Introduction
Cervical cancer (CC) is the third most common cancer
in females worldwide, following breast and colon cancer (1). Squamous cervical cancer (SCC) is the
major pathological type of CC, which is believed to gradually
develop from cervical intraepithelial neoplasia (CIN) (2). One of the causes of CC is persistent
high-risk human papillomavirus (HPV) infection, and HPV type 16
(HPV16) in particular is associated with 55.2% of SCC cases
(3). With widespread screening and
improvements in diagnostic techniques for cervical precancerous
lesions, the incidence of CC has decreased by 65% in certain
Western countries over the past 40 years (4,5).
Nevertheless, invasion and metastasis of SCC remain major causes of
postoperative relapse and mortality.
Tumor invasion and metastasis are a complex
multistep process, involving multiple genes. Weakened adhesion and
increased capacity for migration in tumor cells constitute a basis
for tumor invasion and metastasis (6,7). A
number of studies have indicated that epithelial-mesenchymal
transition (EMT) serves a role in tumor invasion and metastasis
(8–11). In addition, studies have verified the
important role of EMT in solid tumors, including ovarian, breast,
prostate, lung, and liver cancer (12,13). EMT
was first proposed by Greenburg and Hay in 1982 (14) as the process by which epithelial
cells lose their stable structure and their polarity and transform
into freely migrating mesenchymal cells in the cell matrix, under
certain special physiological or pathological conditions. The EMT
process is characterized by the following: Reduction in the
expression of or loss of cell adhesion molecules, including
E-cadherin, transformation of the cytoskeleton from being majorly
composed of epithelial cytokeratin to a cytoskeleton dominated by
vimentin, loss of intercellular junctions, changes in cell
morphology, and improved movement capacity (15). Markers of epithelial cell EMT,
including E-cadherin and β-catenin, and mesenchymal cell markers,
including vimentin and fibronectin, are used to study the
biological behavior of EMT in tissues (16,17). A
number of studies have detected EMT in SCC and evidence from
surgical specimens of SCC suggests that SCC progression is
accompanied by E-cadherin downregulation and vimentin upregulation
(18–21). HPV16 E6 or E7 have been indicated to
regulate EMT in cervical epithelial cells, therefore, promoting
tumor progression and metastasis (22,23).
Nevertheless, the precise involvement and mechanisms of HPV16 in
the EMT of CIN and SCC lesions remain unknown.
In the present study, changes in the EMT indicators,
including E-cadherin, β-catenin, N-cadherin, vimentin, and
fibronectin were studied in clinical tissue specimens, from
low-grade CIN1 to high-grade CIN 2 and 3 (24), in addition to early stage SCC, in
association with HPV16. The association among the EMT indicators
and the clinicopathological features of patients with early stage
SCC was also examined, and their prognostic value in SCC
analyzed.
Materials and methods
Patient sample
Between January 2010 and December 2012, a total of
208 patients (mean age, 44.1±8.6 years; range, 25–61 years)
consulted at The Second Hospital of Hebei Medical University
(Shijiazhuang, China) for abnormal bleeding or increased leucorrhea
discharge. There were 86 cases of SCC, 82 cases of CIN, and 40
cases of leiomyoma with normal cervix. The patients who had
received radiotherapy or chemotherapy were excluded. Among the 86
patients with SCC, 60 had stage Ia-b disease and 26 had stage IIa
disease, according to the International Federation of Gynecology
and Obstetrics (FIGO) 2000 standard (25). These patients underwent radical
hysterectomy and pelvic lymph nodes resection, and the pathological
examination confirmed the SCC in all 86 patients. Among them, 9
patients had parametrial invasion and 37 had lymph node metastasis.
Among the 82 patients with CIN, 22 had CIN grade 1 and 60 had CIN
grade 2–3 lesions according to the 2014 World Health Organization
classification (26). Pathological
examination performed at The Second Hospital of Hebei Medical
University (Shijiazhuang, China) did not reveal any abnormal
findings in the uterine cervix of the control cases, who underwent
hysterectomy due to hysteromyoma.
The project was approved by the Institutional Review
Board of The Second Hospital of Hebei Medical University
(Shijiazhuang, China). Written informed consent was obtained from
each patient prior to recruitment in the present study.
HPV diagnosis
Cervical exfoliated cells of all patients were
clinically collected preoperatively using a cervical brush and were
tested with the Cobas 4800 DNA HPV Test (Roche Molecular Systems,
Pleasanton, CA, USA), according to the manufacturer's protocol.
This test is a polymerase chain reaction fully automated method
detecting separately HPV16, HPV18 and 12 other hrHPV types,
including 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68
(27). All the HPV-positive patients
in the present study were HPV16-positive.
Immunostaining for EMT markers
According to the previously described method
(28), paraffin cervical sections (4
µm) were immersed in xylene for 10 min twice at room temperature,
and hydrated using a graded series of ethanol (100, 95, 80 and 70%)
all for 5 min at room temperature. Antigen retrieval was performed
by immersing the sections in 0.01 M citrated buffer (pH 6.0) in a
pressure cooker and autoclaving for 15 min. Endogenous peroxidase
activity was blocked with 3% H2O2 for 15 min
at room temperature and then incubated with primary antibodies for
2 h at room temperature. Normal cervical tissues were used as the
positive control and PBS was used instead of primary antibody as
the negative control. Mouse anti-human E-cadherin (dilution, 1:100;
cat. no. ab1416) N-cadherin (dilution, 1:500; cat. no. ab98952),
β-catenin (dilution, 1:1,000; cat. no. ab22656), vimentin
(dilution, 1:1,000; cat. no. ab8069), and fibronectin (dilution,
1:100; cat. no. ab6328) monoclonal antibodies all purchased from
Abcam (Cambridge, MA, USA) were used with the streptavidin-peroxide
(SP) kit and diaminobenzidine (DAB) chromogenic reagent kits
(Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China),
according to the manufacturer's protocols. The sections were
incubated with SP for 1 h at 37°C and then stained using DAB for 10
min at room temperature. Specimens were observed under a light
microscope (magnification, ×400) by two pathologists, at The Second
Hospital of Hebei Medical University, separately using five
high-power randomly selected fields for each patient. The
percentage and staining intensity of cells positive for each of the
aforementioned proteins were determined. The scores for the
percentage of positive cells were: 0, no positive cells; 1, ≤25%
positive cells; 2, 26–50% positive cells; 3, 51–75% positive cells;
and 4, >75% positive cells (29).
