CD141+
myeloid DCs (mDCs), and each subset features different pattern
recognition receptor (PRR) profiles, including Toll-like receptors
(TLRs) and C-lectin receptors (CLRs) (9). Adjuvant actions on a number of these
receptors, including the CLR CD207 (also termed langerin), may
promote cross-presentation of antigens, which is a necessary step
to achieve beneficial cell-mediated cytotoxic effects (10–12).
Detailed information on intralesional DC subsets and receptor
repertoires is necessary in order to design effective immunotherapy
for NPC; however, currently there is limited information
available.
The presence of human leukocyte antigen
(HLA)-DR+ and HTA-I+ cells, which are
morphologically and phenotypically similar to antigen-presenting
Langerhans cells, has previously been reported in NPC (13). Similarly, cells with features of the
Langerhans cell type, morphologically or immunohistochemically
using S-100 or HLA-DR/HTA-I (CD1a antibodies), have been
demonstrated in NPC (14–18). Furthermore, Braz-Silva et al
(14) demonstrated a subset of
CD207+ DCs infiltrating EBV-infected areas in NPC.
However, a detailed description of DC subsets, according to current
knowledge in the field, is limited for NPC. In the present study,
using fresh tumor-samples obtained from patients with untreated
NPC, the presence and characteristics of DCs was examined using
multicolor flow-cytometry. It was demonstrated that different
subpopulations of DCs are present in NPC lesions and that
CD1c+ mDCs may have an increased expression of CD207,
compared with other subsets.
Materials and methods
Study design
A total of 5 patients, 3 male and 2 female patients,
(range, 39–67 years of age) with untreated NPC were recruited
between November 2014 and August 2016 at Skåne University Hospital
(Lund, Sweden), a tertiary referral center with a catchment
population of approximately 1.9 million. The inclusion criteria of
the present study was age >18 years and no prior cancer
treatment. Patient characteristics are presented in Table I, including histopathology
classification according to World Health Organization (WHO)
(19) and cancer stage according to
Tumor-Node-Metastasis (TNM) classification of malignant tumors
(20). The Ethics Committee at Lund
University (Lund, Sweden) approved the study protocol and written
informed consent was obtained from all patients prior to
inclusion.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Sex | Age, years | EBER-1 |
Histopathologya | TNMb | TNM
stageb |
|---|
| Female | 67 | Negative | 1 | T2N3bM1 | IV-C |
| Female | 40 | Positive | 2b | T4N1M0 | IV-A |
| Male | 39 | Positive | 2b | T1N1M0 | II |
| Male | 65 | Positive | 2b | T1N1M0 | II |
| Male | 46 | Positive | 2b | T3N2M0 | III |
Sampling
Topical anesthesia and mucosal decongestion was
achieved by nasal/nasopharyngeal administration of a mixture of
tetracain (20 mg/ml) and adrenalin (0.1 mg/ml) using a
spray-device. Tissue samples were obtained using a punch forceps
under endoscopic guidance. Half of the biopsy was sent for routine
pathology work, including EBV-encoded small RNAs-1 (EBER1) in
situ hybridization. The other half was stored in tissue storage
solution at 4°C (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
and transferred to the laboratory.
Cell preparation
Fresh biopsies were cut into ~2 mm pieces and placed
in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with gentamycin (0.1 g/ml; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). Enzymatic digestion was performed by
incubating the tissue suspension at 37°C for 20 min with
Collagenase IV (2 mg/ml) and DNase I (200 Kunitz/ml) (both from
Sigma-Aldrich; Merck KGaA). A single cell suspension was prepared
by filtering the cell suspension through 70 µm cell strainers (BD
Biosciences, San Jose, CA, USA). The number of viable cells were
calculated using 4% Trypan blue solution (Thermo Fisher Scientific,
Inc.) and non-stained cells were counted instantly using Fluovert
FS inverted fluorescence microscope (magnification, ×20; Leitz).
Cell viability was evaluated using Trypan blue-exclusion.
Multicolor flow-cytometry
Single cell suspension was blocked using mouse IgG
(Table II) and incubated for 5 min
at room temperature, followed by incubation at 4°C for 20 min in
Brilliant Stain Buffer (BD Biosciences) in the presence of the
antibody panel indicated in Table
II. Stained cells were run on a BD FACSAria II (BD Biosciences)
and subset frequencies as well as CD207 expression was further
analyzed with FCS Express v4.0 (De Novo Software, Glendale, CA,
USA).
 | Table II.Antibodies utilized. |
Table II.
