Introduction
Cholangiocarcinoma (CCA) is a tumor originating from
biliary epithelial cells. The incidence of CCA in Southeast Asia,
especially in northeast Thailand, is remarkably high with liver
fluke infection as a major risk factor. The prognosis of CCA is
poor with high mortality rates as the majority of patients are
diagnosed at a late stage with a high incidence of metastasis
(1–3). Reliable diagnostic/prognostic markers
are critical for CCA treatment. For CCA diagnosis, there are
several markers which are reported to be effective diagnostic and
prognostic markers such as carcinoembryonic antigen, carbohydrate
antigen (CA) 19-9, CA242, CCA-associated carbohydrate antigen,
mucin glycoproteins and cytokines (4). Even combinations of those markers are
still insufficient for sensitivity and specificity (5).
Recently, using mass spectrometry, Chua-On et
al (6) demonstrated the
different expressions of mitochondrial proteins in cancerous and
adjacent tissues of CCA. Since the main energy of cancer cells is
produced by glucose metabolism, the glucose metabolism
involved-proteins in the mitochondria could be potential
diagnostic/prognostic markers. Among all the candidate proteins,
pyruvate dehydrogenase kinase (PDK) 3 showed the highest fold
increase. PDK is a Ser/Thr kinase that inactivates mitochondrial
pyruvate dehydrogenase by site-specific phosphorylation and plays a
key role in regulation of the Warburg effect in cancer cells
(7). There are four isoforms,
including PDK1, PDK2, PDK3 and PDK4. Their molecular weight range
from 45 kDa (PDK1) to 48 kDa (PDK2, PDK3 and PDK4) and models show
that they have 70% identity of themselves (8). All PDK isoforms are notably expressed
in specific tissues (9). At present,
there is no report of PDK expression in CCA. The aims of this study
were to examine PDK expression in CCA and evaluate whether the PDKs
could be diagnostic/prognostic markers of CCA.
Materials and methods
Selection of PDK from the
mitochondrial protein database
Glucose metabolism-associated proteins were selected
from the CCA mitochondrial proteomic database constructed by
Chua-On et al (6), of which
data were extracted from three CCA tissues and adjacent
non-cancerous tissues using liquid chromatography tandem-mass
spectrometry. Proteins were searched using Mascot against The
National Center for Biotechnology Information human protein
reference database with the human mitochondrial proteins database.
The 282 proteins overexpressed in CCA were classified into their
respective biological functions using Protein Analysis Through
Evolutionary Relationships (PANTHER) software (10).
CCA tissues and sera
Cancerous and adjacent non-cancerous tissues from 15
patients with intrahepatic CCA were kindly provided by the
Cholangiocarcinoma Research Institute (CARI), Khon Kaen University,
Khon Kaen, Thailand. They were immediately snap-frozen in liquid
nitrogen and stored at −80°C until further use. A part of each
frozen tissue section was fixed in 4% buffered formalin and
processed for hematoxylin and eosin staining for histopathological
diagnosis. The mean ± standard deviation (SD) of age, aspartate
transaminase, alanine transaminase and alkaline phosphatase (ALP)
of these patients are 57±5.5 years, 61±6.7, 57±51.4 and 154±100.4
U/l, respectively.
Serum samples from 39 patients with CCA and 20
patients with benign biliary diseases (BBD), mostly cholangitis,
were additionally obtained from The Faculty of Medicine, CARI, Khon
Kaen University. Normal control sera were obtained from 19 healthy
people, who went for check-ups at the Office for Medical Technology
and Physical Therapy Health Service, Faculty of Associated Medical
Sciences, Khon Kaen University. Characteristics of the healthy
controls, and patients with BBD and CCA are summarized in Table I. The serum samples were kept at
−20°C until further use.
 | Table I.Characteristics of the healthy
controls, and the benign biliary disease and cholangiocarcinoma
patients. |
Table I.
Characteristics of the healthy
controls, and the benign biliary disease and cholangiocarcinoma
patients.
| Clinical parameters
(normal range) | Healthy control
(n=19) | BBD (n=20) | CCA (n=39) | P-value |
|---|
| Age, years | 49.8±5.4 (40,59) | 59.3±9.3 (41,74) | 59.3±9.3 (38,77) |
<0.0002b,c |
| Sex, n (%) |
|
|
|
|
| Male | 4
(21.1) | 16 (80.0) | 23 (59.0) |
0.0009b,c |
|
Female | 15 (78.9) | 4 (20.0) | 16 (41.0) |
|
| Liver function
enzymes |
|
|
|
|
| ALT (4–36
U/l) | 19.1±5.5 (8,28) |
88.0±101.4a (8,429) | 57.5±43.0
(2,151) |
<0.0001d,e |
| AST
(12–32 U/l) | 25.5±4.2 (19,31) |
90.0±75.3a
(16,303) | 84.8±76.3
(19,317) |
<0.0001d,e |
| ALP
(42–121 U/l) | 73.6±16.0
(45,105) |
332.8±285.7a (32,991) | 226.9±307.9
(24,1963) |
<0.0001d,e |
The sample sizes for CCA tissues and serum samples
were analyzed using PS program version 3.1.2 (11). This research project was approved by
the Ethical Committee of Khon Kaen University, Thailand (approval
no. HE581431).
