Introduction
Associated to an increase in survival of cancer
patients (1), in recent years it has
been shown a reduction in mortality of oncological patients in the
Intensive Care Unit (ICU) (2,3). Those
patients, however, occupy 15% of all ICU beds, with significant
costs (4,5). In the sub-population of
onco-hematological patients, Acute Respiratory Failure (ARpF) is a
common cause of ICU admission (6,7) and the
strongest risk factor for mortality (8). The economic impact of ARpF, especially
mechanical ventilation (MV), is enormous: Mean costs of
hospitalization in patients with MV for more than 4 days exceeds
US$60,000 and, in prolonged VM (>21 days), $200,000. Those
expenses make up for 12% of hospital costs, in a total of $27
billion dollars in the USA alone (9).
Common causes of ARpF in patients with hematological
malignancy are infections (bacterial and opportunistic), acute
pulmonary edema, alveolar hemorrhage, neoplastic infiltrate,
chemotherapy adverse reactions, specific and unspecific
inflammatory diseases, among other uncommon pathologies (10,11).
In these patients, surgical lung biopsy (SLB) can be
performed when etiology has not been established by less invasive
tests (cultures, serologies) and procedures [bronchoalveolar lavage
(BAL), transbronchial biopsy] (12).
The aim of the present study was to evaluate open
SLB as a diagnostic strategy in onco-hematological patients with
ARpF without defined etiology, in MV, at the ICU. Specifically, we
seek to understand what is the impact of the biopsy in medical
management, what are the etiologies commonly found and what are the
complications and outcomes of this group of patients submitted to
this intervention.
Materials and methods
Study design and setting
Observational, retrospective study, based on
analysis of databases, physical charts and electronic medical
records of patients admitted to the ICU of a dedicated oncology
hospital in southern Brazil. It is an 8 bed general ICU (medical
and surgical) that assists almost exclusively oncological
patients.
Patients admitted to the ICU between 2010 and 2016
were evaluated. Through databases, we selected all
onco-hematological patients with ARpF and submitted to open SLB. We
analyzed also physical charts to get further information regarding
the realization of SLB, treatment plans before and after the
biopsy, histopathology results, complications, and outcomes. The
same pathologist (AGB) that performed the histological studies
revised the microscope slides for this study.
Patients
Patients with onco-hematological disorders admitted
to the general ICU from 2010 to 2016 with bilateral pulmonary
infiltrates and ARpF, receiving MV, that underwent SLB during ICU
stay. There were no exclusion criteria, except 2 patients whose
data from physical charting and electronic medical records were
scarce and insufficient. Based on these inclusion criteria, we
analyze electronic medical records and paper (physical) charting of
all 17 that met the criteria.
Definitions and variables
Onco-hematological disorders: Leukemias, lymphomas,
multiple myeloma, bone marrow aplasia and chronic
lymphoproliferative disorders.
ARpF: Was defined clinically by the assistant ICU
team.
Acute Respiratory Distress Syndrome (ARDS): Was
defined according to the Berlin Consensus Conference (13).
Acute Renal Failure (ARnF): Was defined according to
the AKIN criteria (14).
Sepsis: Was defined according to Sepsis-3 criteria
(15).
The diagnoses of comorbidities were made by the ICU
and onco-hematology healthcare team, without pre-selected criteria
for the study.
During ICU stay, the patients were treated according
to the ICU's own protocols regarding mechanical ventilation and
weaning, sedation, nutrition, as well as management of specific
conditions such as infections, sepsis and choosing antibiotics.
SLB: Open technique was the only one performed at
our study, using mini-thoracotomy access, done at the bedside or
the operating room. It was performed by 2 thoracic surgeons and 1
oncological surgeon experienced in the procedure. The procedure,
indications and their routines remained unchanged throughout the
period relating to the collection of the study.
Statistical methods
A descriptive statistical analysis was performed and
percentages expressed as frequency, mean and standard deviation.
Data were compared using the Chi-square test and
Statistica® 7.0 software (StatSoft, Inc., Tulsa, OK,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Ethical approval and Consent to
participate
This study was conducted in accordance with the
recommendations of Resolution 466/2012 of the Brazilian National
Health Council. The project was approved by UNIOESTE (Western
Paraná State University)'s Committee on Ethics in Research
involving human beings. Due to the retrospective nature of this
study the requirement for patient or the families' written informed
consent was waived.
