Introduction
Epithelioid inflammatory myofibroblastic sarcomas
(EIMSs) were first described by Mariño-Enríquez in 2011 (1), and are rare malignant mesenchymal tumors
commonly identified in the lung, abdominal/pelvic cavity and
retroperitoneal cavity irrespective of age (2). In contrast to the conventional
spindle-cell inflammatory myofibroblastic tumors (IMTs), the
characteristics of EIMSs include extensive epithelial cell
infiltration pathologically and a dismal prognosis (3). Given its low incidence and inadequate
recognition, standard guidelines for EIMSs are presently not
available. Based on experience, surgery is the primary choice for
patients with localized lesions; however, in the event of
recurrence or metastasis, traditional chemotherapeutic drugs such
as anthracyclines are typically ineffective (2).
Similar to 50% of IMTs, EIMSs primarily express the
anaplastic lymphoma kinase (ALK) protein (1). The gene encoding ALK is located on the
short arm of chromosome 2 (2p23) (https://www.ncbi.nlm.nih.gov/gene/238). The protein
typically manifests as RAN-binding protein 2 (RANBP2)-ALK fusion
oncoprotein with distinctive nuclear membrane localization
(4). Therefore, patients expressing
this fusion protein may benefit from treatment with targeted ALK
inhibitors such as crizotinib (5).
Crizotinib is a small molecule ALK kinase inhibitor that has been
approved for use in patients with various types of ALK+
advanced cancer, such as non-small cell lung cancer (6). However, acquired resistance remains
inevitable and largely limits the efficacy of crizotinib (7). Despite this limitation, the mechanism
and therapeutic strategy for crizotinib-resistance in EIMSs have
been rarely documented in the literature.
In the case presented herein article, a chromosomal
ALK-G1269A mutation was identified in a patient with
crizotinib-resistant EIMS by next generation sequencing (NGS) and
Sanger sequencing. Treatment with the chemically distinct ALK
inhibitor AP26113 (brigatinib) was an effective therapeutic
modality in this patient, and a significant partial response was
observed. The aim of the present study was to explore the
resistance mechanisms and develop follow-up treatment strategies
for patients with EIMSs, and to ultimately improve the symptoms and
survival time of these patients.
Case report
A 28-year-old male suffering from recurrent fever,
abdominal distention, night sweats and obvious fatigue since May
2016 presented at our institution in July 2018. Examination with
computed tomography (CT) and magnetic resonance imaging (MRI)
revealed substantial ascites and a huge caking in the abdomen and
pelvis. Radical surgery was impossible due to discrete miliary
nodules on the abdominal wall surface and extensive peritoneal
adhesions, which were revealed by an exploratory laparotomy. Thus,
a mass biopsy was performed, and the patient was diagnosed with
epithelioid inflammatory myofibroblastic sarcoma composed of
predominantly epithelioid cells (Fig.
1A). IHC performed as previously described (8) revealed that ALK was positive
(3+) in tumor cells (Fig.
