Introduction
Osteosarcoma is a primary malignant tumor of bone
that is prone to occur in adolescent children. Its incidence rate
is approximately 10% in primary malignant tumor of bone, and the
main site is at tubular bone of the distal end of the bone and
proximal humerus (1,2). Osteosarcoma has the characteristics of
high malignancy and early distal metastasis that is also the main
cause of death (3,4), which is the cause of the poor prognosis
of osteosarcoma patients and the less than 20% 5-year survival rate
(5). With the development of new
chemotherapy techniques, the survival rate of osteosarcoma patients
has increased, and the 5-year survival rate is up to 80%, but the
5-year survival rate of osteosarcoma patients with metastasis has
not improved significantly (6).
In recent years, the development of molecular
biology has found increasing number of molecules playing an
important role in the development of osteosarcoma (7). As an organism polypeptide growth
factor, bone morphogenetic protein (BMP) has been found to be
highly expressed in osteosarcoma, and it is speculated that BMP can
promote the growth of osteosarcoma cells (8). BMP-2, a member of BMPs family, has been
found to be highly expressed in osteosarcoma (9), but its mechanism in osteosarcoma cells
is not described in detail. MicroRNAs (miRNAs/miRs) have been
intensely researched in tumor molecular biology in recent years,
and large number of studies considered that their abnormal
expression and regulation is the main cause of tumor cell
production and metastasis (10). As
a member of the miR-29 family, a study (11) found that miR-29c can inhibit the
proliferation and metastasis of tumor cells in a variety of tumors.
Therefore, it was speculated that miR-29c is a factor associated
with metastasis of tumor cells (12). However, there are few reports on the
expression of miR-29c in osteosarcoma cells and its effect on the
biological function of osteosarcoma cells.
The expression of BMP-2 and miR-29c in osteosarcoma
cells and their effects were studied in order to provide a new
theoretical basis for the diagnosis and treatment of osteosarcoma
in molecular biology.
Patients and methods
General information
A retrospective analysis of 75 patients with
osteosarcoma who underwent surgery in Tianjin Baodi Hospital
(Tianjin, China) from May 2013 to June 2017 was conducted. The
average age was 21.3±9.4 years. A total of 49 patients were at
stage IIB/III, and 26 patients were at stage I/IIA. A total of 75
osteosarcoma tissues and 51 normal paraneoplastic tissues were
excised with the patient's consent during the surgery. All patients
were diagnosed with osteosarcoma by pathology and signed an
informed consent. Patients with other serious organ diseases and
tumors; with communication and mental disorders; and patients not
cooperating with the study were excluded. All the specimens were
stored in liquid nitrogen tanks immediately after removal.
The study was approved by the Ethics Committee of
Tianjin Baodi Hospital. Patients who participated in this research
had complete clinical data. The signed informed consents were
obtained from the patients or the guardians.
Experimental reagents and
materials
Human osteosarcoma cell line MG-63 was purchased
from the cell bank of Shanghai Institutes of Biological Sciences
(CAS; Shanghai, China); real-time quantitative PCR instrument was
purchased from Bio-Rad Laboratories, Inc., Hercules, CA, USA; fetal
bovine serum (FBS) and 0.25% trypsin were purchased from HyClone;
GE Healthcare Life Sciences (Logan, UT, USA); TRIzol reagent was
purchased from Applied Biosystems; Thermo Fisher Scientific, Inc.,
(Waltham, MA, USA); DMEM medium was purchased from Gibco; Thermo
Fisher Scientific, Inc.; MTT solution was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany); Transwell insert
was purchased from Corning, Inc. (Corning, NY, USA); Matrigel
matrix was purchased from Bejing Biodee Biotechnology Co., Ltd.,
(Beijing, China); CDNA reverse transcription kit, SYBR Green PCR
kit and Lipofectamine 2000 transfection reagent were both purchased
from Invitrogen; Thermo Fisher Scientific, Inc. All primers and
transfection plasmids were synthesized and designed by Sangon
Biotech Co., Ltd. (Shanghai, China).
Expression of BMP-2 mRNA and miR-29c
in osteosarcoma and paraneoplastic tissues
Total RNA of BMP-2 mRNA and miR-29c were extracted
by TRIzol reagent from the osteosarcoma and paraneoplastic tissues.
The purity and concentration of RNA were detected by ultraviolet
spectrophotometer (G-9; Runqee (Shanghai) Instruments Technology
Co., Ltd.). Then, 5 µg total RNA was taken from each to reverse
transcribe cDNA according to the kit instructions. Reaction
parameters: 37°C for 15 min, 42°C for 42 min and 70°C for 5 min.