Results
The majority of the 17 patients submitted to SLB
were young adults (mean: 33.8 years old-min=12; max=58) men
(70.6%), without comorbidities (70.5%) and diagnosed with leukemia
(53%) or lymphoma (41%). Most of them (88%) were submitted to
chemotherapy recently. ICU admission cause was medical in all
patients (no surgical cases), with mean MV time before biopsy of 2
days and total MV time of about 7 days. Immediately before the
biopsy, these patients were being ventilated with high PEEPs (mean:
11 cmH2O), frequently lightly thrombocytopenic (mean
99×103/mm3), using vasoactive drugs and
receiving several antibiotics (88% with 3 or more) with frequent
covering of opportunistic infections-any antifungal in 70.5% of
patients, antiviral in 41% or Trimethoprim-sulfamethoxazole in
41.2%. Most common comorbidities were Systemic Hypertension and
Diabetes Mellitus (Table I).
 | Table I.Characteristics and clinical course of
patients submitted to SLB (n=17). |
Table I.
Characteristics and clinical course of
patients submitted to SLB (n=17).
| Characteristics | Value |
|---|
| Male sex, % | 70.6% |
| Age, years, mean ±
SD | 33.8±16.45 |
| APACHE II admission,
mean ± SD | 24.5±8.95 |
| Type of neoplasm,
% |
|
|
Solid | 0 |
|
Onco-hematological | 100 |
|
Multiple
myeloma | 0 |
|
Lymphoma | 41 |
|
Leukemia | 53 |
|
Othersa | 6 |
| Other
comorbiditiesa | 29.5 |
|
CHF | 6 |
|
DM | 12 |
|
Other neoplasm
(cured or not) | 6 |
|
Othersa | 12 |
| Previous
oncological treatments, % |
|
| Recent
chemotherapy | 88 |
| Recent
or previous radiotherapy | 12 |
| Recent
or previous BMT | 12 |
| Cause of ICU
admission, % |
|
|
Medical | 100 |
| Total MV, days,
mean ± SD |
7.2±4.54 |
| MV time before SLB,
days ± SD | 2.1±2.8 |
| Parameters
immediately prior to SLB |
|
| PEEP,
cmH2O, mean ± SD | 11.0±4.86 |
|
PaO2/FiO2,
mean ± SD | 258.0±89.0 |
|
Norepinephrine dose,
µg/kg/min, mean ± SD | 0.4±0.57 |
| Serum
creatinine, mg/dl, mean ± SD | 1.3±0.60 |
|
Platelets, cells
×103/mm3, mean ± SD | 95.9±53.1 |
| Use of
antibiotics/antifungals/antivirals prior to SLB, % |
|
|
None | 0 |
| 1-2
antibiotics/antifungals | 12 |
| ≥3 | 88 |
|
Glycopeptides | 88.2 |
|
Carbapenems | 70.5 |
|
Amphotericin B or
equinocandins | 52.9 |
|
Trimethoprim-sulfamethoxazole | 41.2 |
|
Gancyclovir | 35.3 |
|
Polymyxin | 17.6 |
|
Fluconazole | 17.6 |
|
3rd - 4th
generation cephalosporin | 12 |
|
Aminoglycosides | 12 |
|
Penicillin +
penicillinase inhibitor | 6 |
|
Acyclovir | 6 |
|
Quinolones | 0 |
| Total ICU length of
stay, days, mean ± SD | 9.5±5.68 |
| ICU mortality,
% | 88.2 |
| Death
prior to histopathology result | 35.3 |
| Hospital mortality,
% | 88.2 |
The most commonly found etiology on histopathology
was infectious (52.3%)-bacterial infections, Cytomegalovirus (CMV)
pneumonitis, and Pneumocystis jirovecii pneumonia accounted
for 17.6% of findings each. Unspecific inflammatory conditions were
the second most common finding (ARDS and unspecific infiltrate),
present in 29.5% of biopsies. Alveolar hemorrhage was shown in 3
biopsies (17.6%), neoplastic infiltrate in 2 (12%) and Pulmonary
Embolism in 1 (6%) (Table II, and
Figs. 1–5).
 | Table II.Biopsy histopathological results
(n=17). |
Table II.