Immunohistochemistry
Paraffin-embedded CCA sections of 4 µm thickness of
15 CCA cases were deparaffinized by soaking in xylene, absolute
ethanol, 95% ethanol and 70% ethanol for 2 min each time. For the
antigen retrieval process, sections were boiled in 1X citrate
buffer (pH 6.0) for 10 min and washed in 1X PBS buffer. Then, the
endogenous peroxidase activity of the sections was blocked with 3%
H2O2 in methanol for 1 h in the dark and
non-specific background binding was blocked by incubation with 20%
fetal bovine serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 2 h. The sections were then incubated with 150 µl rabbit
polyclonal antibody against human PDK1, PDK2, PDK3 and PDK4
(Biorbyt, Cambridge, UK; cat. nos. of the anti-PDK1, -PDK2, -PDK3
and -PDK4 antibodies are orb14422, orb137873, orb312719 and
orb136059, respectively) at a dilution of 1:100 at 4°C overnight.
The sections were washed in 1X PBS-T and incubated with 150 µl
undiluted goat anti-rabbit immunoglobulin G (IgG) antibody with the
EnVision System (Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) for 1 h and the signal was developed with diamino-benzidine
(Dako; Agilent Technologies, Inc.) for 5 min in the dark. The
sections were washed with running tap water until clear and
counterstained with hematoxylin for 10 min. The sections were
dehydrated in an ascending series of ethanol for 2 min at each
concentration, cleared in xylene, mounted with permount and sealed
with a cover glass.
To assess the immunohistochemical staining (IHC)
using H-score, both the intensity of staining (0, no staining; 1+,
weak staining; 2+, moderate staining; and 3+, strong staining) and
the percentage of the stained tumor cells (0–100%) were assessed.
IHC results were observed for ~10 fields per sample to reduce
variation of detection. The H-score was calculated as a sum of the
intensity as follows (12): H-score
= (% of positively stained tumor cells at weak intensity ×1) + (%
of positively stained tumor cells at moderate intensity ×2) + (% of
positively stained tumor cells at strong intensity ×3). Lastly, 10
fields of H-score were averaged leading to a range of 0–300 for the
H-score for each sample.
Western blot analysis
In total, five CCA sera samples (2 µl each) were
mixed with 4X loading dye to a 1:4 ratio and boiled for 5 min. The
protein samples were fractionated on 12.5% SDS-PAGE and run at 120
V for 3 h in a cold room. The electrophoresis was completed when
the dye reached the end of the gel, and the separated proteins were
transferred onto a polyvinylidene difluoride membrane at 300 V for
1 h at room temperature. The membrane was blocked with 5% skimmed
milk in 1X TBS with Tween-20 (TBST) for 1 h at room temperature.
The membrane was then incubated with a 1:500 dilution of primary
antibody, which was a rabbit polyclonal antibody against human PDK3
overnight at 4°C. Next, the membrane was washed three times with 1X
TBST for 10 min each time, and incubated with a 1:10,000 dilution
of anti-rabbit IgG-horseradish peroxidase-conjugated secondary
antibody for 1 h at room temperature, followed by three washes with
1X TBST for 10 min each time. Finally, the chemiluminescence was
detected using an Enhanced Chemiluminescent plus system (GE
Healthcare Life Sciences, Little Chalfont, UK) and visualized using
Image Quant LASmini 400 (GE Healthcare Life Sciences). A KKU-055 (a
CCA cell line) lysate was used as a positive control for PDK3,
which was provided by CARI, Khon Kaen University.
Dot blot assay
The membrane was soaked in 1X TBST for 10 min before
being placed on a machine. All sera of each group (2 µl each) were
transferred to a nitrocellulose membrane with Bio-Dot
Microfiltration Apparatus (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). After air-drying, the membrane was blocked with 5%
skimmed milk in 1X TBST for 1 h at room temperature. The membrane
was then incubated with a 1:500 dilution of rabbit polyclonal
antibody against human PDK3 (Biorbyt) overnight at 4°C. The
detection process was the same as the western blot analysis method.