1B), whereas other indicators, including cytokeratin, smooth
muscle actin, calretinin, epithelial membrane antigen and myogenin
were negative (data not shown). ALK rearrangement was assessed by
fluorescence in situ hybridization (FISH) using a Vysis ALK
Break Apart Probe kit (Abbott Pharmaceutical Co., Ltd., Lake Bluff,
IL, USA), and tests were performed according to the manufacturer's
protocol (8). ALK rearrangement was
defined as positive when >15% tumor cells exhibited split probe
signals. Ten randomly chosen visual fields were observed on each
section under microscope. The proportion of positive stained cells
was ~30% (Fig. 1C). NGS suggested
that RANBP2-ALK gene fusion had occurred (Fig. 1D). Following the exploratory
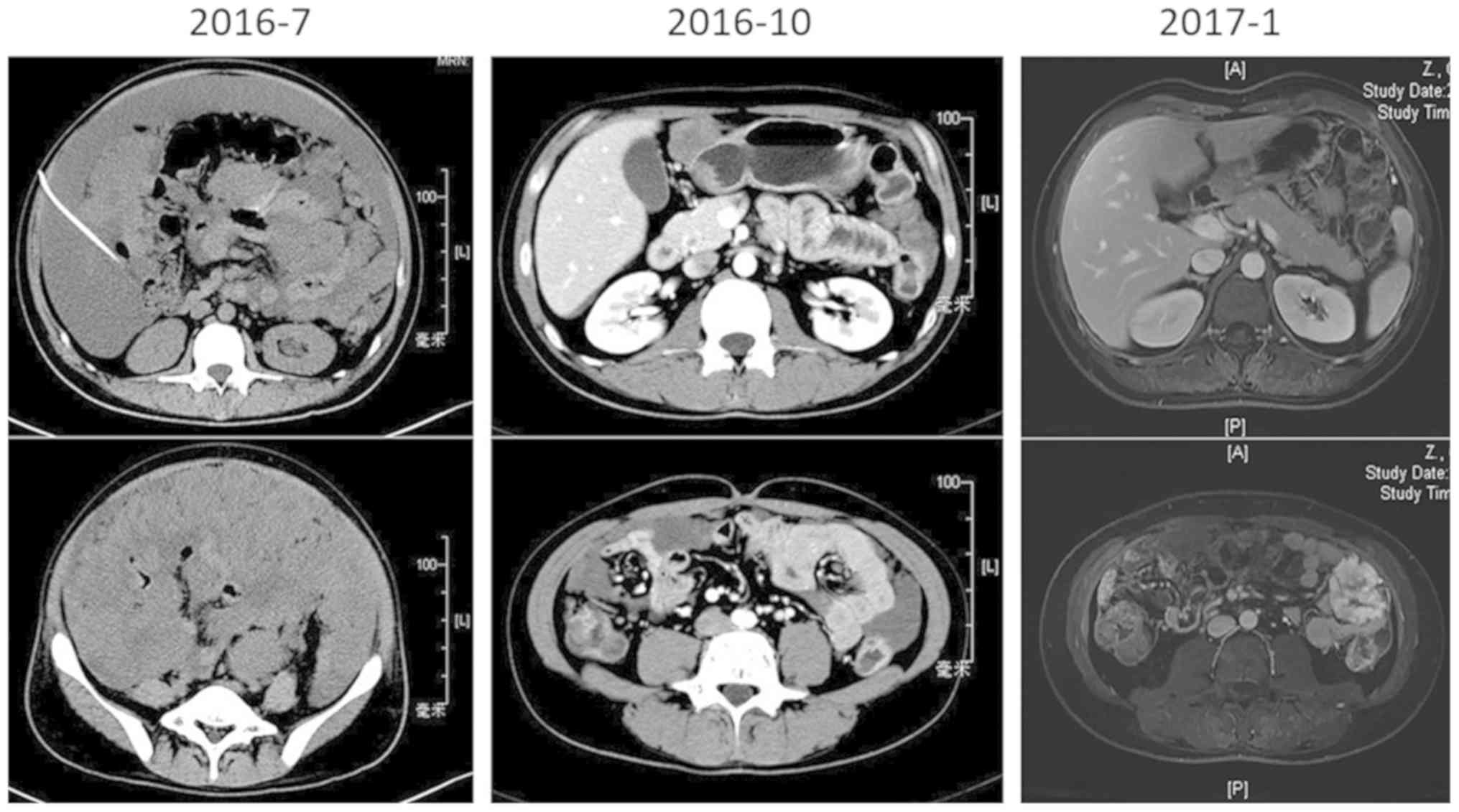
laparotomy, the patient's condition deteriorated rapidly, with
obvious abdominal distention, dyspnea, electrolyte disturbances, a
high level of uric acid (800 µmol/l) and extreme fatigue. From July
2016, crizotinib (200 mg) was administered to the patient twice a
day orally. In September 2016, the patient's previous clinical
symptoms were significantly relieved, and the response evaluation
reached a partial response (PR) according to the Response
Evaluation Criteria in Solid Tumors (RECIST) version 1.1
(http://recist.eortc.org/recist-1-1-2/) (Fig. 2) with slight adverse events, such as
grade 1 edema and myelosuppression according to the NCI Common
Terminology Criteria version 4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/). Until April