Transcribed cDNA was used for PCR amplification, β-actin was used
as the internal reference for BMP-2 mRNA, and U6 was used as an
internal reference for miR-29c. The primer sequences are shown in
Table I. PCR reaction conditions of
BMP-2 mRNA: 40 cycles of predenaturation at 94°C for 4 min, 94°C
for 60 sec, 59°C for 60 sec, then elongation at 72°C for 90 sec;
PCR reaction conditions of miR-29c: 40 cycles of predenaturation at
95°C for 2 min, 95°C for 10 sec, 60°C for 40 sec, then elongation
at 72°C for 90 sec. The relative expression of the gene was
expressed by 2−ΔΔCq (13). Real-time fluorescence quantitative
PCR detection was conducted, and the experiment was repeated 3
times.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Upstream primers | Downstream
primers |
|---|
| BMP-2 |
5′-TTGCGGCTGCTCAGCATGTT-3′ |
5′-TTCCGAGAACAGATGCAAGATG-3′ |
| miR-29c |
5′-ACACTCCAGCTGGGTAGCACCATTTGAAAT-3′ |
5′-TGGTGTCGTGGAGTCG-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
5′-AACGCTTCACGAATTTGCGT-3′ |
Cell culture, passage and
transfection
Human osteosarcoma cells MG-63 were cultured in a
medium containing 10% PBS DMEM at 37°C and 5% CO2. When
the adherent cell confluence reached 85%, 25% trypsin was added for
digestion, then the cells were cultured in medium to complete the
passage. BMP-2 siRNA and miR-29c were transfected into logarithmic
phase cells after passage. Untransfected cells were the blank
group, cells transfected with miR-29c and BMP-2 siRNA were the
experimental groups A and B, respectively. Cells transfected with
miRNA negative control (miR-NC) were the miR-NC negative control
group, cells transfected with non-silent siRNA were the siRNA
negative control group. Lipofectamine 2000 and miR-29c mimics,
BMP-2 siRNA, miR-NC and siRNA were mixed according to the
instructions of Lipofectamine 2000 kit, and incubated at room
temperature for 5 min. Finally, the mixture was mixed with cells
and then transfected at 37°C and 5% CO2. The
transfection efficiency of miR-29c mimics and BMP-2 siRNA in MG-63
cells after 48 h transfection was measured.
MTT assay for cell proliferation
Cells in each group after 48 h of transfection were
inoculated in a 96-well cell culture plate, and approximately 100
µl of cell fluid was inoculated into each well, with a cell density
of 2×103 cell/ml, approximately 200 cells. Next, 20 µl
MTT solution was added into each well on the 1st, 2nd, 3rd and 5th
day, and then cultured in the incubator for 4 h. Then, 150 µl
dimethyl sulfoxide was added, after 10 min of shaking, the
absorbance was measured at the wavelength of 490 nm with a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The experiment was repeated three times.
Transwell inserts for cell invasion in
vitro
Matrigel was first diluted at the ratio of 1:8, then
the upper surface of the bottom membrane of the Transwell chamber
was coated with the dilution. It was placed at 37°C for 30 min to
polymerize Matrigel into a gel, and the basement membrane was
hydrated before use. The transfected MG-63 cells were treated with
starvation for 24 h, then resuspended with FBS free DMEM medium,
and the cell density was adjusted to 1×105/ml. Cells
were seeded in Transwell inserts with the 24-well plate, and each
well was filled with 100 µl of cell suspension. Then, 600 µl DMEM
medium containing 10% FBS was added to the lower chamber of the
24-well plate, and then cultured in the incubator at 37°C for 6 h.
After culture, the supernatant was removed with cotton swabs, then
the chambers were washed with PBS. Cells in the lower chamber were
immobilized with 95% ethanol solution for 15 min at 37°C, then
washed with PBS and stained with 0.1% crystal violet. After
staining, the number of cell migration in random 6 wells was
calculated by microscope (XSP-L130; Shanghai Puqian Optical
Instrument Co., Ltd., Shanghai, China) to get the average value,
and the experiment was repeated three times.
Statistical analysis
SPSS 20.0 software package (IBM Corp., Armonk, NY,
USA) was used for statistical analysis of the experimental data.
The enumeration data were measured by Chi-square test. The
measurement data are presented as the mean ± standard deviation.