The method was modified from a previously described method
(13). A HeLa cell lysate was used
as a positive control. The cell lysate was kindly provided by
Daraporn Chua-orn (Khon Kaen University). The intensities of PDK3
protein in the sera were normalized using PDK3 intensity in the
HeLa cell lysate as a relative expression ratio.
Receiver operating characteristic
(ROC) curve analysis
ROC curve analysis was performed to analyze the
applicability of PDK3 as a tumor marker for CCA. The cut-off value
was considered in the left-top or shoulder of the curve. In this
study, we selected the value that provided the highest likelihood
ratio as the cut-off.
Statistical analysis
Fold change analysis was calculated from the signal
intensity of CCA minus the signal intensity of adjacent
non-cancerous tissue. The data are presented as mean ± standard
deviation and the range (minimum to maximum). A Kruskal-Wallis
normality test was used for the normality of data. The different
values among two and three sample groups were estimated using the
Mann-Whitney and Kruskal Wallis tests, respectively. Bonferroni's
correction was used as the post hoc test. The correlation between
serum PDK3 levels and the clinicopathological parameters of the
patients were analyzed using Fisher's exact test. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism v.5 software (GraphPad Software, Inc., La Jolla, CA,
USA) was used for statistical analyses.
Results
PDK expression in CCA
In our previous study, the different expression of
mitochondrial proteins in cancerous and adjacent normal tissues of
CCA specimens was determined using mass spectrometry, and it was
identified that a total of 281 mitochondrial proteins were
identified to be overexpressed in CCA tissues (6). All 281 proteins were considered to be
significantly different (P<0.05). In this study, we classified
those 281 mitochondrial proteins into their respective biological
function using PANTHER software (10) (analyzed on 21st August 2017). The
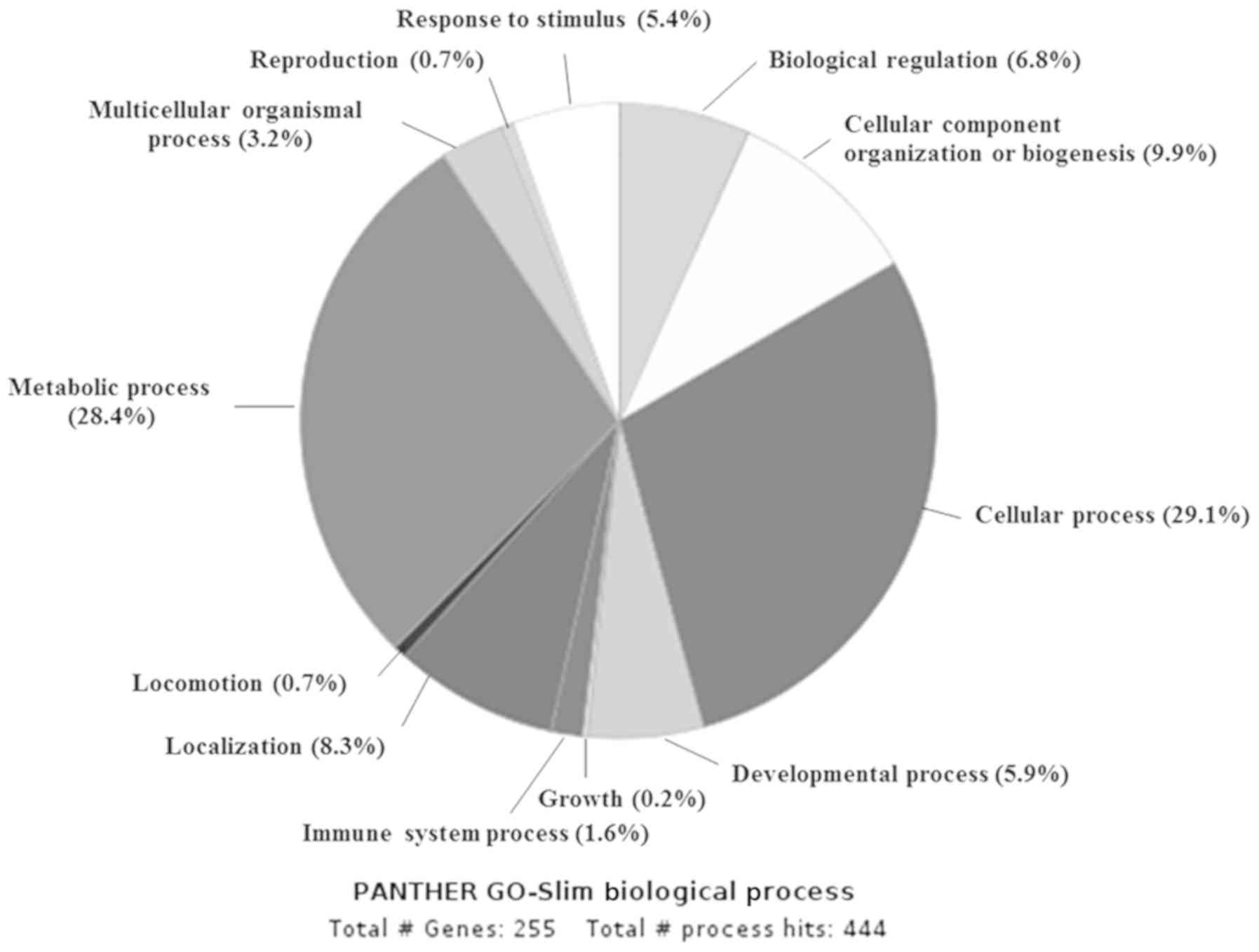
results showed that the majority of the proteins were classified
into cellular process (82, 29.1%), metabolic process (80, 28.4%),
cellular component organization or biogenesis (28, 9.9%),
localization (23, 8.3%) and other processes possibly associated
with cancer, such as biogenesis and immune system (Fig. 1). Then, we focused on the proteins
involved in metabolic process and identified 15 proteins that are
involved in glucose metabolism. Among those 15 proteins, PDK3 was
the most overexpressed protein. In addition, a 6-fold
overexpression of PDK2 was detected (Table II).