2017, the patient presented with abdominal distention, and MRI
scanning confirmed the reappearance of a large amount of ascites,
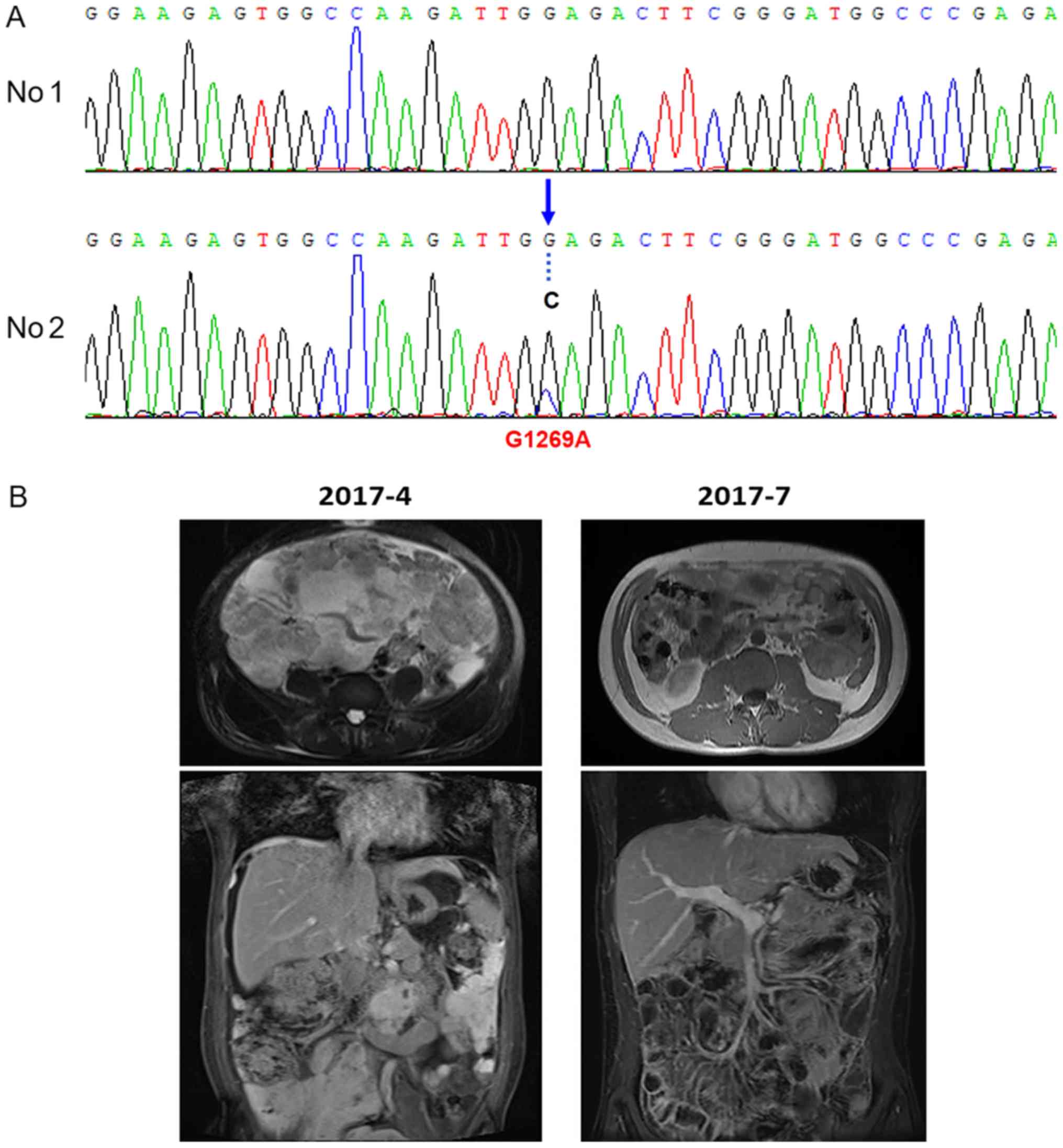
which was classified as progressive disease (PD). NGS, using the
second aspiration biopsy specimen of the abdominal mass identified
RANBP2 as fusion partner and revealed the acquisition of an
ALK-G1269A mutation with a mutational abundance of 22.9% (data not
shown), which was confirmed by Sanger sequencing (Fig. 3A). Primers designed were as shown in
Table I. No additional mutations were
detected. Treatment with AP26113 was initiated in April 2017 (90 mg
daily), and the tumor-associated symptoms resolved rapidly. On July
2017, MRI scanning revealed a 50% reduction in the abdominal mass,
which was classified as a partial response (Fig. 3B). The patient is currently alive.
 | Table I.Sequences for Sanger test. |
Table I.
Sequences for Sanger test.
| Primer | Sequence (5′-3′) | Template strand | Length | Start | Stop | Temperature | GC% | Self
complementarity | Self 3′
complementarity |
|---|
| Forward |
TAAGGCTGTTTCTCTCACAC | Plus | 20 | 213 | 232 | 55.02 | 45.00 | 3.00 | 0.00 |
| Reverse |
CCATAGCCTGAAAAGGAACT | Minus | 20 | 615 | 596 | 55.02 | 45.00 | 3.00 | 1.00 |
| Product length, base
pairs | 403 |
|
|
|
|
|
|
|
|
Discussion
Herein, we identified a chromosomal ALK-G1269A
mutation in a rare and special case of a patient with EIMS who
responded to treatment with AP26113, following the failure of