With regard to staining intensity, the scores were: 1, weak; 2,
moderate; and 3, strong. If the product of percentage and scoring
intensity scores was >3, the specimen was considered to be
immunohistochemically positive for the corresponding marker
(30).
Statistical analysis
Continuous data were presented as mean ± standard
deviation and analyzed using one-way analysis of variance with the
Tukey's post hoc test. Categorical data were presented as
frequencies and analyzed using the χ2 test. The
associations among HPV16 and E-cadherin, β-catenin, N-cadherin,
vimentin, and fibronectin were analyzed using Spearman's
correlation coefficient. Survival curves were generated by the
Kaplan-Meyer method and the survival rates were compared using the
log-rank test. Univariate and multivariate survival analyses were
performed using the Cox regression model for overall survival (OS)
and disease-free survival (DFS) time. Data were analyzed using SPSS
v.21.0 (IBM Corp., Armonk, NY, USA). Two-sided P<0.05 values
were considered to indicate a statistically significant
difference.
Results
Characteristics of the patients
Table I presents the
characteristics of the patients. Patients with CIN were
significantly younger compared with the controls and patients with
early stage SCC (P<0.001). The rate of HPV16 infection
significantly gradually increased from the controls (0%), to CIN1
(9.1%), CIN2-3 (41.7%), and SCC (94.2%) (P<0.001).
 | Table I.Clinical characteristics of the
patients. |
Table I.
Clinical characteristics of the
patients.
| Variables | Normal control | CIN1 | CIN2-3 | SCC | P-value |
|---|
| No. | 40 | 22 | 60 | 86 |
|
| Age (mean ±
SD) | 48.8±2.9 | 33.5±6.5 | 37.0±5.8 | 49.6±5.9 | <0.001 |
| HPV16 infection
(%) | 0 | 9.1 (2/22) | 41.7 (25/60) | 94.2 (81/86) | <0.001 |
| FIGO stage |
|
|
|
|
|
|
Ia-b | – | – | – | 60 |
|
|
IIa | – | – | – | 26 |
|
| aHistological grade |
|
|
|
|
|
| G1 | – | – | – | 22 |
|
|
G2-3 | – | – | – | 64 |
|
| Parametrial
invasion |
|
|
|
|
|
|
Yes | – | – | – | 9 |
|
| No | – | – | – | 77 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
Yes | – | – | – | 37 |
|
| No | – | – | – | 49 |
|
| Recurrence |
|
|
|
|
|
|
Yes | – | – | – | 23 |
|
| No | – | – | – | 63 |
|
Expression of E-cadherin, β-catenin,
N-cadherin, vimentin, and fibronectin in cervical lesions
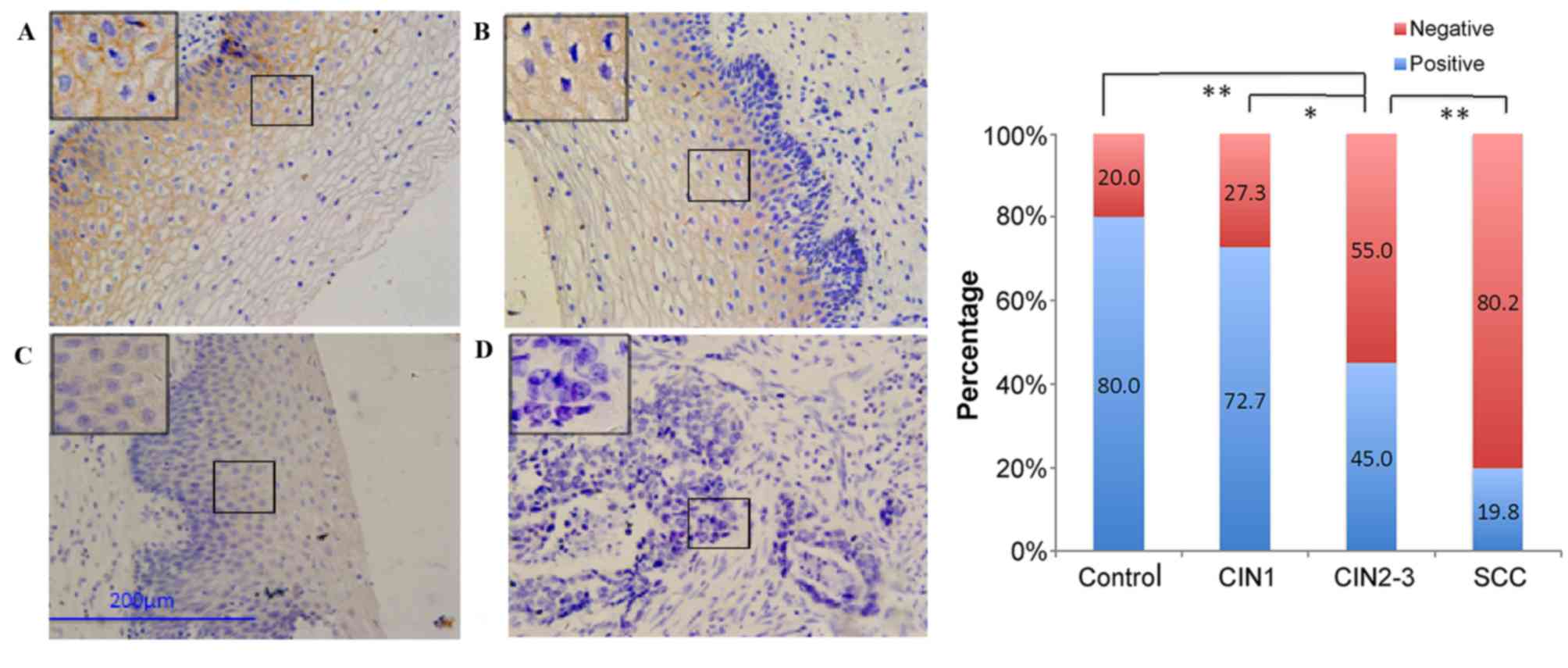
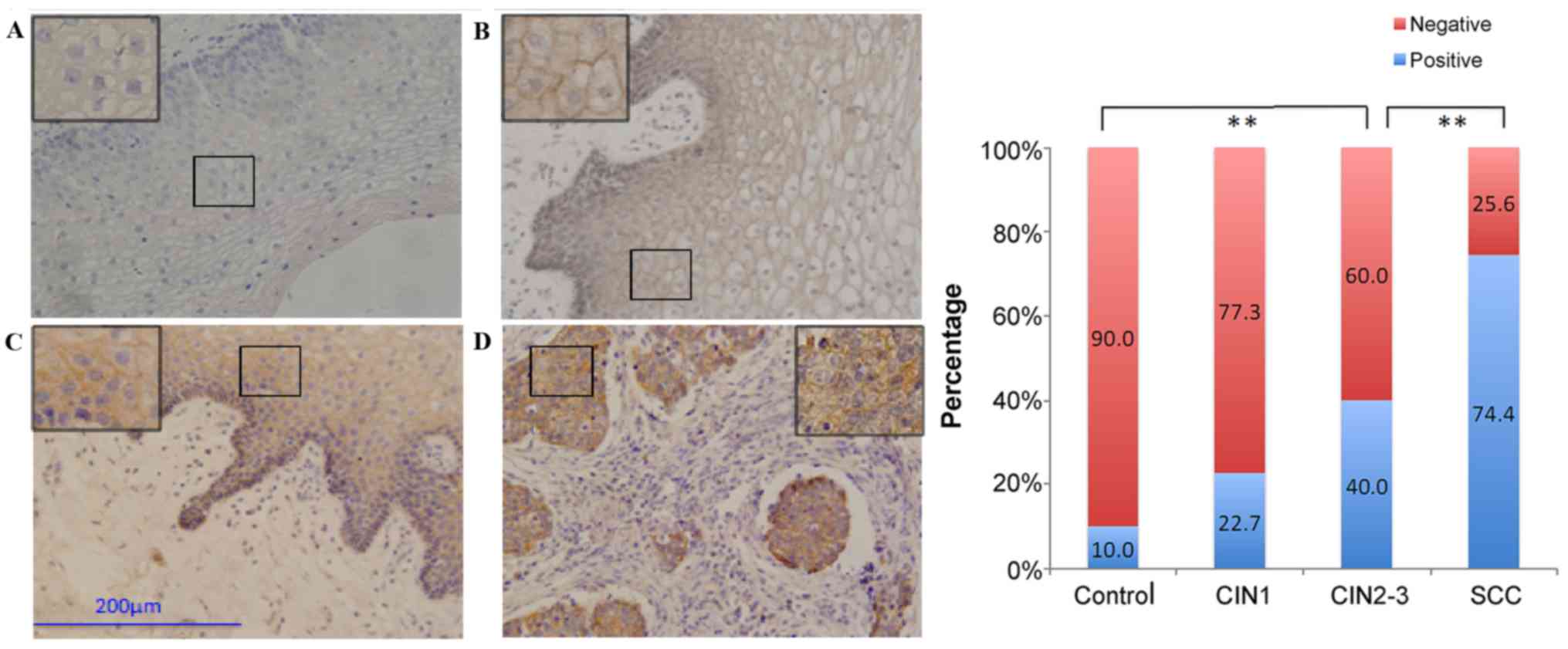
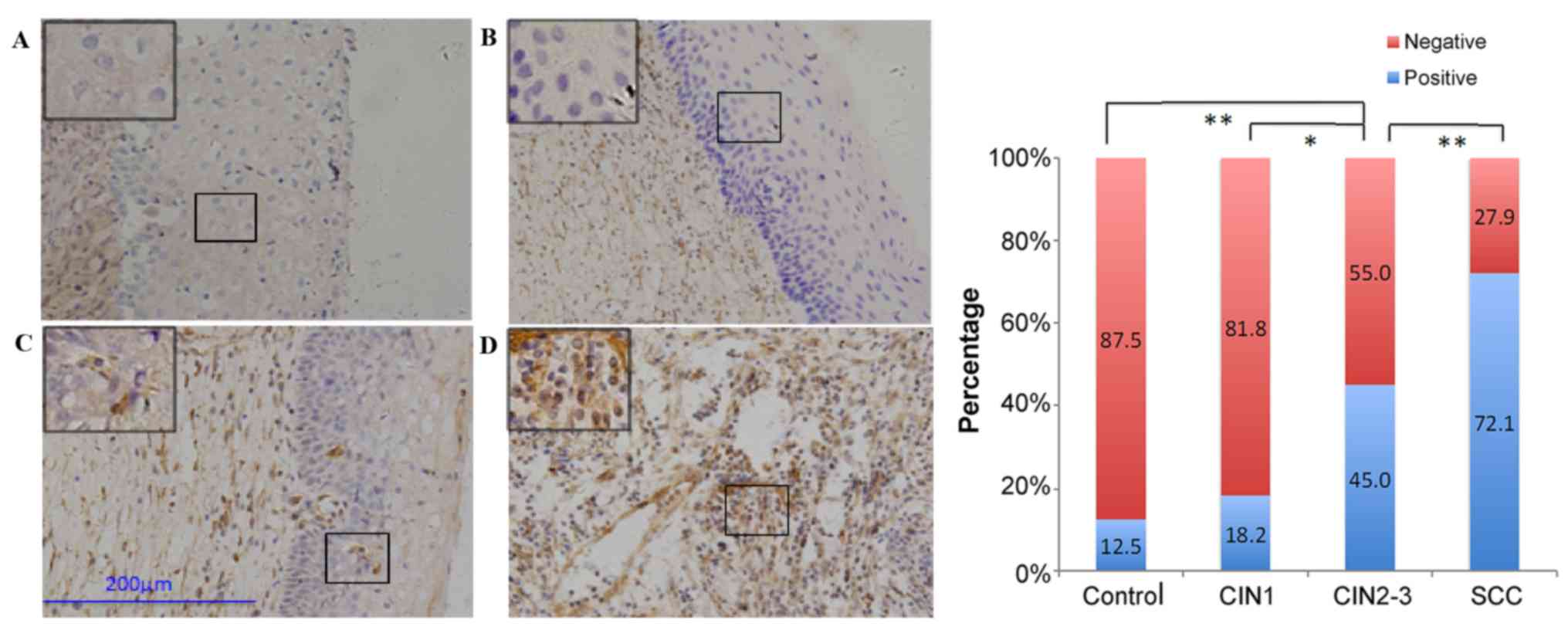
Normally, brown granules in the membrane of
epithelial cells were considered to indicate positive expression
for E-cadherin, β-catenin, and N-cadherin, while brown granules in
the cytoplasm of extracellular matrix were considered to indicate
positive expression for vimentin and fibronectin (20,31,32).