 | Table II.Candidate mitochondrial proteins
involved in the glucose metabolism of cholangiocarcinoma. |
Table II.
Candidate mitochondrial proteins
involved in the glucose metabolism of cholangiocarcinoma.
| Protein name | Gene symbol | Fold change of
CCAa |
|---|
| Pyruvate
dehydrogenase kinase 3 | PDK3 | 27 |
| Cytochrome c
oxidase assembly protein 3 homolog | COX15 | 11 |
| Cytochrome c
oxidase subunit 5A | COX5A | 10 |
| ATP synthase
subunit epsilon | ATP5E | 11 |
| Cytochrome c
oxidase subunit 5b | COX5B | 7 |
| Complex I
intermediate-associated protein 30 | CIA30 | 7 |
| Succinate
dehydrogenase | SDH | 7 |
| Pyruvate
dehydrogenase kinase 2 | PDK2 | 6 |
| Acetyl-CoA
carboxylase 2 | ACC2 | 8 |
| NADH dehydrogenase
[ubiquinone] 1 alpha subcomplex subunit 2 | NDUFA2 | 4 |
| ATP synthase
subunit alpha, mitochondrial isoform | ATP5A | −1 |
| Cytochrome b-c1
complex subunit 1 | UQCRC1 | −3 |
| ATP synthase
mitochondrial F1 complex assembly factor 1 | ATPAF1 | 4 |
| Isocitrate
dehydrogenase | IDH | −4 |
| Cytochrome c
oxidase subunit I | COX1 | 0 |
High expression of PDK1, PDK2 and PDK3
in CCA tissues
To evaluate the expression of four PDK isoforms in
CCA, immunohistochemistry was performed on 15 CCA tissues from
patients containing cancerous and adjacent non-cancerous tissues.
The results showed that PDK1, 2 and 3 were strongly positive in the
cancer cells but only weakly positive in adjacent non-cancerous
tissues. However, PDK4 was negative in both cancerous and
non-cancerous adjacent tissues (Fig.
2A). The intensities of immunostaining of PDK1, PDK2 and PDK3,
expressed by H-scores (mean ± SD), were 269±34, 268±32 and 268±20,
respectively, in CCA tissues. Whereas, the H-scores of adjacent
non-cancerous tissues were 38±28, 30±21 and 54±11, respectively
(P-value <0.0001; Mann-Whitney U test; Fig. 2B).