Independent t-test was used for the comparison between the two
groups, one-way ANOVA was used for multigroup comparison, and
Bonferroni was used for post hoc comparison. GraphPad Prism 6
software was used to draw figures in this experiment. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of BMP-2 mRNA and miR-29c
in osteosarcoma and paraneoplastic tissues
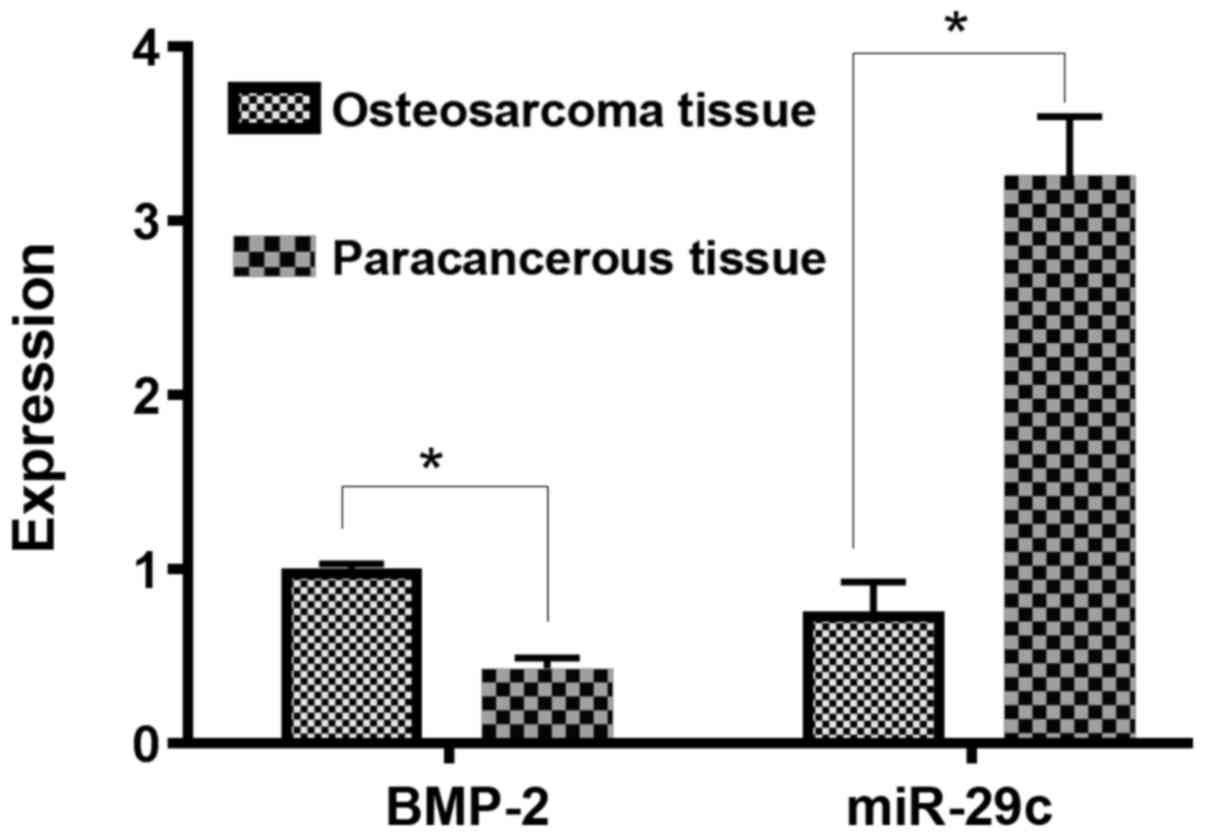
The relative expression of BMP-2 mRNA in
osteosarcoma tissue (0.979±0.053) was significantly higher than
that in paraneoplastic tissue (0.431±0.062), and the difference was
statistically significant (P<0.05). The relative expression of
miR-29c in osteosarcoma tissue (0.733±0.195) was significantly
lower than that in paraneoplastic tissue (3.261±0.341), and the
difference was statistically significant (P<0.05; Fig. 1).
Relative expression of BMP-2 mRNA and
miR-29c in cells of each group after transfection
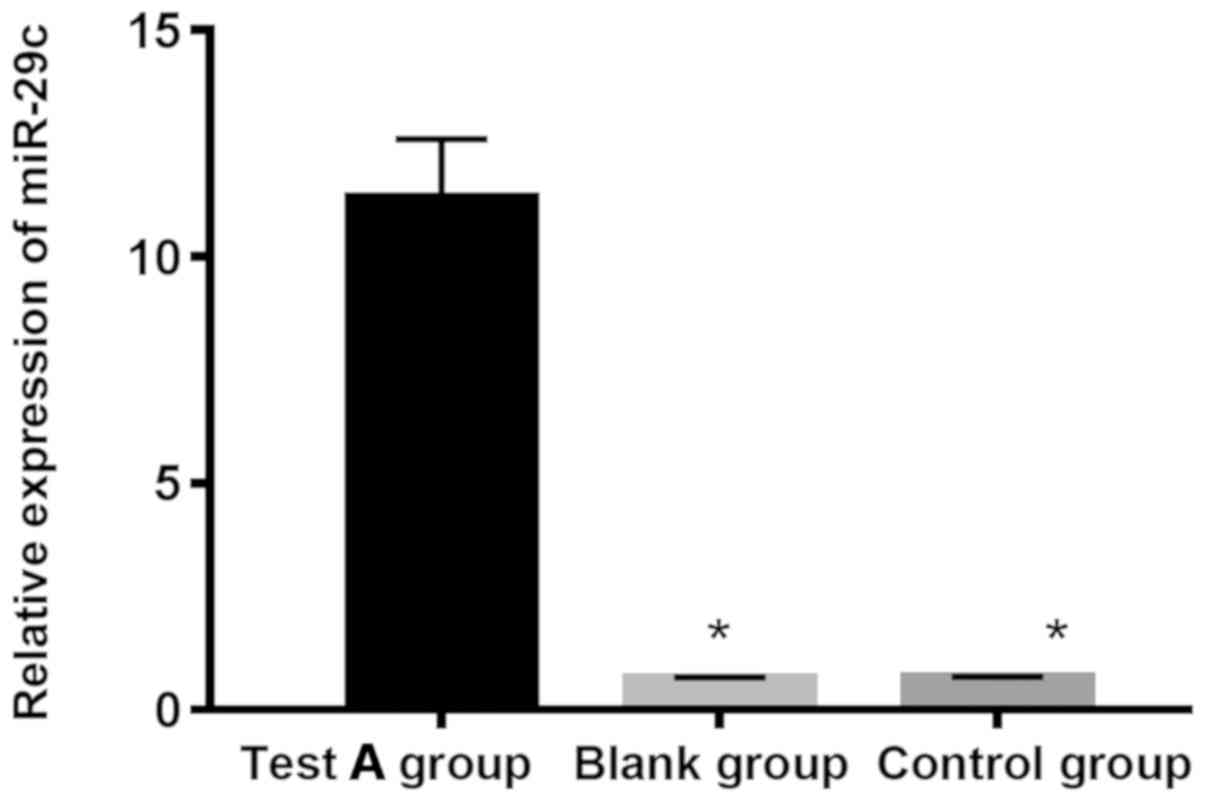
The expressions of miR-29c in experimental group A,
miR-NC negative control and blank groups were 11.319±1.276,
0.688±0.027 and 0.714±0.021, respectively. The expression of
miR-29c in experimental group A was significantly higher than that
in miR-NC negative control and blank groups (P<0.05), and there