E-cadherin expression was the highest in normal cervical tissue,
and decreased with disease progression in the order of CIN1,
CIN2-3, and SCC, respectively (Fig.
1). β-catenin indicated abnormal expression in the CIN/SCC
cases in the form of weakened membranous staining and increased
cytoplasmic staining (Fig. 2).
Similar to E-cadherin expression, the percentage of patients with
positive β-catenin expression decreased with disease progression.
The expression of N-cadherin (Fig.
3), vimentin (Fig. 4), and
fibronectin (Fig. 5) exhibited an
opposite trend, therefore, positive staining rate increased with
disease progression. The expression of all five proteins was not
significantly different between the control and CIN1 groups. The
expression of E-cadherin, β-catenin, and vimentin was significantly
different (P=0.024–0.026), but the expression of N-cadherin and
fibronectin was not significantly different between the CIN1 and
CIN2-3 groups. However, the differences were significant for
comparisons among all the other groups (P≤0.002).
Clinicopathological features and
prognostic values of EMT indicators
During the median follow-up of 60 months (range,
10–90 months), 23/86 (26.7%) patients had a SCC recurrence. Among
those 23 patients, 20 (87.0%) succumbed to cancer progression. The
5-year DFS and OS rates were 73.3 and 76.7%, respectively.
Patients with SCC with negative E-cadherin and
negative β-catenin expression had significantly shorter OS times
(P=0.014 and P=0.043, respectively) and shorter DFS times (P=0.025,
and P=0.045, respectively) (Fig. 6).
Patients with SCC with lymph node metastasis and parametrial
invasion also had significantly shorter OS times (P=0.001 and
P=0.015, respectively) and shorter DFS times (P=0.002 and P=0.021,
respectively) (Fig. 7). The other
clinical features, including age, FIGO stage, and histological
grade, and the other three EMT indicators, including N-cadherin,
vimentin, and fibronectin indicated no significant differences.
Among patients with SCC, univariate survival
analyses revealed that SCC with lymph node metastasis, parametrial
invasion, negative E-cadherin, and negative β-catenin were
correlated with shorter OS (P=0.003, P=0.022, P<0.001, and
P<0.001, respectively; Table II)
and DFS (P=0.004, P=0.030, P<0.001, and P<0.001,
respectively; Table II).
 | Table II.Univariate survival analysis of
factors associated with OS and DFS for patients with SCC. |
Table II.
Univariate survival analysis of
factors associated with OS and DFS for patients with SCC.
|
| OS |
| DFS |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.026 | 0.557–1.892 | 0.934 | 1.055 | 0.311–3.583 | 0.931 |
| FIGO stage | 0.840 | 0.540–1.307 | 0.840 | 0.676 | 0.280–1.633 | 0.385 |
| Histological
grade | 1.385 | 0.891–2.154 | 0.147 | 1.850 | 0.767–4.464 | 0.171 |
| Lymph node
metastasis | 4.151 | 1.606–10.731 | 0.003b | 4.043 | 1.567–10.432 | 0.004b |
| Parametrial
invasion | 1.804 | 1.090–2.986 | 0.022a | 1.744 | 1.055–2.883 | 0.030a |
| E-cadherin | 0.159 | 0.081–0.312 |
<0.001b | 0.156 | 0.080–0.307 |
<0.001b |
| β-catenin | 0.575 | 0.422–0.784 |
<0.001b | 0.332 | 0.179–0.616 |
<0.001b |
| N-cadherin | 1.216 | 0.705–2.097 | 0.481 | 1.491 | 0.502–4.433 | 0.472 |
| Vimentin | 1.689 | 0.915–3.116 | 0.094 | 2.655 | 0.782–9.108 | 0.118 |
| Fibronectin | 1.662 | 0.802–3.445 | 0.172 | 2.733 | 0.636–11.733 | 0.176 |
The multivariate analysis indicated that among
patients with SCC, lymph node metastasis, parametrial invasion, and
negative E-cadherin expression were independent prognostic
predicators for OS (P=0.010, P=0.007 and P<0.001, respectively;
Table III) and DFS (P=0.009,
P=0.011 and P<0.001, respectively; Table III).
 | Table III.Multivariate survival analysis of OS
and DFS in SCC patients. |
Table III.
Multivariate survival analysis of OS
and DFS in SCC patients.
|
| OS |
| DFS |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Lymph node
metastasis | 3.544 | 1.361–9.280 | 0.010a | 3.612 | 1.385–9.415 | 0.009b |
| Parametrial
invasion | 2.014 | 1.208–3.355 | 0.007b | 1.935 | 1.165–3.214 | 0.011a |
| E-cadherin | 0.163 | 0.066–0.403 |
<0.001b | 0.168 | 0.068–0.414 |
<0.001b |
| β-catenin | 0.611 | 0.176–2.120 | 0.438 | 0.625 | 0.176–2.216 | 0.466 |
Correlation of HPV16 infection and EMT
proteins
The Cobas 4800 HPV test indicated that no case in
the control group was HPV16 positive, and 27/82 (32.9%) patients
with CINs had HPV16 infection. Correlation analysis indicated that
the expression rate of β-catenin was not significantly correlated
to HPV16 infection (P=0.941), while the expression rates of
E-cadherin, N-cadherin, vimentin and fibronectin were significantly
correlated to HPV16 infection (P≤0.001; Table IV). E-cadherin had a negative
relationship with HPV16 infection (rs=−0.424), while
N-cadherin, vimentin, and fibronectin had positive relationships
with HPV16 infection (rs=0.404, 0.417, and 0.355,
respectively). Among early stage SCC cases, 81/86 (94.2%) had a
HPV16 infection, but no significant relationship was indicated
between HPV16 infection and the five EMT proteins (P>0.05).
 | Table IV.Correlations between HPV16 infection
and expression of E-cadherin, β-catenin, N-cadherin, vimentin, and
fibronectin in CIN tissues (n=82). |
Table IV.