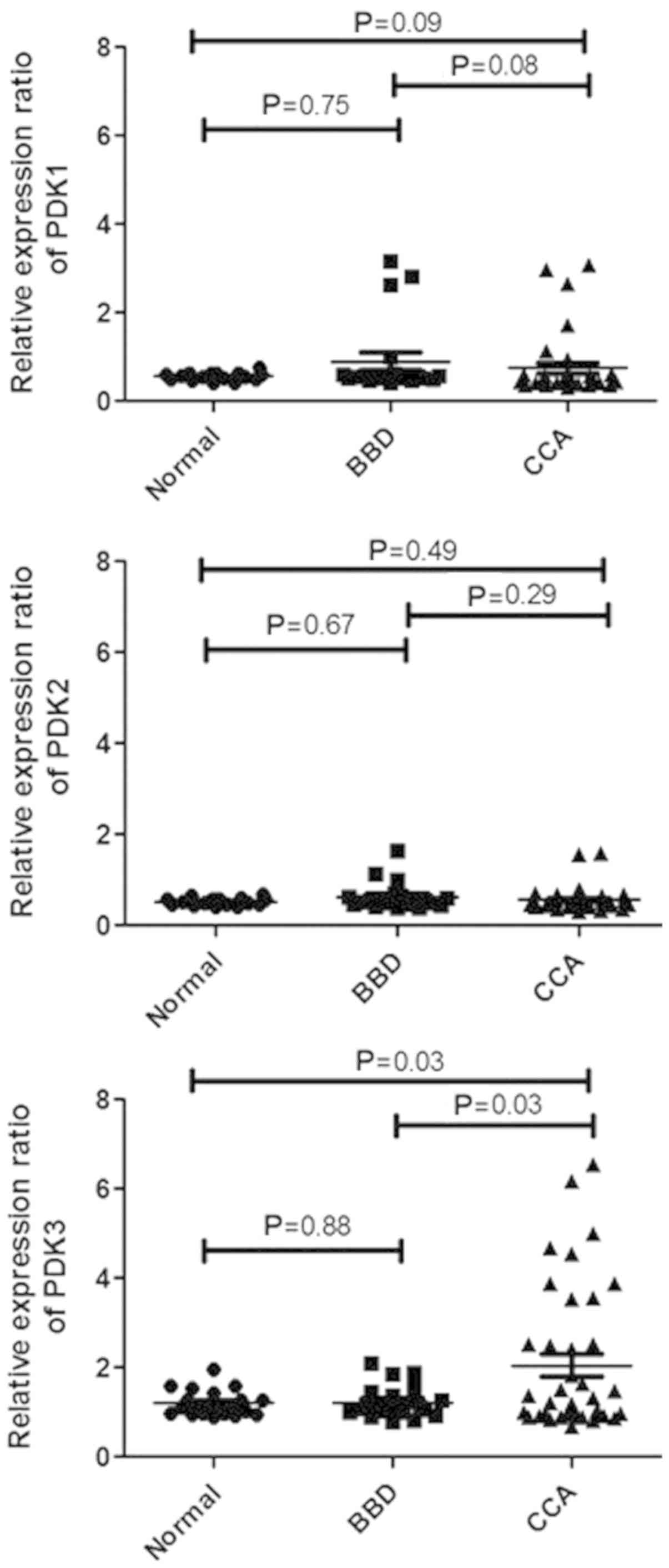
High expression of PDK3 in the sera of
patients with CCA
The levels of PDK1, PDK2 and PDK3 in the sera were
measured in 39 patients with CCA, 20 patients with BBD and 19
normal controls using dot blot analysis. We confirmed that PDK3
expression in sera was not influenced by age and sex by statistical
testing in all three groups (Tables
SI and SII). As shown in
Fig. 3, PDK3 was significantly
higher in CCA sera compared with normal sera (P=0.03). There was no
significant difference in PDK3 levels between BBD and normal sera.
Furthermore, the comparison of the PDK3 levels in the sera revealed
that it was significantly higher in CCA sera compared with the BBD
group sera at a P-value of 0.03. For PDK1 and PDK2, the result
showed no significant difference between CCA vs. normal control and
CCA vs. BBD. To confirm that the dot blot positivity was in fact
detecting PDK3, we performed western blot analysis for five
representative dot-blot positive CCA sera. PDK3 was detected in all
five CCA sera as a 48 kDa band. The cell lysate of the KKU-055 CCA
cell line was used as a positive control for PDK3 (Fig. S1).