was no significant difference between negative control and blank
groups (P>0.05). The expression of BMP-2 mRNA in experimental
group B, siRNA negative control and blank groups was 0.102±0.013,
0.981±0.173 and 0.932±0.231, respectively. The expression of BMP-2
mRNA in experimental group B was significantly lower than that in
siRNA negative control and blank groups (P<0.05), and there was
no significant difference between siRNA negative control and blank
groups (P>0.05; Figs. 2 and
3).
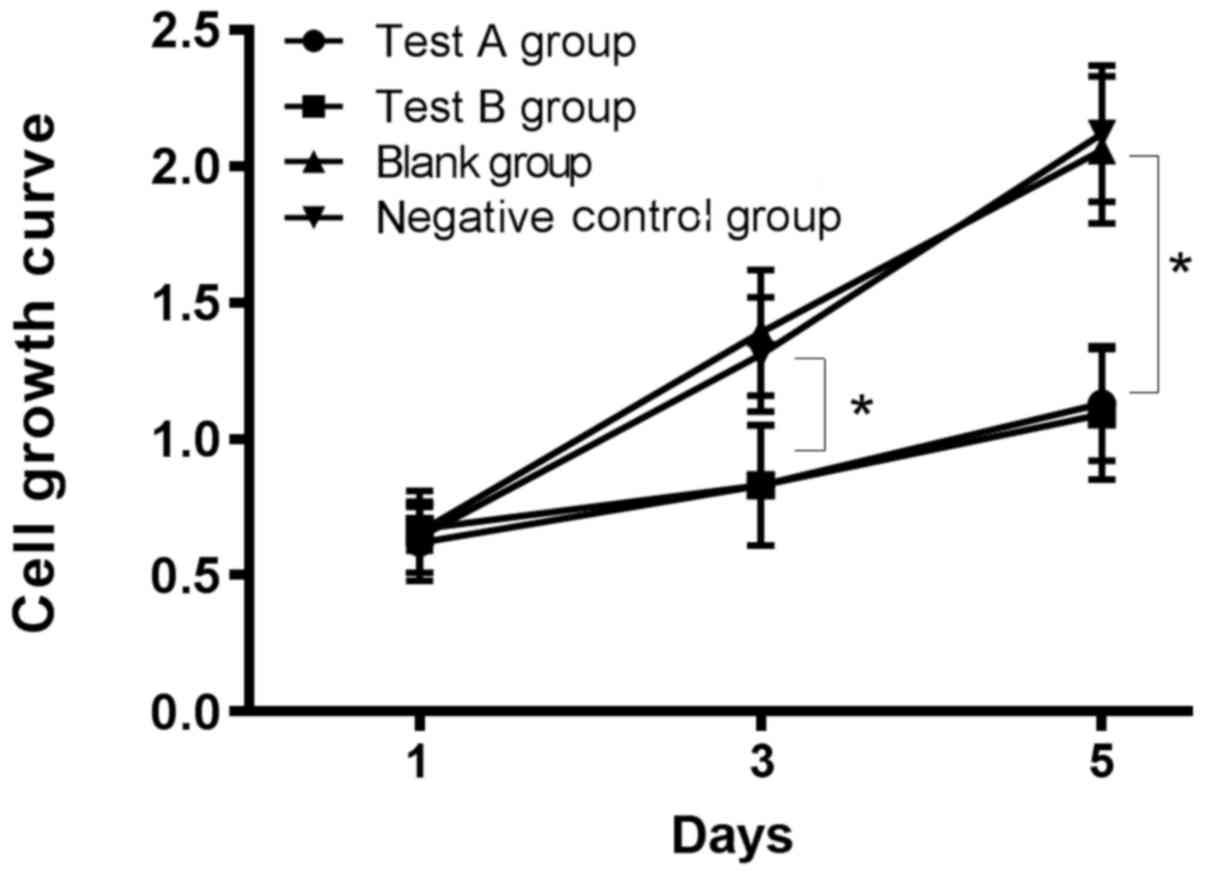
Comparison of cell proliferation in
each group
There was no significant difference in proliferation
ability among the five groups on the 1st and 3rd days after
transfection (P>0.05), but the proliferation ability in
experimental groups A and B was significantly lower than that in
the blank, miR-NC negative control and siRNA negative control
groups on the 5th day, and the difference was statistically
significant (P<0.05). There was no significant difference in
proliferation ability between experimental groups A and B on the
1st, 3rd and 5th days (P>0.05), and there was also no
significant difference between the blank, the negative control and
the siRNA negative control groups on the 1st, 3rd and 5th days