Correlations between HPV16 infection
and expression of E-cadherin, β-catenin, N-cadherin, vimentin, and
fibronectin in CIN tissues (n=82).
|
| HPV16 |
|
|
|---|
|
|
|
|
|
|---|
| Marker | Positive
(n=27) | Negative
(n=55) | rs | P-value |
|---|
| E-cadherin | 6 | 37 | −0.424 |
<0.001a |
| β-catenin | 13 | 26 | 0.008 | 0.941 |
| N-cadherin | 17 | 12 | 0.404 |
<0.001a |
| Vimentin | 18 | 13 | 0.417 |
<0.001a |
| Fibronectin | 20 | 20 | 0.355 | 0.001a |
Discussion
The precise involvement and mechanisms of HPV16 in
EMT of CIN and SCC remain unknown. Therefore, the present study
aimed to examine the expression of EMT indicators and their
relationship with HPV16 in CIN and early stage SCC, and their
prognostic value in early stage SCC. The results indicated that EMT
occurs during the progression of CINs to early stage SCC, and is
correlated to HPV16 infection in CINs. Lymph node metastasis and
parametrial invasion are poor prognostic factors for early stage
SCC, while positive E-cadherin expression may serve as a protective
prognostic factor for early stage SCC.
E-cadherin is a calcium-dependent cell adhesion
molecule serving a critical role among epithelial cells as its loss
has been reported to contribute to cancer metastasis (33). During EMT, the downregulation of
E-cadherin is a main indicator for intercellular junctions loss
(20). β-catenin is a member of the
catenin family and serves a vital role in cadherin adhesion
functions (34). In the present
study, it was indicated that the expression level of E-cadherin and
β-catenin were reduced with disease progression, in a gradual
manner. In addition, the expression pattern of β-catenin differed
on a molecular level, as the expression level in the cell membrane
declined, while its expression level in the cytoplasm was
upregulated with the progression of cervical lesions. These results
are in accordance to a previous study (35), in addition to the previous findings
reporting that Wnt signaling inhibits the degradation process of
β-catenin by phosphorylating and inhibiting GSK3β in tumors,
thereby allowing β-catenin to accumulate in the cytosol and enter
the nucleus, binding to the T-cell factor and driving transcription
(36).
Accordingly, in the present study, the expression
levels of E-cadherin and β-catenin in patients with early stage SCC
with intravascular tumor thrombus and lymph node metastasis were
significantly decreased compared with the expression levels in
patients with SCC, but without tumor thrombus or lymph node
metastasis. Therefore, from the aforementioned findings, it is
suggested that downregulation of E-cadherin and β-catenin serves a
role in the occurrence and development of SCC. Based on previous
studies and the present results, reduced expression levels of
E-cadherin and β-catenin are suggested to weaken intercellular
adhesion, cause intercellular junction loss, morphological changes,
and increase mobility of abnormal cells, leading to cancer
metastasis (6,8,11). In
the present study, the expression of E-cadherin was a protective
prognostic factor in patients with early stage SCC, supporting the
concept that low EMT leads to a limited number of cells
participating in the metastatic spread.
N-cadherin and E-cadherin are members of the
cadherin family, but they exhibit opposite effects, where
E-cadherin mediates the adhesion between epithelial cells (33), as indicated above, and N-cadherin
promotes cell movement (37). The
‘cadherin switch’ is characterized by reduced expression of
E-cadherin and increased expression of N-cadherin and is believed
to be one of the most important processes of EMT and its
involvement in the metastatic spread of cancer cells (37). A number of studies have reported that
abnormal expression of N-cadherin in epithelial tissues could
promote morphological changes and EMT of cancer cells (38–41). In
the present study, the expression level of N-cadherin was very low
in normal cervical tissues, in comparison to its expression level
in the CIN tissues, while N-cadherin exhibited an even higher
expression in SCC tissues. Its positive expression rate in the SCC
group with parametrial invasion was significantly higher compared
with the SCC group without parametrial invasion, and its positive
expression rate in the group with lymph node metastasis was
significantly higher compared with the group without metastasis.
These results are supported by a previous study in nasopharyngeal
carcinoma that indicated that the expression of N-cadherin was
correlated with lymph node metastasis (42). In conclusion, these findings suggest
the important role of N-cadherin in SCC progression.
Vimentin (type III; 57 kD) is an intermediate
filament that is found in the mesenchymal cells of various types of
tissue during their developmental stages and maintains cell and
tissue integrity (43). Increased
abnormal expression of vimentin causes compositional changes in
cytoskeletal proteins and transforms cuboidal epithelial cells into
fusiform fibroblasts for easy migration (44,45). It
also leads to reoriented microtubule polarity and increased EMT
phenotypes, due to increased β1-integrin and the loss of junction
protein E-cadherin (46). A number
of studies have reported that vimentin was expressed abnormally in
epithelial malignant tumors, including ovarian, breast, colon,
esophageal, and prostate cancer (47–51).
Fibronectin is a non-collagen glycoprotein present
in the extracellular matrix. Fibronectin regulates cell adhesion,
proliferation, differentiation, and morphological maintenance of
tissues under normal physiological conditions. It is associated
with tumor invasion and metastasis in various pathological
conditions (52,53). In cervical lesions, fibronectin
correlates positively with alpha v beta 6 expression, an
unfavorable prognostic factor of SCC (32). In the present study, vimentin and
fibronectin exhibited a gradual increase in expression with the
progression from CIN1 to CIN3, and expression significantly
increased in SCC lesions. The expression levels of vimentin and
fibronectin in the SCC group with tumor thrombi were significantly
higher compared with the group without tumor thrombi. In addition,
their expression levels in the group with lymph node metastasis
were higher compared with the group without metastasis. These
findings suggest that vimentin and fibronectin promote the
progression of cervical lesions, and that their upregulated
expression promotes the malignant-invasive behavior of SCC
cells.