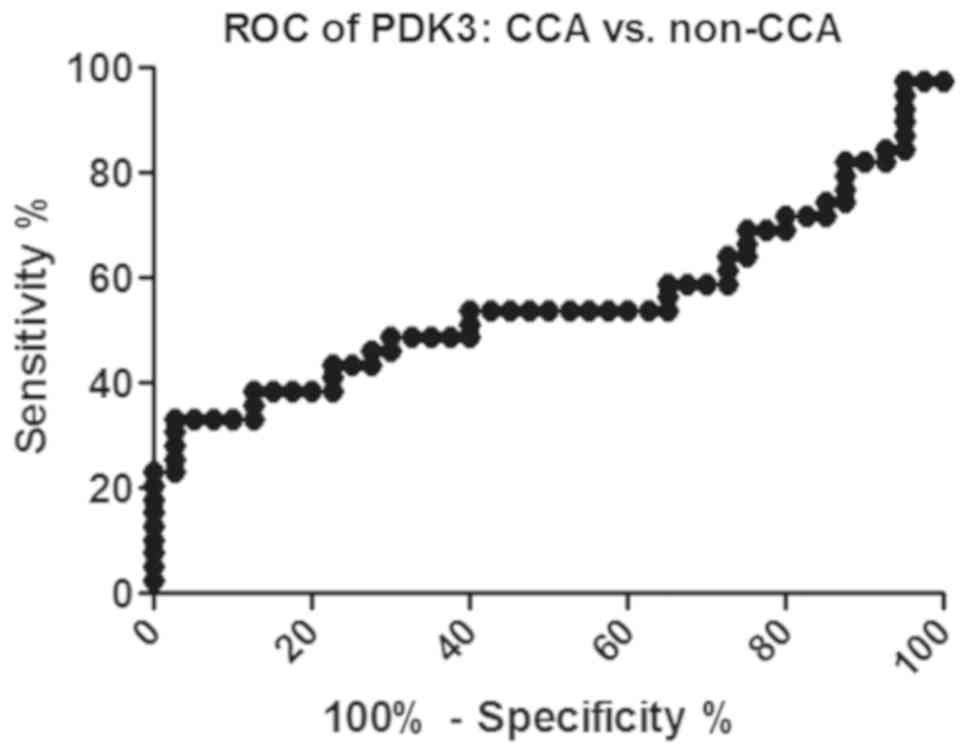
PDK3 as a CCA tumor marker
The cut-off of PDK3 was calculated from an ROC curve
and the value that provided the highest likelihood ratio was used
as the cut-off. The PDK3 cut-off of CCA from non-CCA was 2.253
(Fig. 4). We found 33.0%
sensitivity, 97.5% specificity, 92.9% positive predictive value,
60.0% negative predictive value and 65.8% accuracy.
Correlation of high PDK3 and short
survival
We investigated the correlation between serum PDK3
levels with clinicopathological parameters of patients with CCA
(Table III). Using the median
cut-off, we found a negative correlation between the serum PDK3
level and the ALP level at a P-value of 0.0307 (Fig. 5A). Moreover, the serum PDK3 level was
negatively correlated with the survival time of patients, as shown
in Fig. 5B with a P-value of 0.0314
(Table III). This negative
correlation indicated that PDK3 could be a prognostic marker for
patients with CCA; the higher the PDK3 level, the shorter the
survival time.
 | Table III.Correlation between low and high
pyruvate dehydrogenase kinase 3 levels and the clinicopathological
characteristics of patients with cholangiocarcinoma. |
Table III.
Correlation between low and high
pyruvate dehydrogenase kinase 3 levels and the clinicopathological
characteristics of patients with cholangiocarcinoma.
|
|
| Low PDK3 | High PDK3 |
|---|
|
|
|
|
|
|---|
| Clinical
parameters | n | r-value | P-value | r-value | P-value |
|---|
| Age (years) | 39 | 0.03168 | 0.8976 | −0.0144 | 0.9521 |
| Liver function
enzymes |
| ALT
(U/l) | 39 | 0.1986 | 0.4150 | −0.2878 | 0.2185 |
| AST
(U/l) | 39 | 0.2405 | 0.3213 |
0.0239 | 0.9205 |
| ALP
(U/l) | 39 | 0.2368 | 0.3441 | −0.4837 | 0.0307a |
| Total
bilirubin (mg/dl) | 39 | 0.5035 | 0.0280a | −0.0140 | 0.9532 |
| Direct
bilirubin (mg/dl) | 39 | 0.4622 | 0.0463a | −0.0350 | 0.8836 |
| Tumor markers |
| CEA
(ng/ml) | 26 | 0.3571 | 0.2310 | −0.0572 | 0.8527 |
| CA 19-9
(U/ml) | 32 | −0.1096 | 0.6861 |
0.1900 | 0.4810 |
|
Survival time (days) | 32 | 0.0161 | 0.9529 | −0.5385 | 0.0314a |
Discussion
Proteomic analysis on the expression of
mitochondrial proteins revealed that the proteins involved in
glucose metabolism are overexpressed in CCA. In particular, PDK3
showed the highest (27-fold) change in >15 proteins. By
immunohistochemistry, the overexpression of PDK1, PDK2 and PDK3 was
identified in CCA tissues compared with the adjacent non-cancerous
tissues. From the existing literature, PDK1 and PDK2 are
hypothesized to be strongly expressed in cancer cells because PDK1
is mainly associated with aerobic glycolysis, which is the
preferred state for cancer cells to produce adenosine triphosphate,
even in sufficient oxygen support (14,15). For
PDK2, there is a study that demonstrated PDK2 expression in the
majority of normal tissues (9). We
studied the expression of all PDK isoforms and found that PDK1,
PDK2 and PDK3, but not PDK4, were significantly overexpressed in 15
CCA tissues. PDK1 overexpression was reported in gastric, renal and
colon cancers (16–18). PDK2 was highly expressed in head and
neck cancers (19). The high
expression of PDK3 was reported in colon cancer (18). PDK3 inhibits pyruvate dehydrogenase
activity via phosphorylation of the E1 subunit of pyruvate
dehydrogenase E1 component subunit α, somatic form, mitochondrial,
and thereby regulates glucose metabolism and aerobic glycolysis in
cancer. Moreover, there are previous studies that demonstrated that
PDK3 is involved in anticancer resistance (20,21).