(P>0.05; Table II and Fig. 4).
 | Table II.Comparison of cell proliferative
ability in each group. |
Table II.
Comparison of cell proliferative
ability in each group.
| Time | Experimental group
A | Experimental group
B | Blank group | miR-NC negative
control group | siRNA negative
control group | F | P-value |
|---|
| 1st day | 0.62±0.14 | 0.67±0.08 | 0.66±0.15 | 0.64±0.13 | 0.65±0.12 | 0.070 |
0.978 |
| 3rd day |
0.83±0.22a |
0.82±0.21a | 1.39±0.23 | 1.31±0.21 | 1.23±0.24 | 3.881 |
0.117 |
| 5th day |
1.13±0.21a |
1.09±0.24a | 2.06±0.27 | 2.12±0.25 | 2.09±0.27 | 13.97 | <0.050 |
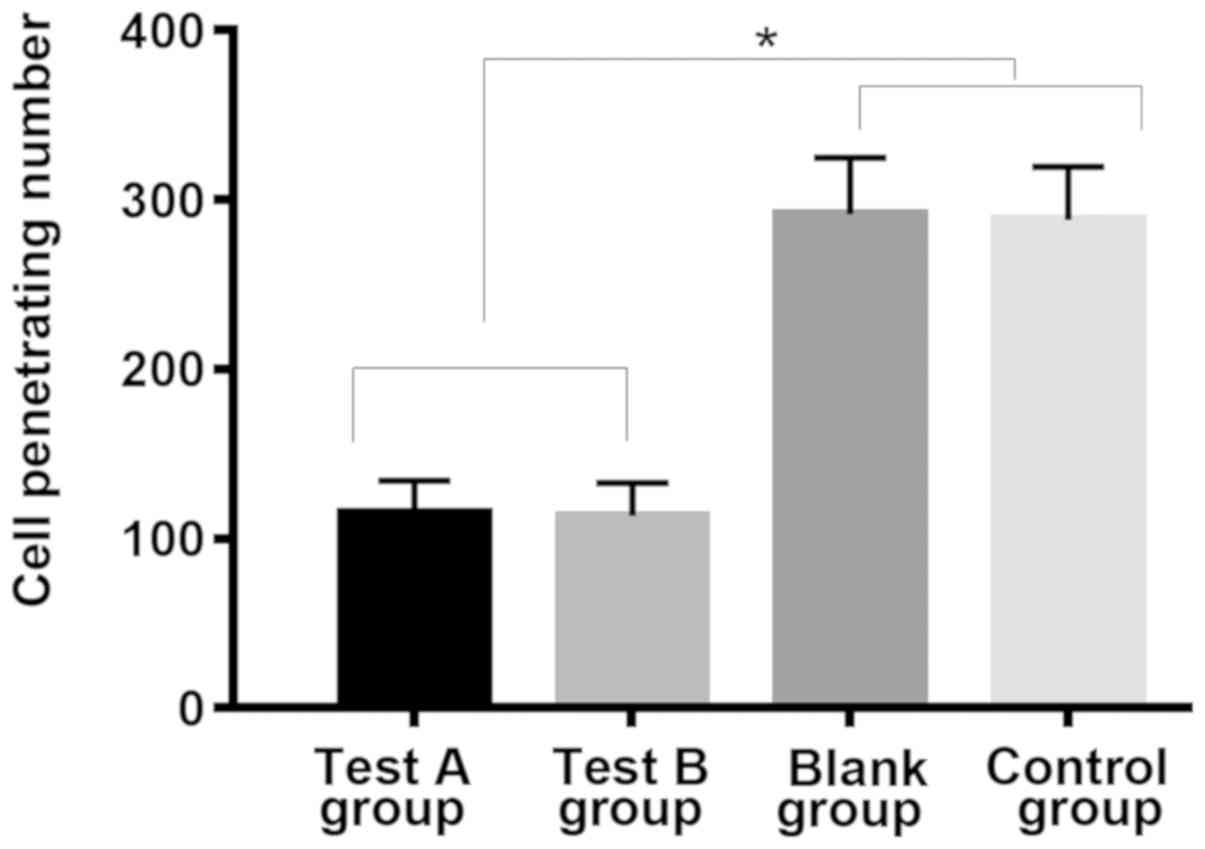
Comparison of cell invasion in each
group
The number of cell transmembranes in the
experimental group A (115.19±18.91) and group B (113.62±19.13) were
significantly lower than that in the blank group (291.35±33.51),
the miR-NC negative control group (288.21±31.33) and the siRNA
negative control group (289.75±30.96), and the difference was
statistically significant (P<0.05). However, there was no
significant difference in the number of cell transmembranes between
experimental group A and B, and between the blank, miR-NC negative
control and siRNA negative control groups (P>0.05; Table III and Fig. 5).