Biopsy histopathological results
(n=17).
| No pathological
findings | %a |
|---|
| CMV | 17.6 |
| ARDS | 23.5 |
| Bacterial
pneumonia | 17.6 |
| Alveolar
hemorrhage | 17.6 |
| Pneumocystis
jirovecii | 17.6 |
| Neoplastic
infiltrate | 12 |
| PE | 6 |
| Unspecific
infiltrate | 6 |
| Others | 0 |
There were only minor complications related to the
procedure, but no major or significative early complications
(Table III).
 | Table III.Complications associated with the
procedure (n=17). |
Table III.
Complications associated with the
procedure (n=17).
| Complications | Incidence, % |
|---|
| Hemoptysis,
minor | 0 |
| Hemoptysis,
major | 0 |
|
Pneumotorax/broncho-pleural fistula | 0 |
| Minor bleeding from
the thorax draina | 41 |
| Major bleeding from
the thorax drainb | 9 |
| Need to
re-operation | 0 |
| Worsening of
hypoxemia/need for increased ventilatory parameters | 9 |
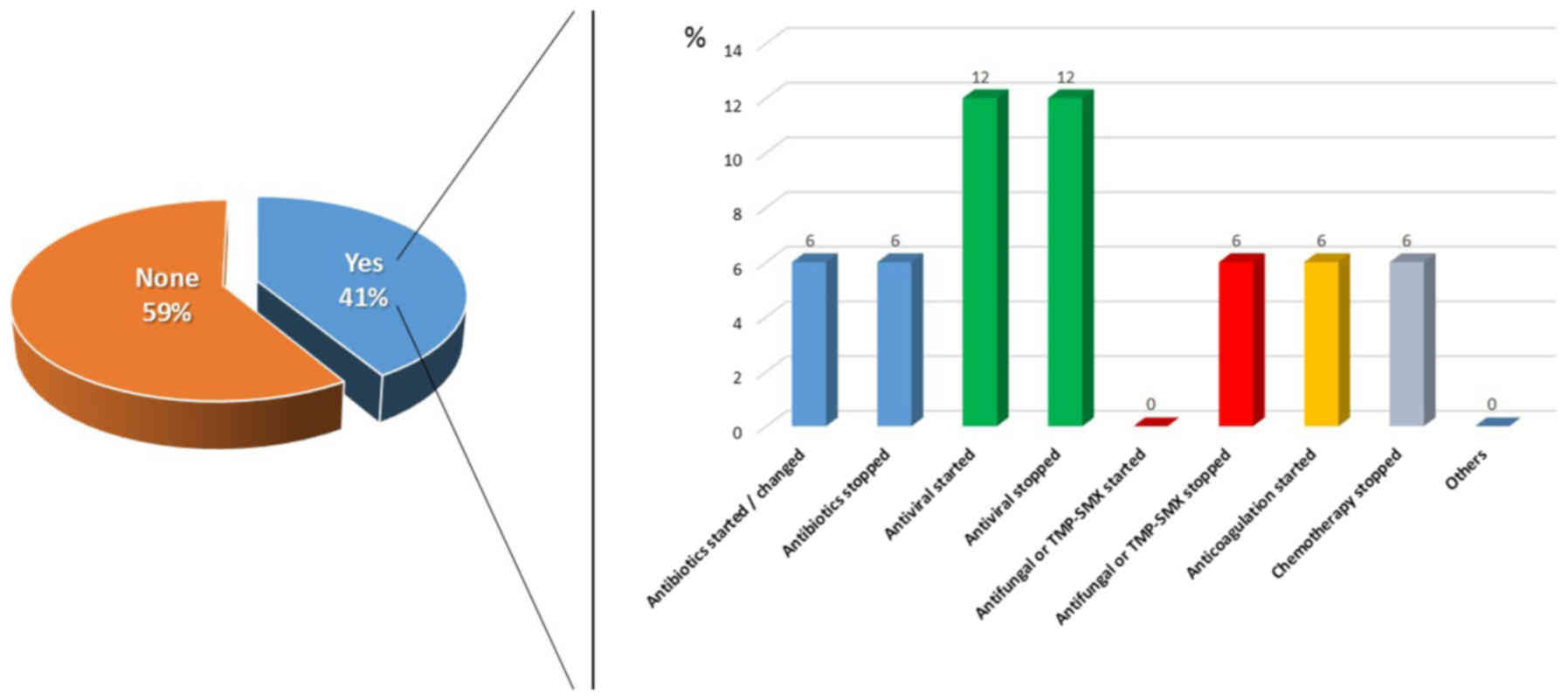
Therapeutic modifications occurred in 41% of
patients. However, it has to be considered that 35.3% patients died
before the histology results were available. Thus, there were
therapeutic modifications in 63.3% of patients whose biopsy's
results were available before death. The most common therapeutic
impact was changing in antibiotics-starting or stopping antibiotics
(1 patient each), antivirals (2 patients) and antifungals (1
patient). In one patient it was started anticoagulation and in
another chemotherapy was discontinued (Fig. 1).