However, there was no indication that N-cadherin,
vimentin and fibronectin were independent prognostic factors of
early stage SCC. This could be due to the fact that the effects of
E-cadherin and β-catenin are stronger and may obscure the effects
of N-cadherin, vimentin and fibronectin. Another possibility is
that these factors are covariate. Additional studies are necessary
to determine their exact prognostic significance in SCC.
Nonetheless, there were no significant differences observed in the
present study in the five EMT indicators among different SCC
stages. This may be due to the relatively small sample size of the
present study. In addition, EMT indicators may have a higher
association with parametrial invasion and lymph node metastasis
compared with FIGO stages. In the present study, lymph node
metastasis and parametrial invasion were indicated to be poor
prognostic factors for SCC. As these factors have already been
identified in a number of studies (54–56),
this suggests that the group of patients it the present study may
be representative of the general population of patients with
SCC.
The association between HPV16 infection and SCC
occurrence has been extensively studied (22,57–59).
HPV16, along with HPV18, was identified as an important factor to
the development of cervical cancer in China in 2009 (60). A number of studies have confirmed
that HPV16, similar to other tumor-causing viruses, including
hepatitis B, Epstein-Barr virus and hepatitis C, have the ability
to downregulate E-cadherin and inhibit Langerhans cell adhesion and
immune surveillance capability (61,62),
causing increased expression of N-cadherin in epithelial cells
(63), therefore, participating in
EMT. The expression levels of E-cadherin in the cell membrane of
HPV16-positive keratinocytes increased following the silencing of
HPV16 E7 gene (64). This suggests
that the HPV16 oncogene may lead to cervical lesions through
cellular EMT. In the present study, 32.9% (27/82) of the CIN cases
had HPV16 infection, and HPV16 infection was negatively correlated
with the expression level of E-cadherin and positively correlated
with the expression level of N-cadherin, vimentin, and fibronectin.
The expression of E-cadherin gradually decreased, while the
expression of N-cadherin, vimentin, and fibronectin gradually
increased with HPV16 infection in the progression of CIN lesions.
Therefore, cervical epithelial cells may lose their morphology and
characteristics during EMT and gain invasive properties, leading to
the formation of progressive cervical lesions and, subsequently,
SCC. There are a number of studies indicating the impact of the HPV
E6 oncogene in the canonical Wnt/β-catenin signaling pathway
(65–67). E6 can augment the Wnt/β-catenin/T
cell factor (TCF) signaling response, however, it does not
significantly alter β-catenin stability and expression (66). In addition, the ability of E6 to
activate TCF response may be dependent, as well as independent, of
β-catenin translocation (66). This
may explain the lack of association of β-catenin and HPV16 in the
present study.
However, in the present study no significant
associations between HPV16 infection and the five EMT indicators
were observed in the 86 cases of SCC. There are a number of reasons
why this may have occurred, including the relatively small sample
size of the present study. It may also be due to the fact that once
HPV16 prompts CIN progression through EMT, it will affect SCC cells
through other pathways, rather than EMT. Another possibility is
that other oncogenic effects of HPV16 are stronger compared with
EMT process. For example, HPV E6 and E7 oncogenes have the ability
to degrade pRb, inactivate P53, interact with PDZ-containing
proteins, regulate multiple epigenetic factors, encode miRNAs, to
allow viral persistence, evade host immune surveillance, and
deregulate cell cycle and apoptosis control (68,69).
This was a single-center study and the number of
patients was limited. In addition, the panel of proteins was
limited. Further studies on a more comprehensive panel of factors
potentially involved in EMT, invasion, and prognosis are required.
In addition, in the present study only the protein levels were
examined, therefore mRNA levels should also be examined in the
future.
This is the first study, to the best of our
knowledge, to systematically examine the expression of the five
representative EMT markers in normal cervical tissue, CIN1, CIN2-3
and SCC. The expression of the epithelial markers E-cadherin and
β-catenin reduced gradually, while the expression of the
mesenchymal markers N-cadherin, vimentin, and fibronectin increased
gradually during the progression of cervical squamous cell lesions.
Patients with SCC with lymph node metastasis, parametrial invasion,
negative E-cadherin, and negative β-catenin expression had shorter
OS and DFS. Lymph node metastasis and parametrial invasion are
independent poor prognostic factors, while positive E-cadherin
expression may serve as an independent protective prognostic factor
for early stage SCC. In addition, during the process of CIN, the
rate of HPV16 infection was negatively correlated with the
expression of E-cadherin and positively correlated with N-cadherin,
vimentin, and fibronectin. Therefore, HPV16 may cause CIN
progression via an EMT-based pathway in cervical squamous
epithelial cells. Nevertheless, the association between EMT and
high-risk HPV require further investigation with a larger sample
size of patients, in addition to the study of the underlying
molecular mechanisms involved.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant, no. 81101974).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ conceived the study, participated in study design
and coordination, and assisted with drafting of the manuscript. XL
performed the pathological study and drafted the manuscript. XY
performed the clinical follow-up and the statistical analysis. JZ
performed the histological examination and interpreted the data. BS
contributed towards data analysis and the revision of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of The Second
Hospital of Hebei Medical University (Hebei, China) approved the
project. All methods were performed in accordance with the relevant
guidelines and regulations. Written informed consent was received
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lertkhachonsuk AA, Yip CH, Khuhaprema T,
Chen DS, Plummer M, Jee SH, Toi M and Wilailak S; Asian Oncology
Summit 2013, : Cancer prevention in Asia: Resource-stratified
guidelines from the Asian Oncology Summit, 2013. Lancet Oncol.
14:e497–e507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stanley M: Pathology and epidemiology of
HPV infection in females. Gynecol Oncol 117 (2 Suppl). S5–S10.