 | Table III.Comparison of the number of cell
transmembranes in each group. |
Table III.
Comparison of the number of cell
transmembranes in each group.
| Indicator | Experimental group
A | Experimental group
B | Blank group | miR-NC negative
control group | siRNA negative
control group | F | P-value |
|---|
| Cell transmembrane
number |
115.19±18.91a |
113.62±19.13a | 291.35±33.51 | 288.21±31.33 | 289.75±30.96 | 36.55 | <0.050 |
Discussion
Osteosarcoma, a primary malignant bone tumor,
originates from mesenchymal stem cells (14). Most of its biological behavior is
highly malignant with the characteristics of early metastasis, high
mortality and high disability rate (15). A study (16) has shown that metastasis of
osteosarcoma is one of the main causes of failure in treatment and
death of patients. The metastasis mechanism of malignant tumors is
that the tumor cells that are detached from lesion enter the blood
or lymphatic system and the body circulation, and then proliferate
and form metastasis focus in the metastatic organs, which is
basically consistent with the metastasis mechanism of osteosarcoma
(17). In recent years, studies
(18,19) have found that the occurrence,
development and metastasis of osteosarcoma are related to the
abnormal expression of genes, and BMP-2 and miR-29c are two of
these factors. In the development of the skeletal system, BMPs, an
important growth factor, have been a hot topic in the occurrence
and development of osteosarcoma (20). A previous study on the detection of
BMPs in osteosarcoma tissues of osteosarcoma patients found that
the BMP-2, BMP-6 and other BMPs were highly expressed (21). At present, there is no detailed
description of the cell proliferation signaling pathway and
migration mechanism of BMP-2 in osteosarcoma. However, a study of
the effect of BMP-2 on osteoblasts proliferation (22) has found that BMP-Smad signaling
pathway plays an important role in the proliferation of
osteoblasts. miR-29c is a member of the miRNA-29 family. A study
found that miR-29c can inhibit the migration of nasopharyngeal
carcinoma tumor cells in nasopharyngeal carcinoma, and also
explained its mechanism is to inhibit the migration of
nasopharyngeal carcinoma cells by targeting activator TIAM1 in Rho
(23). A large number of studies
have found that miR-29c can inhibit tumor metastasis in leukemia
(24) and other malignancies. It has
also been suggested that in granulocytic leukemia, miR-29c can
inhibit the combination of CDK26 and downstream factors by
targeting CDK26, thereby inhibiting the proliferation of tumor
cells (25). Although the expression
of miR-29c in osteosarcoma was studied and found that the
expression of miR-29c is low in osteosarcoma, its mechanism on
osteosarcoma cells has not been described in detail (26). Besides, there are few studies on the
effects of BMP-2 on osteosarcoma cells. Therefore, the expression
of BMP-2 and miR-29c in osteosarcoma and its effect on osteosarcoma
cells were investigated in this study, in order to provide
theoretical basis and data of the relationship between miR-29c and
BMP-2 for future studies, and new ideas and directions for the
diagnosis and treatment of osteosarcoma.