The mean ICU length of stay was of 9.5 days, with
high ICU and in-hospital mortality (88.2%). Approximately 1/3
(35.3%) of patients died before the availability of biopsy
results.
Discussion
The indication for SLB in onco-hematological
patients with ARpF is limited, according to the literature.
Patients with ARpF of unknown etiology after less invasive tests,
with clinical deterioration and BAL that is inconclusive, with low
diagnostic yield or high risk of complications, are generally the
circumstances in which this procedure is indicated by the
literature (12,16–18).
In this present study, lung biopsy has evidenced a
specific diagnosis in 70.5% of patients, similar to the 60–65% of
previous studies (16–20). Infection was the most common finding
(about 70% of biopsies), despite previous studies showing
unspecific infiltrates (fibrosis, interstitial pneumonitis, ARDS)
as the most prevalent biopsy finding, even though infections were
still common (16–18). Among infections, the opportunistic
ones were the most commonly found (52.8%) (16–20).
Previous studies have had inconsistent findings in
terms of etiology prevalence. Infection was usually reported as the
most common cause of ARpF in oncohematological patients. Among
infections, bacterial and mixed are the most common previously
reported etiologies. Opportunistic infections-such as fungal, viral
and P. jirovecii are also frequently described (up to 30%).
Non-infectious causes were less frequent, but very relevant
management-wise-acute pulmonary edema (most incident
non-infectious), alveolar hemorrhage, pulmonary embolism, TRALI,
radiation pneumonitis, neoplastic infiltrate, Bronchiolitis
obliterans/Organizing Pneumonia (BOOP) and chemotherapy-related
adverse reactions (10,11).
In our study, fungal infections were uncommon
(17.6%)-only 2 patients with P. jirovecii and none with
Aspergillosis-, differently from other studies that have shown
fungal infections as the most common and Aspergillosis as a
frequent finding (17,18,20). A
possible reason for no Aspergillosis findings is that we had few
(two) patients that had underwent BMT, a population with a high
incidence of that infection (21).
Regardless of that, the high prevalence of antifungals and
Trimethoprim-Sulfamethoxazole (70.5 and 41.2%, respectively) in our
patients raised our attention as well. Other infectious causes,
such as CMV and bacterial pneumonia, were found in our study and
reported by previous literature with highly variable incidence.
Unlike previous studies, we did not find any biopsy showing
tuberculosis.
Specific inflammatory findings, such as BOOP and
granulomatous reactions were not found in our case series, even
though they are reported as frequent in this patient group by the
literature. Unspecific findings (ARDS and interstitial fibrosis)
were found in 29.5% of biopsies, which is a similar prevalence to
that of other studies (about 1/3). Alveolar hemorrhage was more
prevalent (17.6%- 3 biopsies) in our study than reported
previously. Neoplastic infiltrates, present in 2 biopsies (12%),
was a frequent finding in the literature (16–20,22,23).
Therapeutic modifications can be defined as starting
or stopping of therapeutic strategies or adoption of exclusive
palliative care (therapeutic limitation). The rate of therapeutic
modification due to the biopsy results was 41%, but, taking into
account only the patients that were alive when the
histopathological results were available, the rate was higher:
63.3%. This data corroborates previous findings, in which
therapeutic modification was performed in 40–70% of patients, with
specific diagnosis associated with lower mortality (16–20,22,23).