crizotinib. Given the rarity of EIMSs, no large, well-powered
clinical trials are available, with only ~40 cases being reported
to date. In 2016, Yu et al (2)
reviewed the clinical characteristics and pathological features of
25 cases of EIMS. The present study summarized an additional 18
cases of EIMS reported since 2016 (Table
II). From these reports, certain factors, including age, sex,
tumor location, tumor size, proliferation index, mitosis and
necrosis degree, were not associated with clinical progression.
However, positive ALK expression was closely associated with
recurrence or metastasis. The positive rate of ALK rearrangements
is presently unknown in EIMSs. However, according to the reported
cases, several ALK fusion proteins, including RNA binding protein 2
(RANBP2)-ALK, tropomyosin 3 (TPM3)-ALK, TRK-fused Gene (TFG)-ALK
and ribosome binding protein 1 (RRBP1)-ALK, were identified in EIMS
(Table II). RANBP2-ALK fusion
oncoproteins exhibit distinctive nuclear membrane localization,
indicating that they may be sensitive to targeted kinase
inhibitors, such as crizotinib (3,9–11). In the present case, the young male was
diagnosed with EIMS characterized with predominant epithelioid
cells and ALK positivity via immunohistochemistry. In addition,
FISH using break apart probes revealed ALK rearrangement, and the
NGS assay suggested that RANBP2-ALK gene fusion had occurred. These
findings are consistent with the diagnostic criteria of EIMS.
 | Table II.Clinical features of 18 cases of EIMS
since 2016. |
Table II.