The content of BMP-2 and miR-29c in osteosarcoma
tissue and paraneoplastic tissue of patients with osteosarcoma was
measured, and it was found that the relative expression of BMP-2 in
osteosarcoma tissue was significantly higher than that in
paraneoplastic tissue, and the difference was statistically
significant (P<0.05). The relative expression of miR-29c in
osteosarcoma tissue was significantly lower than that in
paraneoplastic tissue, and the difference was statistically
significant (P<0.05). Guo et al (27) found that BMP-2 could be detected in
all osteosarcoma tissues, and BMP-2 was associated with cancer
metastasis. Di Fiore et al (28) found that miR-29c expression was lower
in osteosarcoma tissue than that in normal tissue. Our conclusions
are consistent with the above studies. miR-29c and BMP-2 siRNA were
transfected into human osteosarcoma MG-63 cells respectively, then
the proliferation and invasive abilities of cells in these groups
were compared after successful transfection. The results showed
that there was no significant difference in proliferation ability
among the five groups on the 1st and 3rd day after transfection,
but the proliferation ability in the experimental groups A and B
was significantly lower than that in the blank, miR-NC negative
control and siRNA negative control groups on the 5th day. There was
no significant difference in proliferation ability between the
experimental groups A and B, the blank, the miR-NC negative control
and the siRNA negative control groups. The number of cell
transmembranes in the experimental groups A and B was significantly
lower than that in the blank, the miR-NC negative control and the
siRNA negative control groups. However, there was no significant
difference in the number of cell transmembranes between
experimental groups A and B, and between the blank, the miR-NC
negative control and the siRNA negative control groups. These
results suggest that interfering with the expression of BMP-2 and
overexpression of miR-29c can inhibit the proliferation and
invasion of osteosarcoma cells. In the study of the mechanism of
miR-29c on colon cancer cells (29),
it was found that miR-29c inhibited cell proliferation and
metastasis by acting on its target genes PHLDB2 and p53, which was
consistent with our conclusions. However, there are no reports on
the association between miR-29c and BMP-2, and only a few reports
on the effect of BMP-2 on osteosarcoma cells. We also found that
there was no correlation between the two factors by searching their
relationship through online software, but whether the two factors
regulate osteosarcoma cells through other signaling pathways
remains to be further explored.
In conclusion, BMP-2 is over-expressed in
osteosarcoma tissues, and miR-29c is under-expressed in
osteosarcoma tissues. Interfering with the expression of BMP-2 and
overexpression of miR-29c can inhibit the proliferation and
invasion of osteosarcoma cells, which indicates that BMP-2 and
miR-29c may be involved in the regulation of proliferation and
metastasis of osteosarcoma cells and could be used as new molecular
target markers for the diagnosis and treatment of osteosarcoma.
However, the detailed mechanism of osteosarcoma cells still need to
be explored.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC was in charge of the study, performed PCR and
wrote the manuscript. XC and YZ were responsible for MTT assay.
Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Tianjin Baodi Hospital (Tianjin, China). Patients who participated
in this study had complete clinical data. Signed informed consents
were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fagioli F, Biasin E, Mereuta OM, Muraro M,
Luksch R, Ferrari S, Aglietta M and Madon E: Poor prognosis
osteosarcoma: new therapeutic approach. Bone Marrow Transplant. 41
(Suppl 2):S131–S134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin BC, Huang D, Yu CQ, Mou Y, Liu YH,
Zhang DW and Shi FJ: MicroRNA-184 modulates doxorubicin resistance
in osteosarcoma cells by targeting BCL2L1. Med Sci Monit.
22:1761–1765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacci G, Balladelli A, Palmerini E,
Alberghini M, Pollastri P, Galletti S, Mercuri M and Picci P:
Neoadjuvant chemotherapy for osteosarcoma of the extremities in
preadolescent patients: the Rizzoli Institute experience. J Pediatr
Hematol Oncol. 30:908–912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li W, Xie P and Ruan WH: Overexpression of
lncRNA UCA1 promotes osteosarcoma progression and correlates with
poor prognosis. J Bone Oncol. 5:80–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bacci G, Ruggieri P, Bertoni F, Ferrari S,
Longhi A, Biagini R, Zavatta M, Versari M and Forni C: Local and
systemic control for osteosarcoma of the extremity treated with
neoadjuvant chemotherapy and limb salvage surgery: the Rizzoli
experience. Oncol Rep. 7:1129–1133. 2000.PubMed/NCBI
|
|
7
|
Chen G, Fang T, Huang Z, Qi Y, Du S, Di T,
Lei Z, Zhang X and Yan W: MicroRNA-133a inhibits osteosarcoma cells
proliferation and invasion via targeting IGF-1R. Cell Physiol
Biochem. 38:598–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu P, Man Y, Wang Y and Bao Y: Mechanism
of BMP9 promotes growth of osteosarcoma mediated by the Notch
signaling pathway. Oncol Lett. 11:1367–1370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang L, Shrestha S, LaChaud G, Scott MA
and James AW: Review of microRNA in osteosarcoma and
chondrosarcoma. Med Oncol. 32:6132015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang J, Yu C, Chen M, Zhang H, Tian S and
Sun C: Reduction of miR-29c enhances pancreatic cancer cell
migration and stem cell-like phenotype. Oncotarget. 6:2767–2778.