Recently, studies on SLB in patients with pulmonary infiltrates and
ARpF, in a population with 50% of immunocompromised patients (but
not onco-hematological), has shown the importance of SLB in the
diagnosis of potentially reversible diseases, particularly through
the usage of high dose corticoids, and, consequentially, with the
potential to lower mortality rate (22).
Gay et al (16) have studied necropsies of oncological
patients with lung infiltrates, adding diagnostic accuracy.
Unfortunately, due to cultural and organizational problems
(especially due to low societal acceptance of necropsies), we do
not have access in our institution to necropsies done for
scientific purposes; thus, we could not compare our SLB findings
with those of post-mortem histopathological study. Also in relation
to the transbronchial biopsy it was not possible to make a
comparison with the data of the patients submitted to SLB, since
the majority of the patients had high levels of PEEP and several
had coagulation disorders/thrombocytopenia, for which surgical
procedure was considered the safest option, both to contain
possible bleeding and to prevent aerial fistulae.
However, it is necessary to outline that previous
understanding of ‘therapeutic modification’ could underestimate the
value of the SLB. Even in patients with unspecific findings, the
information provided by the biopsy could have relevance in terms of
avoiding further diagnostic and/or therapeutic interventions that
could have been pursued if there was no histopathological
confirmation. Particularly, it could lead to the complete
suspension of aggressive and ‘heroic’ efforts, allowing palliative
and end-of-life care to provide dignity to the patients and family
members. A relevant fact is that about one third of the patients
died before the histopathological result. This denotes the high
severity of the patients (since the histopathological result in our
institution is available between 36–96 h on average), but also
possibly a delay in indicating and performing the procedure. In any
case, the authors intentionally kept these patients, not only by
the number of patients, but also to demonstrate a ‘real life’
situation (even if with non-ideal results).
In relation to SLB complications, in our study, no
death occurred during the biopsy or that had been directly
attributed to the procedure-for example, due to hemorrhagic shock
or pneumothorax. Several authors had not reported, as well,
attributable deaths to SLB, however, there has been literature
reports of need for reoperation and increased time of MV (16–19,22).
There was no report in our study of pneumothorax and a very low
incidence of major hemorrhages. No patient needed reoperation.
Previous studies have shown 10–20% of complication in this
population, and recently published study (in a non-oncological
population) evidenced 12% of minor complications. Most commonly
reported complications are bleeding and pneumothorax (including
bronchopleural fistula), but there is also the chance of worsening
respiratory failure and need to MV (in non-mechanically ventilated
patients). In our study all patients were in MV already (16–19,22). It
should be emphasized, however, that due to the high mortality and
intrinsic severity of the patients, possibly early or late
unrecognized complications (such as worsening respiratory function
or secondary infections) could have occurred, even if not
recognized as secondary to the procedure itself.
Another question that warrants further analyzes is
the impact of SLB in mortality. In our study, we found a mortality
rate of almost 90% (no deaths were directly attributable to the
procedure or its complications), higher than previously reported
(18–45%). Nevertheless, it is relevant to outline that in our study
all the patients were in MV with high PEEP levels, which denotes
higher severity and mortality (16–20,22,23).
Even though the authors could not correlate
performing SLB and mortality, this was higher than previously
reported (16–18). We believe that the high mortality we
found could be secondary to several factors, such as: 1) Higher
illness severity when compared to previous SLB population-which had
a share of non-intubated patients. In our sample, all patients were
intubated, most on high dose vasopressors and high PEEP levels. 2)
The characteristics and eventual deficiencies in treatment and
management. It has been shown, for example, that sepsis and ARDS
patients in low to mid-income countries have worst outcomes than in
high-income countries (24–26).
This study has several limitations that preclude the
eventual generalization of its findings. It was a single center
study, which makes the sample size (n) relatively small, but in
line with the size of previous studies. This reduced size of the
sample could justify the incidence of diseases (such as the lack of
patients with tuberculosis) or even the low incidence of
complications. Besides, for its local characteristics and possibly
for a common limitation in developing countries, there was a
paucity of less invasive procedures (such as BAL and serological
tests in determined situations), and without performing necropsies
in deceased patients (for eventual comparison), due to
unavailability and/or high associated costs. However, this
limitation puts it closer to the reality of developing countries.