Clinical features of 18 cases of EIMS
since 2016.
| Age/sex | Site | ALK
rearrangement | Fusion type | Crizotinib | Follow up
(months) | Reference |
|---|
| 15/F | Ovary | + | RANBP2-ALK | Yes | ND | I9 |
| 42/M | Abdominal cavity | + | RANBP2-ALK | Yes | Recurred at 8 m, AWD
at 40 m |
|
| 10/F | Abdominal cavity | + | TFG-ALK | No | ANED at 81 m |
|
| 34/M | Liver | + | RANBP2-ALK | No | DOD at 5 m |
|
| 62/M | Abdominal cavity | + | RRBP1-ALK | No | DOD at 2 m |
|
| 14/M | Pelvis | + | TPM3-ALK | No | DOD at 3 m | 10 |
| 76/F | Abdominal cavity | + | RANBP2-ALK | No | DOD at 4 m |
|
| 30/M | Abdominal cavity | + | ND | No | DOD at 8 m |
|
| 26/M | Abdominal cavity | + | RRBP1-ALK | No | Recurred at 7 and 16
m, AWD at 16 m |
|
| 39/F | Abdominal cavity | + | RRBP1-ALK | No | Recurred and AWD at
10 m |
|
| 7/M | Abdominal cavity | + |
RRBP1-ALK-negative | No | DOD at 36 m |
|
| 16/F | Lung | + |
RRBP1-ALK-negative | Yes | Recurred at 1 m, AWD
at 48 m |
|
| 52/F | Small bowel
mesentery | + | ND | No | DOD at 8 m | 11 |
| 37/F | Rectum | + | ND | No | ANED at 8 m | 2 |
| 55/M | Mesentery of
ileum | + | ND | No | ANED AT 10 m |
|
| 22/M | Mesentery of
colon | + | ND | Yes | Recurred at 2 m,
AWD at 14 m |
|
| 58/F | Omentum | + | ND | No | Recurred at 2 m,
DOD at 8 m |
|
| 15/F | Transverse
colon | + | ND | No | ANED at 7 m |
|
Crizotinib has a significant effect in EIMS patients
with ALK-rearrangement; however, it is difficult to avoid acquired
resistance. In the present case report, the patient with EIMS was
resistant to crizotinib after 9 months. Secondary NGS detection
revealed an ALK point mutation G1269A in addition to RANBP2-ALK
fusion, suggesting that the base G was changed to C at position
3806 of the ALK gene. Consequently, the coded amino acid was
changed from glycine to alanine at position 1269, which was located
at the end of the adenosine triphosphate (ATP) binding pocket. The
steric hindrance effect of the mutation affects ALK protein binding
to oxazolidine, therefore ALK G1269A is an acquired resistance
mutation in EIMS. However, only a few cases describing the
mechanisms of crizotinib-resistance have been reported in IMTs and
even fewer in EIMSs, as summarized in Table III (12–14).
 | Table III.ALK crizotinib-resistance reported in
IMTs or EIMSs. |
Table III.
ALK crizotinib-resistance reported in
IMTs or EIMSs.
| Case | Age/sex | Site | Disease | ALK fusion | Treatment 1 | Follow Up 1 | Secondary
mutation | Treatment 2 | Follow Up 2 | (Refs.) |
|---|
| 1 | NA | NA | IMT | RANBP2-ALK | Crizotinib | NA | F1174L | High dosage of
crizotinib; | In vitro
(effective) | 14 |
|
|
|
|
|
|
| TAE684 |
|
|
|
|
| 2 | 32/M | Lung | IMT | TPM3-ALK | Crizotinib | PFS(8m) | Numerous genes | Ceritinib | AWD (8 m); ANED (18
m) | 12 |
| 3 | 36/F | Lung | IMT | DCTN1-ALK | Crizotinib | PFS(29m) | G1269A | Ceritinib | PFS (3 m) | 13 |
|
|
|
|
|
|
|
|
| Oncology, 2017 |
|
|
| 4 | 28/M | Abdomen | EIMS | RANBP2-ALK | Crizotinib | PFS(9m) | ALK-G1269A | AP26113 | AWD at 8 m | Present study |
In addition to G1269A, other secondary mutations,
such as L1152R, C1156Y, F1174L, L1196M, G1202R and S1206Y, were
also identified in vitro, xenograft models or patients with
ALK-rearranged tumors, such as non-small-cell lung cancers
(7). These patients were sensitive to
high dosages of crizotinib or second-generation ALK inhibitors,
such as ceritinib and alectinib (15,16). In
the present article, AP26113 was still effective in the patient
with crizotinib resistance. AP26113 is also a second-generation ALK
inhibitor with a double inhibitory effect on ALK and EGFR (17). Similar to crizotinib, AP26113 could
relieve the clinical symptoms of the patient, without obvious side
effects. Thus, the quality of life of this patient was greatly
improved. Previously, certain novel agents, such as heat shock
protein (HSP) 90 inhibitors, have been demonstrated to be effective
in overcoming crizotinib-resistance in preclinical models and Phase
I–II studies (14). With a deeper
understanding of EIMS, more molecular mechanisms and potential
treatments will be available in the future.