Antibodies utilized.
| Antibody | Conjugate | Clone | Cat. no. | Supplier | Dilution | Stock
concentration, µg/ml |
|---|
| CD45 | APC-H7 | 2D1 | 560178 | BD Biosciences | 1:50 | 200 |
| CD3 | FITC | UCHT1 | 5553332 | BD Biosciences | 1:25 | 12.5 |
| CD14 | FITC | TüK4 | MHCD1401 | Thermo Fisher
Scientific, Inc. | 1:50 | Not available |
| CD56 | FITC | NCAM16.2 | 345811 | BD Biosciences | 1:50 | 6 |
| CD19 | Brilliant Blue515
(BB515) | HIB19 | 564456 | BD Biosciences | 1:25 | 100 |
| HLA-DR | PerCp-Cy5.5 | L243 | 307630 | BioLegend Inc., San
Diego, CA, USA | 1:250 | 200 |
| CD11c | Brilliant Violet
421 (BV421) | B-ly6 | 562561 | BD Biosciences | 1:50 | 50 |
| CD1c | APC | L161 | 17-0015-42 | eBioscience; Thermo
Fisher Scientific, Inc. | 1:50 | 6 |
| CD141 | Brilliant Violet
510 (BV510) | 1A4 | 563298 | BD Biosciences | 1:100 | 100 |
| CD123 | PE-Cy7 | 6H6 | 306010 | BioLegend Inc. | 1:100 | 200 |
| CD207 | PE | DCGM4 | IM3577 | Immunotech; Beckman
Coulter, Inc., Brea, CA, USA | 1:50 | 25 |
| ChromPure mouse
IgG, whole molecule | None | Not available | 015-000-003 | Jackson
ImmunoResearch Laboratories, Inc., West Grove PA, USA | 1:19500 | 5900 |
Viable cells were gated in a forward scatter
(FSC)-side scatter (SSC) plot and doublet exclusion was then
performed using FSC-area vs. FSC-height. Leukocytes were
subsequently identified as CD45+ cells, out of which
HLA-DR+ lineage- (CD3, CD14, CD56 and CD19) cells were
gated. mDCs and pDCs were identified based on the expression of
CD11c and CD123, respectively, and these subsets collectively were
the total number of DCs. mDCs were further subdivided into
CD141+, CD1c+ and
CD1c−CD141− cells. The applied gating
strategy is indicated in Fig. 1. Cell
surface expression of CD207 was assessed using a similar gating
strategy (Fig. 1). A fluorescence
minus one (FMO) control was used for the PE channel (an identical
additional sample prepared without CD207-staining antibodies) when
sufficient numbers of cells were available to perform two staining
preparations from the same sample (the DC populations are present
in very low frequencies and ≥1×106 live CD45+
cells are required in order to distinguish the populations and to
gate CD207+ cells). The gate for CD207+ cells
was set based on the FMO sample if available; otherwise the gating
was compared with samples with an FMO to ensure appropriate gate
settings.
Statistics
Descriptive data was presented as mean ± standard
deviation where appropriate. A χ2 test was performed to
analyze the difference in DC subset frequencies. The statistical
analysis was performed in GraphPad prism 6.0 software (GraphPad
Software, La Jolla, CA) and VassarStats (vassarstats.net). P<0.05 was considered to indicate
a statistically significant difference.