2015.PubMed/NCBI
|
|
12
|
Zhu W, He J, Chen D, Zhang B, Xu L, Ma H,
Liu X, Zhang Y and Le H: Expression of miR-29c, miR-93, and miR-429
as potential biomarkers for detection of early stage non-small lung
cancer. PLoS One. 9:e877802014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Velletri T, Xie N, Wang Y, Huang Y, Yang
Q, Chen X, Chen Q, Shou P, Gan Y, Cao G, et al: P53 functional
abnormality in mesenchymal stem cells promotes osteosarcoma
development. Cell Death Dis. 7:e20152016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan WD, Wang P, Feng S, Xue Y and Zhang
B: MicroRNA-497 inhibits cell proliferation, migration, and
invasion by targeting AMOT in human osteosarcoma cells. OncoTargets
Ther. 9:303–313. 2016. View Article : Google Scholar
|
|
16
|
Mirabello L, Koster R, Moriarity BS,
Spector LG, Meltzer PS, Gary J, Machiela MJ, Pankratz N, Panagiotou
OA, Largaespada D, et al: A genome-wide scan identifies variants in
NFIB associated with metastasis in patients with osteosarcoma.
Cancer Discov. 5:920–931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi C, Li X, Xu W and Chen A: Relationship
between the expression of MTA-1 gene and the metastasis and
invasion in human osteosarcoma. J Huazhong Univ Sci Technolog Med
Sci. 25:445–447. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Park P, Zhang H, La Marca F,
Claeson A, Valdivia J and Lin CY: BMP-2 inhibits the tumorigenicity
of cancer stem cells in human osteosarcoma OS99-1 cell line. Cancer
Biol Ther. 11:457–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong Q, Fang J, Pang Y and Zheng J:
Prognostic value of the microRNA-29 family in patients with primary
osteosarcomas. Med Oncol. 31:372014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu L, van der Valk M, Cao J, Han CY, Juan
T, Bass MB, Deshpande C, Damore MA, Stanton R and Babij P:
Sclerostin expression is induced by BMPs in human Saos-2
osteosarcoma cells but not via direct effects on the sclerostin
gene promoter or ECR5 element. Bone. 49:1131–1140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gobbi G, Sangiorgi L, Lenzi L, Casadei R,
Canaider S, Strippoli P, Lucarelli E, Ghedini I, Donati D, Fabbri
N, et al: Seven BMPs and all their receptors are simultaneously
expressed in osteosarcoma cells. Int J Oncol. 20:143–147.
2002.PubMed/NCBI
|
|
22
|
Huo L, Liu K, Pei J, Yang Y, Ye Y, Liu Y,
Sun J, Han H, Xu W and Gao Y: Fluoride promotes viability and
differentiation of osteoblast-like Saos-2 cells via BMP/Smads
signaling pathway. Biol Trace Elem Res. 155:142–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu N, Tang LL, Sun Y, Cui RX, Wang HY,
Huang BJ, He QM, Jiang W and Ma J: MiR-29c suppresses invasion and
metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer
Lett. 329:181–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stamatopoulos B, Meuleman N, Haibe-Kains
B, Saussoy P, Van Den Neste E, Michaux L, Heimann P, Martiat P,
Bron D and Lagneaux L: microRNA-29c and microRNA-223
down-regulation has in vivo significance in chronic lymphocytic
leukemia and improves disease risk stratification. Blood.
113:5237–5245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawrie CH, Ballabio E, Dyar OJ, Jones M,
Ventura R, Chi J, Tramonti D, Gooding S, Boultwood J, Wainscoat JS,
et al: MicroRNA expression in chronic lymphocytic leukaemia. Br J
Haematol. 147:398–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao S, Cheng C, Chen H, Li M, Liu K and
Wang G: IGF1 3′UTR functions as a ceRNA in promoting angiogenesis
by sponging miR-29 family in osteosarcoma. J Mol Histol.
47:135–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo W, Gorlick R, Ladanyi M, Meyers PA,
Huvos AG, Bertino JR and Healey JH: Expression of bone
morphogenetic proteins and receptors in sarcomas. Clin Orthop Relat
Res. 365:175–183. 1999. View Article : Google Scholar
|
|
28
|
Di Fiore R, Drago-Ferrante R, Pentimalli
F, Di Marzo D, Forte IM, D'Anneo A, Carlisi D, De Blasio A,
Giuliano M, Tesoriere G, et al: MicroRNA-29b-1 impairs in
vitro cell proliferation, self-renewal and chemoresistance of
human osteosarcoma 3AB-OS cancer stem cells. Int J Oncol.
45:2013–2023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen G, Zhou T, Li Y, Yu Z and Sun L: p53
target miR-29c-3p suppresses colon cancer cell invasion and
migration through inhibition of PHLDB2. Biochem Biophys Res Commun.
487:90–95. 2017. View Article : Google Scholar : PubMed/NCBI
|