Acknowledgements
The authors would like to thank Ms. Huiyan Li from
the institute of Precision Medicine, 3D Medicines Inc. (Shanghai,
China) for their excellent technical assistance with NGS and Sanger
sequencing.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81502003) and the
Shanghai Committee of Science and Technology (grant nos.
14ZR1406500 and 15411961900).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX, YY and TL conceived and designed the study. XX,
HL and KP performed the experiments. LC and YH provided the FISH
results. YF and YS performed the operation on the patient. XX wrote
the paper. YY and TL reviewed and edited the manuscript. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhongshan Hospital Affiliated to Fudan University (Shanghai,
China). The research involved one patient and their tissues, and
informed consent for use of their clinical images and tissues was
obtained from the patient.
Patient consent for publication
Identifying information, including names, initials,
date of birth or hospital numbers, images or statements were not
included in the manuscript. And the patient has provided written
informed consent for publication.
Competing interests
The authors have declared that they have no
competing interests.
Glossary
Abbreviations
Abbreviations:
|
EIMS
|
epithelioid inflammatory
myofibroblastic sarcoma
|
|
ALK
|
anaplastic lymphoma kinase
|
|
IMT
|
inflammatory myofibroblastic tumor
|
|
NGS
|
next-generation sequencing
|
|
H&E
|
hematoxylin and eosin
|
|
FFPE
|
formalin fixed paraffin embedded
|
|
PCR
|
polymerase chain reaction
|
|
IHC
|
immunohistochemistry
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
PR
|
partial response
|
|
PD
|
progressive disease
|
References
|
1
|
Mariño-Enríquez A, Wang WL, Roy A,
Lopez-Terrada D, Lazar AJ, Fletcher CD, Coffin CM and Hornick JL:
Epithelioid inflammatory myofibroblastic sarcoma: An aggressive
intra-abdominal variant of inflammatory myofibroblastic tumor with
nuclear membrane or perinuclear ALK. Am J Surg Pathol. 35:135–144.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu L, Liu J, Lao IW, Luo Z and Wang J:
Epithelioid inflammatory myofibroblastic sarcoma: A
clinicopathological, immunohistochemical and molecular cytogenetic
analysis of five additional cases and review of the literature.
Diagn Pathol. 11:672016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Q, Kan Y, Zhao Y, He H and Kong L:
Epithelioid inflammatory myofibroblastic sarcoma treated with ALK
inhibitor: A case report and review of literature. Int J Clin Exp
Pathol. 8:15328–15332. 2015.PubMed/NCBI
|
|
4
|
Kruczynski A, Delsol G, Laurent C,
Brousset P and Lamant L: Anaplastic lymphoma kinase as a
therapeutic target. Expert Opin Ther Targets. 16:1127–1138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kimbara S, Takeda K, Fukushima H, Inoue T,
Okada H, Shibata Y, Katsushima U, Tsuya A, Tokunaga S, Daga H, et
al: A case report of epithelioid inflammatory myofibroblastic
sarcoma with RANBP2-ALK fusion gene treated with the ALK inhibitor,
crizotinib. Jpn J Clin Oncol. 44:868–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heigener DF and Reck M: Crizotinib. Recent
Results Cancer Res. 211:57–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voena C and Chiarle R: The battle against
ALK resistance: Successes and setbacks. Expert Opin Investig Drugs.