2010. View Article : Google Scholar
|
|
3
|
Clifford GM, Smith JS, Plummer M, Muñoz N
and Franceschi S: Human papillomavirus types in invasive cervical
cancer worldwide: A meta-analysis. Br J Cancer. 88:63–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang J, Wei LH, Li YL, Wu RF, Xie X, Feng
YJ, Zhang G, Zhao C, Zhao Y and Chen Z: Detection of TERC
amplification in cervical epithelial cells for the diagnosis of
high-grade cervical lesions and invasive cancer: A multicenter
study in China. J Mol Diagn. 12:808–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geiger TR and Peeper DS: Metastasis
mechanisms. Biochim Biophys Acta. 1796:293–308. 2009.PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhau HE, Odero-Marah V, Lue HW, Nomura T,
Wang R, Chu G, Liu ZR, Zhou BP, Huang WC and Chung LW: Epithelial
to mesenchymal transition (EMT) in human prostate cancer: Lessons
learned from ARCaP model. Clin Exp Metastasis. 25:601–610. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang MH, Chen CL, Chau GY, Chiou SH, Su
CW, Chou TY, Peng WL and Wu JC: Comprehensive analysis of the
independent effect of twist and snail in promoting metastasis of
hepatocellular carcinoma. Hepatology. 50:1464–1474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gjerdrum C, Tiron C, Høiby T, Stefansson
I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT,
et al: Axl is an essential epithelial-to-mesenchymal
transition-induced regulator of breast cancer metastasis and
patient survival. Proc Natl Acad Sci USA. 107:1124–1129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kowalski PJ, Rubin MA and Kleer CG:
E-cadherin expression in primary carcinomas of the breast and its
distant metastases. Breast Cancer Res. 5:R217–R222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ngan CY, Yamamoto H, Seshimo I, Tsujino T,
Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, et
al: Quantitative evaluation of vimentin expression in tumour stroma
of colorectal cancer. Br J Cancer. 96:986–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang J, Zhou H, Peng Y, Xie X, Li R, Liu
Y, Xie Q and Lin Z: β-catenin expression negatively correlates with
WIF1 and predicts poor clinical outcomes in patients with cervical
cancer. Biomed Res Int. 2016:49239032016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Zhou Y, Jiang W, Yang X, Zhu J,
Feng D, Wei Y, Li M, Yao F, Hu W, et al: Significance of
E-cadherin, β-catenin, and vimentin expression as postoperative
prognosis indicators in cervical squamous cell carcinoma. Hum
Pathol. 43:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li XL, Jiang J and Lu SY:
Epithelial-mesenchymal transition and gynecologic oncology. Chin J
Obstet Gynecol. 47:549–551. 2012.(In Chinese).
|
|
22
|
Chen X, Bode AM, Dong Z and Cao Y: The
epithelial-mesenchymal transition (EMT) is regulated by oncoviruses
in cancer. FASEB J. 30:3001–3010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cyprian FS, Al-Farsi HF, Vranic S, Akhtar
S and Al Moustafa AE: Epstein-barr virus and human papillomaviruses
interactions and their roles in the initiation of
epithelial-mesenchymal transition and cancer progression. Fron
Oncol. 8:1112018. View Article : Google Scholar
|
|
24
|
Lapierre SG, Sauthier P, Mayrand MH,
Dufresne S, Petignat P, Provencher D, Drouin P, Gauthier P, Dupuis
MJ, Michon B, et al: Human papillomavirus (HPV) DNA triage of women
with atypical squamous cells of undetermined significance with
cobas 4800 HPV and Hybrid Capture 2 tests for detection of
high-grade lesions of the uterine cervix. J Clin Microbiol.
50:1240–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benedet JL, Bender H, Jones H III, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO committee
on gynecologic oncology. Int J Gynecol Obstet. 70:209–262. 2000.
View Article : Google Scholar
|
|
26
|
Lax SF, Horn LC and Löning T:
Categorization of uterine cervix tumors: What's new in the 2014 WHO
classification. Pathologe. 37:573–584. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Isidean SD, Coutlée F and Franco EL: cobas
4800 HPV Test, a real-time polymerase chain reaction assay for the
detection of human papillomavirus in cervical specimens. Expert Rev
Mol Diagn. 14:5–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jang TJ, Jung KH and Choi EA: Id-1 gene
downregulation by sulindac sulfide and its upregulation during
tumor development in gastric cancer. Int J Cancer. 118:1356–1363.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fedchenko N and Reifenrath J: Different
approaches for interpretation and reporting of immunohistochemistry
analysis results in the bone tissue-a review. Diagn Pathol.
9:2212014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi D, Jiang K, Fu Y, Fang R, Liu XI and
Chen J: Overexpression of SPARC correlates with poor prognosis in
patients with cervical carcinoma and regulates cancer cell
epithelial-mesenchymal transition. Oncol Lett. 11:3251–3258. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li B, Shi H, Wang F, Hong D, Lv W, Xie X
and Cheng X: Expression of E-, P- and N-cadherin and its clinical
significance in cervical squamous cell carcinoma and precancerous
lesions. PLoS One. 11:e01559102016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hazelbag S, Kenter GG, Gorter A, Dreef EJ,
Koopman LA, Violette SM, Weinreb PH and Fleuren GJ: Overexpression
of the alpha v beta 6 integrin in cervical squamous cell carcinoma
is a prognostic factor for decreased survival. J Pathol.
212:316–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stewart CJ and McCluggage WG:
Epithelial-mesenchymal transition in carcinomas of the female
genital tract. Histopathology. 62:31–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeanes A, Gottardi CJ and Yap AS:
Cadherins and cancer: How does cadherin dysfunction promote tumor
progression? Oncogene. 27:6920–6929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hazan RB, Qiao R, Keren R, Badano I and
Suyama K: Cadherin switch in tumor progression. Ann N Y Acad Sci.