Results
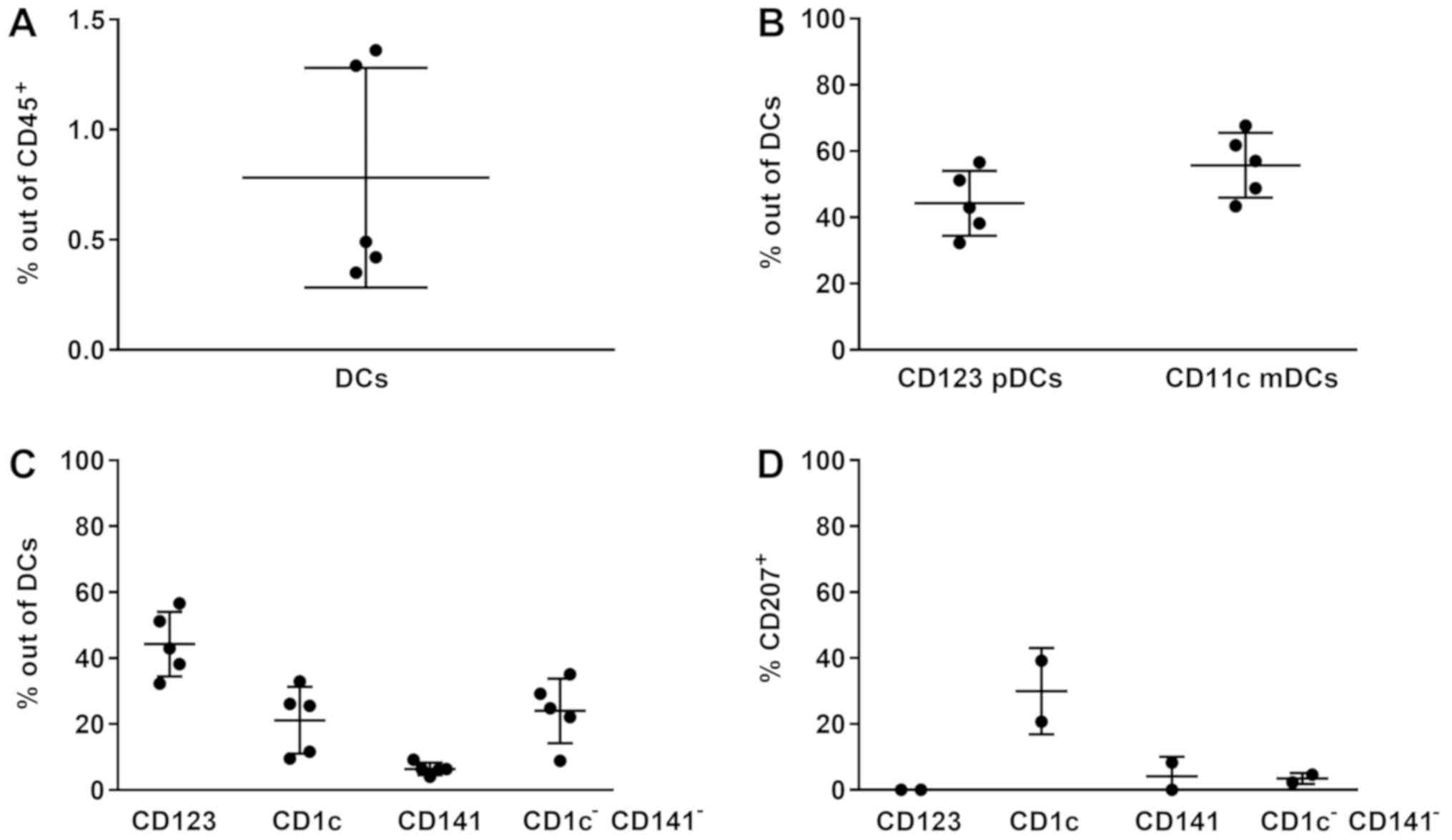
DCs, including CD11c+ mDCs and
CD123+ pDCs, were detected in all NPC lesions (n=5),
contributing to a frequency mean average of 0.78±0.50% out of the
CD45+ leukocytes in situ (Fig. 2A). A significant difference was
observed between the CD123+ pDCs and CD11c+
mDCs (P<0.01; Fig. 2B), indicating
a different presence of aforementioned DCs in the lesions.
Different DC subpopulations, including
CD123+ pDCs, CD1c+ mDCs, CD141+
mDCs and CD1c−CD141− mDCs, were observed,
constituting 44.27±9.78, 21.17±10.21, 6.42±1.84 and 24.03±9.79% of
DCs, respectively (Fig. 2C), and the
differences in frequency in these subpopulations were significant
(P<0.001). CD207 expression in the different subsets was
increased in CD1c+ DCs (n=2), suggesting a selective
expression of CD207 in this subset (Fig.
2D).
Discussion
In the present study, an intralesional presence of
DCs in untreated NPC and their subpopulation frequencies was
demonstrated. This includes CD1c+ and CD141+
mDCs that are of interest in the context of cross-presentation of
antigens (21). Furthermore, a
notable expression of the CLR CD207 in CD1c+ mDCs is
suggested, which is of interest for the identical reason. The
results are of relevance to future study designs and to therapeutic
attempts to instruct DCs to facilitate antigen-specific
immunological responses against NPC.
The present data demonstrates that DCs are present
in NPC, which verifies previous morphological observations
(13–18). Furthermore, it was indicated for the
first time that specific subpopulations, previously characterized
in blood as CD1c+ and CD141+ mDCs as well as
CD123+ pDCs (9),
infiltrate NPC lesions. mDCs and pDCs, being antigen-presenting
cells and representing an important association between innate and
adaptive immunity, may thus be regarded as treatment targets in
conditions such as NPC. Indeed, such a therapeutic potential is
underscored by the present description of intralesional DC subsets,
which may be selectively targeted to achieve a desired
cross-presentation of antigens and resulting CTL responses. It may
also be of interest to investigate the prognosis of NPC in
association with the presence of DC subsets, due to conflicting
information being presented for DCs (or DC-like cells) in this
context (18,22).