21:1751–1754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su D, Zhang D, Chen K, Lu J, Wu J, Cao X,
Ying L, Jin Q, Ye Y, Xie Z, et al: High performance of targeted
next generation sequencing on variance detection in clinical tumor
specimens in comparison with current conventional methods. J Exp
Clin Cancer Res. 36:1212017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang H, Langstraat CL, Visscher DW, Folpe
AL and Schoolmeester JK: Epithelioid inflammatory myofibroblastic
sarcoma of the ovary with RANB2-ALK fusion: Report of a case. Int J
Gynecol Pathol. 37:468–472. 2018.PubMed/NCBI
|
|
10
|
Lee JC, Li CF, Huang HY, Zhu MJ,
Mariño-Enríquez A, Lee CT, Ou WB, Hornick JL and Fletcher JA: ALK
oncoproteins in atypical inflammatory myofibroblastic tumours:
Novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic
sarcoma. J Pathol. 241:316–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang N, Yang QJ, Deng YT, Feng X, Xia HS,
Zhang YG, Wang MW, Wu D, Zhou H and Guo F: Epithelioid inflammatory
myofibroblastic sarcoma of small bowel mesentery: Report of a case.
Zhonghua Bing Li Xue Za Zhi. 46:201–202. 2017.(In Chinese).
PubMed/NCBI
|
|
12
|
Mansfield AS, Murphy SJ, Harris FR,
Robinson SI, Marks RS, Johnson SH, Smadbeck JB, Halling GC, Yi ES,
Wigle D, et al: Chromoplectic TPM3-ALK rearrangement in a patient
with inflammatory myofibroblastic tumor who responded to ceritinib
after progression on crizotinib. Ann Oncol. 27:2111–2117. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michels SYF, Scheel AH, Wündisch T,
Heuckmann JM, Menon R, Puesken M, Kobe C, Pasternack H, Heydt C,
Scheffler M, et al: ALKG1269A mutation as a potential mechanism of
acquired resistance to crizotinib in an ALK-rearranged inflammatory
myofibroblastic tumor. NPJ Precis Oncol. 1:42017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki T, Okuda K, Zheng W, Butrynski J,
Capelletti M, Wang L, Gray NS, Wilner K, Christensen JG, Demetri G,
et al: The neuroblastoma-associated F1174L ALK mutation causes
resistance to an ALK kinase inhibitor in ALK-translocated cancers.
Cancer Res. 70:10038–10043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishio M, Murakami H, Horiike A, Takahashi
T, Hirai F, Suenaga N, Tajima T, Tokushige K, Ishii M, Boral A, et
al: Phase I study of ceritinib (ldk378) in Japanese patients with
Advanced, Anaplastic lymphoma kinase-rearranged non-small-cell lung
cancer or other tumors. J Thorac Oncol. 10:1058–1066. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katayama R, Lovly CM and Shaw AT:
Therapeutic targeting of anaplastic lymphoma kinase in lung cancer:
A paradigm for precision cancer medicine. Clin Cancer Res.
21:2227–2235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang WS, Liu S, Zou D, Thomas M, Wang Y,
Zhou T, Romero J, Kohlmann A, Li F, Qi J, et al: Discovery of
brigatinib (AP26113), a phosphine oxide-containing, potent, orally
active inhibitor of anaplastic lymphoma kinase. J Med Chem.
59:4948–4964. 2016. View Article : Google Scholar : PubMed/NCBI
|