We also studied the serum PDK levels in normal
controls, and patients with BBD and CCA. Until now, the PDK level
in the serum or plasma has not been determined in healthy controls
and patients with diseases. As PDK is a mitochondrial protein, it
was surprising that PDK1, 2 and 3 were detected in the sera,
especially in the sera of patients with CCA. When the secretion
abilities of the PDKs were determined using SignalP 4.1 server with
a cut-off D-score of 0.45, the D-score of the PDKs was 0.126, which
suggested that PDKs are not secreted proteins (22). As SignalP is a server, which detects
signal peptides of transmembrane regions, PDKs may be secreted from
CCA through other pathways (22).
While all CCA tissues were positive for PDK1, 2 and 3 by
immunohistochemistry, only less than a half of CCA sera were
positive for PDK3. This suggested that PDK3 was not always secreted
from CCA for circulation and this secreted PDK3 has significance in
some clinical aspects. Since the high serum PDK3 level is
associated with the poor prognosis of the patients, the role of
PDK3 in tumor invasion/metastasis as well as tumor proliferation
requires examination in the future.
Although PDK3 provided good specificity to CCA in
the IHC analysis of tumor tissues, its serum level presented a
rather low sensitivity for discrimination of CCA and non-CCA. This
discrepancy is due to the low detection rate of PDK3 in the sera of
patients with CCA. In this aspect, PDK3 is not a good diagnostic
marker. Nevertheless, when the correlation of the PDK3 intensity
and clinical parameters of patients was analyzed, a significant
correlation was observed between PDK3 and three CCA biomarkers,
namely, ALP, total bilirubin and direct bilirubin. Our previous
studies revealed that, in CCA, the total serum bile acid correlates
with total bilirubin, and total serum bile acid and intercellular
adhesion molecule 1 correlate with ALP (23,24).
Although the possible mechanisms underlying this phenomenon remain
unclear, an inverse correlation between the PDK3 level and survival
times was observed. Therefore, the destructive nature of CCA may be
associated with PDK3 release for circulation. The PDK3 level in
serum could be an effective prognostic marker of CCA. However,
limitation of this study was the small sample size so age- and
sex-matched groups were unable to be gathered for CCA, BBD patients
and normal controls.
In our study, PDK3 levels in the sera of CCA
patients were significantly higher than both normal and BBD.
Although serum PDK3 level was high in less than half of the CCA
patients, high PDK3 level was significantly correlated with poor
prognosis. These results indicated that PDK3 might be used as a
prognostic marker of CCA.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Yukifumi
Nawa (Tropical Diseases Research Centre, Faculty of Medicine, Khon
Kaen University, Khon Kaen, Thailand), for editing the
manuscript.
Funding
The project was supported by grants from Khon Kean
University (Khon Kaen, Thailand; grant no. 601804), the Centre for
Research and Development of Medical Diagnostic Laboratories,
Faculty of Associated Medical Sciences and the Cholangiocarcinoma
Research Institute, Khon Kaen University, the Research Fund for
Supporting Lecturer to Admit High Potential Student to Study and
Research on His Expert Program Year 2014, Graduate School, Khon
Kaen University (grant no. 571H107) and the Publication Clinic of
the Research Affairs, Khon Kaen University.
Availability of data and materials
The datasets used and analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
SSa performed the experiments and analyzed the data.
TP, TL, WS and SP revised and conceived the study. SR developed the
mass spectrometry database. DCO contributed to the CCA
mitochondrial data analysis. SW and CW contributed to the study
design and were involved in drafting the manuscript. OS and SSu
collected the CCA tissue samples. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This research project was approved by the Ethical
Committee of Khon Kaen University, Thailand (no. HE581431). Written
inform consent was obtained from all patients at the time of sample
collection by the Cholangiocarcinoma Research Institute.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan SA, Toledano MB and Taylor-Robinson
SD: Epidemiology, risk factors, and pathogenesis of
cholangiocarcinoma. HPB (Oxford). 10:77–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sripa B, Brindley PJ, Mulvenna J, Laha T,
Smout MJ, Mairiang E, Bethony JM and Loukas A: The tumorigenic
liver fluke Opisthorchis viverrini-multiple pathways to cancer.