Considering DCs as treatment targets pertinent to
NPC, it is important to reflect on how they can be instructed to
facilitate cross-presentation of antigens. In this context,
different DC subsets express distinct PRR profiles (9) and, in vaccination/active immunotherapy,
adjuvant effects are mediated via these receptors. As a result, and
depending on which PRR is activated, DCs receive directional
information. A key consideration is that information on DC receptor
repertories may be required to be subset specific. This is
reflected by the fact that different DC populations may conduct
opposing actions, including immunosuppression and immunoactivation.
Accordingly, stimulation of a receptor that is not restricted to a
specific DC subset may produce conflicting effects. Furthermore,
experimental evidence indicates that stimulation of a number of
receptors, including CD207, as demonstrated in CD1c+ DCs
in the present study, may facilitate cross-presentation of antigens
(10).
The present study warrants future attempts to
outline a complete map of PRRs on DC subpopulations in NPC and
other head and neck cancer types, focusing on those PRRs that
facilitate combined humoral and cellular antigen-specific
responses, including TLR2, TLR4, Dectin-1, Dectin-2, CD206, DEC205,
C-type lectin domain containing 9a, DC-specific intercellular
adhesion molecule 3-grabbing non-integrin and X-C motif chemokine
receptor 1 (23). In the present
study, the sample size was small, both in regard to patient numbers
and tissue amount, reflecting the fact that this cancer type is
rare and indicating that the number of cells available for flow
cytometry analysis were too low for a wider profiling using this
technique. Nevertheless, such examinations, and possibly a
comparison to control tissue, are necessary and may, for example,
be initiated by biomarker identification using single-cell
RNA-sequencing methods (24). In this
context, a comparison between EBV+ and EBV- NPC may also
be warranted as well as between NPC and control tissue.
In conclusion, a number of DC-subpopulations are
present in NPC lesions. CLR CD207, as a selective endocytic marker
on CD1c+ mDCs, may be targeted for therapeutic purposes
to facilitate cross-presentation of antigens, serving potential
cell-mediated antitumor effects. The present study leaves
CD1c−CD141− mDCs, which have been recently
identified (24,25), open for further characterization, as
they comprise a notable fraction of mDCs residing in the NPC
samples.
Acknowledgements
The authors would like to thank Dr Fredrik Andersson
(Department of Pathology, Skåne University Hospital, Lund, Sweden),
for reviewing the histopathology results, and Dr Margareta Nilsson
(Department of Diagnostic Radiology, Skåne University Hospital,
Lund, Sweden), for reviewing the imaging results.
Funding
Funding was received from the Swedish National
Health Service, the Swedish Association for Otorhinolaryngology
Head and Neck Surgery, and Laryngfonden.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSN, KL, ML and LG conceived the study. JSN
conducted collection of samples and drafting of the manuscript. MA
and KL conducted the laboratory work. All authors were involved in
the preparation and revision of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee at Lund University and written informed consent was
obtained.
Patient consent for publication
Patient written consent for publication was
obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chua ML, Wee JT, Hui EP and Chan AT:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee AW, Sze WM, Au JS, Leung SF, Leung TW,
Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al: Treatment results
for nasopharyngeal carcinoma in the modern era: The Hong Kong
experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zackrisson B, Mercke C, Strander H,
Wennerberg J and Cavallin-Ståhl E: A systematic overview of
radiation therapy effects in head and neck cancer. Acta Oncol.
42:443–461. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chua D, Huang J, Zheng B, Lau SY, Luk W,
Kwong DL, Sham JS, Moss D, Yuen KY, Im SW and Ng MH: Adoptive
transfer of autologous Epstein-Barr virus-specific cytotoxic T
cells for nasopharyngeal carcinoma. Int J Cancer. 94:73–80. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comoli P, Pedrazzoli P, Maccario R, Basso
S, Carminati O, Labirio M, Schiavo R, Secondino S, Frasson C,
Perotti C, et al: Cell therapy of stage IV nasopharyngeal carcinoma
with autologous Epstein-Barr virus-targeted cytotoxic T
lymphocytes. J Clin Oncol. 23:8942–8949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL,
Cheng YF, Chen CL, Chang YS, Lee SP, Rickinson AB and Tam PK:
Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic
cells induces functional CD8+ T-cell immunity and may
lead to tumor regression in patients with EBV-positive
nasopharyngeal carcinoma. Cancer Res. 62:6952–6958. 2002.PubMed/NCBI
|
|
7
|
Smith C, Tsang J, Beagley L, Chua D, Lee
V, Li V, Moss DJ, Coman W, Chan KH, Nicholls J, et al: Effective
treatment of metastatic forms of Epstein-Barr virus-associated
nasopharyngeal carcinoma with a novel adenovirus-based adoptive
immunotherapy. Cancer Res. 72:1116–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collin M and Haniffa M: Dendritic cells
and dendritic cell subsets. Encyclopedia of Immunobiology. Academic
Press; Oxford: pp. 345–352. 2016, View Article : Google Scholar
|
|
9
|
Lundberg K, Rydnert F, Greiff L and
Lindstedt M: Human blood dendritic cell subsets exhibit
discriminative pattern recognition receptor profiles. Immunology.