In addition to it, its design, a retrospective, observational, the
reliability of data can be affected by the absence of previous
uniformization of concepts to their registry, in addition to
possibly time-related diagnosis and management changes (although
the authors did not detect significant changes in the medical
records evaluation). Also, it cannot establish the impact of the
intervention (SLB) in morbidity and mortality, due to its design
(observational, non-interventionist). Despite that, the goal of
this study was to evaluate the ‘real life’ of an oncology ICU in a
developing country, being appropriate for this objective.
Onco-hematological patients submitted to SLB in the
ICU had an infectious process, mainly opportunistic, as the most
common biopsy finding, followed by unspecific infiltrate.
Therapeutic modification could be made in one third of the
patients. However, hospital mortality in these patients was very
high, possibly due to their illness severity. SLB helps the
therapeutic management of these patients with few complications
related to the intervention.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
EMC and PADD designed the study, collected and
analyzed the data, and wrote the manuscript. AGB, DAP, ADC and RCS
analyzed the data, wrote the manuscript and reviewed the
manuscript. All the authors read and approved the final
manuscript.
Ethical approval and consent to
participate
This study was conducted in accordance with the
recommendations of Resolution 466/2012 of the Brazilian National
Health Council. The project was approved by Universidade Estadual
do Oeste do Paraná Committee on Ethics in Research involving human
beings (Cascavel, Brazil). Due to the retrospective nature of the
study the requirement for patients' written informed consent was
waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APACHE
|
acute physiology and chronic health
evaluation score
|
|
ARDS
|
acute respiratory distress
syndrome
|
|
ARnF
|
acute renal failure
|
|
ARpF
|
acute respiratory failure
|
|
BMI
|
body mass index
|
|
BMT
|
bone marrow transplant
|
|
BOOP
|
bronchiolitis obliterans/organizing
pneumonia
|
|
CHF
|
congestive heart failure
|
|
ICU
|
intensive care unit
|
|
MV
|
mechanical ventilation
|
|
PaO2
|
pressure of arterial oxygen
|
|
PEEP
|
positive end-expiratory pressure
|
|
SLB
|
surgical lung biopsy
|
|
SD
|
standard deviation
|
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and
Prevalence Worldwide in 2012 v1.0. Int Agency Res Cancer Lyon,
France: 2013
|
|
2
|
Brenner H: Long-term survival rates of
cancer patients achieved by the end of the 20th century: A period
analysis. Lancet. 360:1131–1135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angus DC, Barnato AE, Linde-Zwirble WT,
Weissfeld LA, Watson RS, Rickert T and Rubenfeld GD; Robert Wood
Johnson Foundation ICU End-Of-Life Peer Group, : Use of intensive
care at the end of life in the United States: An epidemiologic
study. Crit Care Med. 32:638–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schellongowski P, Sperr WR, Wohlfarth P,
Knoebl P, Rabitsch W, Watzke HH and Staudinger T: Critically ill
patients with cancer: Chances and limitations of intensive care
medicine-a narrative review. ESMO Open. 1:e0000182016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nazer L, Al-Shaer M and Hawari F: Drug
utilization pattern and cost for the treatment of severe sepsis and
septic shock in critically ill cancer patients. Intern J Clin
Pharm. 35:1245–1250. 2013. View Article : Google Scholar
|
|
6
|
Groeger JS, Glassman J, Nierman DM,
Wallace SK, Price K, Horak D and Landsberg D: Probability of
mortality of critically ill cancer patients at 72 h of intensive
care unit (ICU) management. Support Care Canc. 11:686–695. 2003.
View Article : Google Scholar
|
|
7
|
Benoit DD, Vandewoude KH, Decruyenaere JM,
Hoste EA and Colardyn FA: Outcome and early prognostic indicators
in patients with a hematologic malignancy admitted to the intensive
care unit for a life-threatening complication. Crit Care Med.