Trends Parasitol. 28:395–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pattanapairoj S, Silsirivanit A, Muisuk K,
Seubwai W, Cha'on U, Vaeteewoottacharn K, Sawanyawisuth K,
Chetchotsak D and Wongkham S: Improve discrimination power of serum
markers for diagnosis of cholangiocarcinoma using data mining-based
approach. Clin Biochem. 48:668–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wongkham S and Silsirivanit A: State of
serum markers for detection of cholangiocarcinoma. Asian Pac J
Cancer Prev. 13 (Suppl):S17–S27. 2012.
|
|
6
|
Chua-On D, Proungvitaya T, Techasen A,
Limpaiboon T, Roytrakul S, Wongkham S, Wongkham C, Somintara O,
Sungkhamanon S and Proungvitaya S: High expression of
apoptosis-inducing factor, mitochondrion-associated 3 (AIFM3) in
human cholangiocarcinoma. Tumour Biol. 37:13659–13667. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Icard P and Lincet H: A global view of the
biochemical pathways involved in the regulation of the metabolism
of cancer cells. Biochim Biophys Acta. 1826:423–433.
2012.PubMed/NCBI
|
|
8
|
Saunier E, Benelli C and Bortoli S: The
pyruvate dehydrogenase complex in cancer: An old metabolic
gatekeeper regulated by new pathways and pharmacological agents.
Int J Cancer. 138:809–817. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bowker-Kinley MM, Davis WI, Wu P, Harris
RA and Popov KM: Evidence for existence of tissue-specific
regulation of the mammalian pyruvate dehydrogenase complex. Biochem
J. 329:191–196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mi H, Poudel S, Muruganujan A, Casagrande
JT and Thomas PD: PANTHER version 10: Expanded protein families and
functions, and analysis tools. Nucleic Acids Res. 44:D336–D342.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dupont WD and Plummer WD Jr: Power and
sample size calculations. A review and computer program. Control
Clin Trials. 11:116–128. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma H, Lu Y, Marchbanks PA, Folger SG,
Strom BL, McDonald JA, Simon MS, Weiss LK, Malone KE, Burkman RT,
et al: Quantitative measures of estrogen receptor expression in
relation to breast cancer-specific mortality risk among white women
and black women. Br Cancer Res. 15:R902013. View Article : Google Scholar
|
|
13
|
Tan LD, Xu YY, Yu Y, Li XQ, Chen Y and
Feng YM: Serum HER2 level measured by dot blot: A valid and
inexpensive assay for monitoring breast cancer progression. PLoS
One. 6:e187642011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeoung NH: Pyruvate dehydrogenase kinases:
Therapeutic targets for diabetes and cancers. Diabetes Metab J.
39:188–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun
J, Ham IH and Han SU: Expression of pyruvate dehydrogenase kinase-1
in gastric cancer as a potential therapeutic target. Int J Oncol.
42:44–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim HY, Yip YM, Chiong E, Tiong HY,
Halliwell B, Esuvaranathan K and Wong KP: Metabolic signatures of
renal cell carcinoma. Biochem Biophys Res Commun. 460:938–943.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu CW, Lin SC, Chien CW, Lin SC, Lee CT,
Lin BW, Lee JC and Tsai SJ: Overexpression of pyruvate
dehydrogenase kinase 3 increases drug resistance and early
recurrence in colon cancer. Am J Pathol. 179:1405–1414. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roh JL, Park JY, Kim EH, Jang HJ and Kwon
M: Activation of mitochondrial oxidation by PDK2 inhibition
reverses cisplatin resistance in head and neck cancer. Cancer Lett.
371:20–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gudi R, Bowker-Kinley MM, Kedishvili NY,
Zhao Y and Popov KM: Diversity of the pyruvate dehydrogenase kinase
gene family in humans. J Biol Chem. 270:28989–28994. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu CW, Lin SC, Chen KF, Lai YY and Tsai
SJ: Induction of pyruvate dehydrogenase kinase-3 by
hypoxia-inducible factor-1 promotes metabolic switch and drug
resistance. J Biol Chem. 283:28106–28114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petersen TN, Brunak S, von Heijne G and
Nielsen H: SignalP 4.0: Discriminating signal peptides from
transmembrane regions. Nat Methods. 8:785–786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sombattheera S, Proungvitaya T, Limpaiboon
T, Wongkham S, Wongkham C, Luvira V and Proungvitaya S: Total serum
bile acid as a potential marker for the diagnosis of
cholangiocarcinoma without jaundice. Asian Pac J Cancer Prev.
16:1367–1370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Janan M, Proungvitaya S, Limpaiboon T,
Proungvitaya T, Roytrakul S, Wongkham C, Jearanaikoon P, Chur-in S
and Wongkham S: Serum adhesion molecule-1 (ICAM-1) as a potential
prognostic marker for cholangiocarcinoma patients. Asian Pac J
Cancer Prev. 13 (Suppl):S107–S114. 2012.
|