142:279–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joffre OP, Segura E, Savina A and
Amigorena S: Cross-presentation by dendritic cells. Nat Rev
Immunol. 12:557–569. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valladeau J, Ravel O, Dezutter-Dambuyant
C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C,
Schmitt D, Davoust J, et al: Langerin, a novel C-type lectin
specific to Langerhans cells, is an endocytic receptor that induces
the formation of Birbeck granules. Immunity. 12:71–81. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Idoyaga J, Cheong C, Suda K, Suda N, Kim
JY, Lee H, Park CG and Steinman RM: Cutting edge: Langerin/CD207
receptor on dendritic cells mediates efficient antigen presentation
on MHC I and II products in vivo. J Immunol. 180:3647–3650. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas JA, Iliescu V, Crawford DH, Ellouz
R, Cammoun M and de-Thé G: Expression of HLA-DR antigens in
nasopharyngeal carcinoma: An immunohistological analysis of the
tumour cells and infiltrating lymphocytes. Int J Cancer.
33:813–819. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Braz-Silva PH, Vitale S, Butori C, Guevara
N, Santini J, Magalhaes M, Hofman P and Doglio A: Specific
infiltration of langerin-positive dendritic cells in EBV-infected
tonsil, Hodgkin lymphoma and nasopharyngeal carcinoma. Int J
Cancer. 128:2501–2508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammar S, Bockus D, Remington F and Bartha
M: The widespread distribution of Langerhans cells in pathologic
tissues: An ultrastructural and immunohistochemical study. Hum
Pathol. 17:894–905. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lauriola L, Michetti F, Sentinelli S and
Cocchia D: Detection of S-100 labelled cells in nasopharyngeal
carcinoma. J Clin Pathol. 37:1235–1238. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma CX, Jia TC, Li XR, Zhand ZF and Yiao
CB: Langerhans cells in nasopharyngeal carcinoma in relation to
prognosis. In Vivo. 9:225–229. 1995.PubMed/NCBI
|
|
18
|
Zong YS, Zhang CQ, Zhang F, Ruan JB, Chen
MY, Feng KT and Yu ZF: Infiltrating lymphocytes and accessory cells
in nasopharyngeal carcinoma. Jpn J Cancer Res. 84:900–905. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thompson LD: Update on nasopharyngeal
carcinoma. Head Neck Pathol. 1:81–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH, Gospodarowicz MK and Wittekind
C: UICC: TNM Classification of Malignant Tumours. 7th.
Wiley-Blackwell; New Jersey: 2009
|
|
21
|
Segura E, Durand M and Amigorena S:
Similar antigen cross-presentation capacity and phagocytic
functions in all freshly isolated human lymphoid organ-resident
dendritic cells. J Exp Med. 210:1035–1047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang CS, Chang JH, Hsu NC, Lin HY and
Chung CY: Expression of CD80 and CD86 costimulatory molecules are
potential markers for better survival in nasopharyngeal carcinoma.
BMC Cancer. 7:882007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin W, Duluc D, Joo H and Oh S: Dendritic
cell targeting vaccine for HPV-associated cancer. Cancer Cell
Microenviron. 3:e14822016.PubMed/NCBI
|
|
24
|
Villani AC, Satija R, Reynolds G,
Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S,
Lazo S, et al: Single-cell RNA-seq reveals new types of human blood
dendritic cells, monocytes, and progenitors. Science. 356(pii):
eaah45732017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abolhalaj M, Askmyr D, Sakellariou CA,
Lundberg K, Greiff L and Lindstedt M: Profiling dendritic cell
subsets in head and neck squamous cell tonsillar cancer and benign
tonsils. Sci Rep. 8:80302018. View Article : Google Scholar : PubMed/NCBI
|