31:104–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pastores SM and Voigt LP: Acute
respiratory failure in the patient with cancer: Diagnostic and
management strategies. Crit Care Clin. 26:21–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooke CR: Economics of mechanical
ventilation and respiratory failure. Crit Care Clin. 28:39–55.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vadde R and Pastores SM: Management of
acute respiratory failure in patients with hematological
malignancy. J Intens Care Med. 31:627–641. 2016. View Article : Google Scholar
|
|
11
|
Azoulay E, Mokart D, Lambert J, Lemiale V,
Rabbat A, Kouatchet A, Vincent F, Gruson D, Bruneel F,
Epinette-Branche G, et al: Diagnostic strategy for hematology and
oncology patients with acute respiratory failure: Randomized
controlled trial. Am J Respir Crit Care Med. 182:1038–1046. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azoulay E and Schlemmer B: Diagnostic
strategy in cancer patients with acute respiratory failure. Intens
Care Med. 32:808–822. 2006. View Article : Google Scholar
|
|
13
|
ARDS Definition Task Force, ; Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
14
|
Mehta RL, Kellum JA, Shah SV, Molitoris
BA, Ronco C, Warnock DG and Levin A; Acute Kidney Injury Network, :
Acute Kidney Injury Network: Report of an initiative to improve
outcomes in acute kidney injury. Crit Care. 11:R312007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gay J, Lemiale V, Meignin V, Bron C, De
Bazelaire C, Schnell D, Canet E, Seguin A and Azoulay E: Diagnostic
contribution from pulmonary biopsies in hematology patients with
acute respiratory failure from undetermined etiology. Minerva
Anestesiol. 79:853–860. 2013.PubMed/NCBI
|
|
17
|
Zihlif M, Khanchandani G, Ahmed HP and
Soubani AO: Surgical lung biopsy in patients with hematological
malignancy or hematopoietic stem cell transplantation and
unexplained pulmonary infiltrates: Improved outcome with specific
diagnosis. Am J Hematol. 78:94–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White DA, Wong PW and Downey R: The
utility of open lung biopsy in patients with hematologic
malignancies. Am J Resp Crit Care Med. 161:723–729. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Snyder CL, Ramsay NK, Mcglave PB, Ferrell
KL and Leonard AS: Diagnostic open-lung biopsy after bone marrow
transplantation. J Pediatr Surg. 25:871–877. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kramer MR, Berkman N, Mintz B, Godfrey S,
Saute M and Amir G: The role of open lung biopsy in the management
and outcome of patients with diffuse lung disease. Ann Thor Surg.
65:198–202. 1998. View Article : Google Scholar
|
|
21
|
Hayes-Jordan A, Benaim E, Richardson S,
Joglar J, Srivastava DK, Bowman L and Shochat SJ: Open lung biopsy
in pediatric bone marrow transplant patients. J Pediatr Surg.
37:446–452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gerard L, Bidoul T, Castanares-Zapatero D,
Wittebole X, Lacroix V, Froidure A, Hoton D and Laterre PF: Open
lung biopsy in nonresolving acute respiratory distress syndrome
commonly identifies corticosteroid-sensitive pathologies,
associated with better outcome. Crit Care Med. 46:907–914. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hilbert G, Gruson D, Vargas F, Valentino
R, Gbikpi-Benissan G, Dupon M, Reiffers J and Cardinaud JP:
Noninvasive ventilation in immunosuppressed patients with pulmonary
infiltrates, fever, and acute respiratory failure. N Engl J Med.
344:481–487. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rudd KE, Kissoon N, Limmathurotsakul D,
Bory S, Mutahunga B, Seymour CW, Angus DC and West TE: The global
burden of sepsis: Barriers and potential solutions. Crit Care.
22:2322018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Becker JU, Theodosis C, Jacob ST, Wira CR
and Groce NE: Surviving sepsis in low-income and middle-income
countries: New directions for care and research. Lancet Infect Dis.
9:577–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laffey J, Madotto F, Bellani G, Pham T,
Fan E, Brochard L, Amin P, Arabi Y, Bajwa EK, Bruhn A, et al:
Geo-economic variations in epidemiology, patterns of care, and
outcomes in patients with acute respiratory distress syndrome:
Insights from the LUNG SAFE prospective cohort study. Lancet Resp
Med. 5:627–638. 2017. View Article : Google Scholar
|