1014:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Costa LC, Leite CF, Cardoso SV, Loyola AM,
Faria PR, Souza PE and Horta MC: Expression of
epithelial-mesenchymal transition markers at the invasive front of
oral squamous cell carcinoma. J Appl Oral Sci. 23:169–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakajima S, Doi R, Toyoda E, Tsuji S, Wada
M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, et al: N-cadherin
expression and epithelial-mesenchymal transition in pancreatic
carcinoma. Clin Cancer Res. 10:4125–4133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun H, Liu M, Wu X, Yang C, Zhang Y, Xu Z,
Gao K and Wang F: Overexpression of N-cadherin and β-catenin
correlates with poor prognosis in patients with nasopharyngeal
carcinoma. Oncol Lett. 13:1725–1730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Coulombe PA and Wong P: Cytoplasmic
intermediate filaments revealed as dynamic and multipurpose
scaffolds. Nat Cell Biol. 6:699–706. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lang SH, Hyde C, Reid IN, Hitchcock IS,
Hart CA, Bryden AA, Villette JM, Stower MJ and Maitland NJ:
Enhanced expression of vimentin in motile prostate cell lines and
in poorly differentiated and metastatic prostate carcinoma.
Prostate. 52:253–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singh S, Sadacharan S, Su S, Belldegrun A,
Persad S and Singh G: Overexpression of vimentin: Role in the
invasive phenotype in an androgen-independent model of prostate
cancer. Cancer Res. 63:2306–2311. 2003.PubMed/NCBI
|
|
46
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015.PubMed/NCBI
|
|
47
|
Colomiere M, Findlay J, Ackland L and
Ahmed N: Epidermal growth factor-induced ovarian carcinoma cell
migration is associated with JAK2/STAT3 signals and changes in the
abundance and localization of alpha6beta1 integrin. Int J Biochem
Cell Biol. 41:1034–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vora HH, Patel NA, Rajvik KN, Mehta SV,
Brahmbhatt BV, Shah MJ, Shukla SN and Shah PM: Cytokeratin and
vimentin expression in breast cancer. Int J Biol Markers. 24:38–46.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shirahata A, Sakata M, Sakuraba K, Goto T,
Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y and
Hibi K: Vimentin methylation as a marker for advanced colorectal
carcinoma. Anticancer Res. 29:279–281. 2009.PubMed/NCBI
|
|
50
|
Usami Y, Satake S, Nakayama F, Matsumoto
M, Ohnuma K, Komori T, Semba S, Ito A and Yokozaki H:
Snail-associated epithelial-mesenchymal transition promotes
oesophageal squamous cell carcinoma motility and progression. J
Pathol. 215:330–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wei J, Xu G, Wu M, Zhang Y, Li Q, Liu P,
Zhu T, Song A, Zhao L, Han Z, et al: Overexpression of vimentin
contributes to prostate cancer invasion and metastasis via src
regulation. Anticancer Res. 28:327–334. 2008.PubMed/NCBI
|
|
52
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Birchler MT, Milisavlijevic D, Pfaltz M,
Neri D, Odermatt B, Schmid S and Stoeckli SJ: Expression of the
extra domain B of fibronectin, a marker of angiogenesis, in head
and neck tumors. Laryngoscope. 113:1231–1237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang L, Zheng M, Liu JH, Xiong Y, Ding H,
Tang L and Wang HY: Risk factors and prognosis of IB-IIB cervical
carcinoma with common iliac lymph node metastasis. Chin J Cancer.
29:431–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li C, Liu W and Cheng Y: Prognostic
significance of metastatic lymph node ratio in squamous cell
carcinoma of the cervix. Onco Targets Ther. 9:3791–3797.
2016.PubMed/NCBI
|
|
56
|
Liu Y, Zhao LJ, Li MZ, Li MX, Wang JL and
Wei LH: The number of positive pelvic lymph nodes and multiple
groups of pelvic lymph node metastasis influence prognosis in stage
IA-IIB cervical squamous cell carcinoma. Chin Med J (Engl).
128:2084–2089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ajila V, Shetty H, Babu S, Shetty V and
Hegde S: Human papilloma virus associated squamous cell carcinoma
of the head and neck. J Sex Transm Dis. 2015:7910242015.PubMed/NCBI
|
|
58
|
Bulk S, Berkhof J, Bulkmans NW, Zielinski
GD, Rozendaal L, van Kemenade FJ, Snijders PJ and Meijer CJ:
Preferential risk of HPV16 for squamous cell carcinoma and of HPV18
for adenocarcinoma of the cervix compared to women with normal
cytology in The Netherlands. Br J Cancer. 94:171–175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cerasuolo A, Annunziata C, Tortora M,
Starita N, Stellato G, Greggi S, Maglione MG, Ionna F, Losito S,
Botti G, et al: Comparative analysis of HPV16 gene expression
profiles in cervical and in oropharyngeal squamous cell carcinoma.
Oncotarget. 8:34070–34081. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen W, Zhang X, Molijn A, Jenkins D, Shi
JF, Quint W, Schmidt JE, Wang P, Liu YL, Li LK, et al: Human
papillomavirus type-distribution in cervical cancer in China: The
importance of HPV 16 and 18. Cancer Causes Control. 20:1705–1713.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Laurson J, Khan S, Chung R, Cross K and
Raj K: Epigenetic repression of E-cadherin by human papillomavirus
16 E7 protein. Carcinogenesis. 31:918–926. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cheng YM, Chou CY, Hsu YC, Chen MJ and
Wing LY: The role of human papillomavirus type 16 E6/E7
oncoproteins in cervical epithelial-mesenchymal transition and
carcinogenesis. Oncol Lett. 3:667–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hellner K, Mar J, Fang F, Quackenbush J
and Munger K: HPV16 E7 oncogene expression in normal human
epithelial cells causes molecular changes indicative of an
epithelial to mesenchymal transition. Virology. 391:57–63. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Caberg JH, Hubert PM, Begon DY, Herfs MF,
Roncarati PJ, Boniver JJ and Delvenne PO: Silencing of E7 oncogene
restores functional E-cadherin expression in human papillomavirus
16-transformed keratinocytes. Carcinogenesis. 29:1441–1447. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang M, Wang M, Li X, Xie Y, Xia X, Tian
J, Zhang K and Tang A: Wnt signaling in cervical cancer? J Cancer.
9:1277–1286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bello JO, Nieva LO, Paredes AC, Gonzalez
AM, Zavaleta LR and Lizano M: Regulation of the Wnt/β-catenin
signaling pathway by human papillomavirus E6 and E7 oncoproteins.
Viruses. 7:4734–4755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sominsky S, Kuslansky Y, Shapiro B,
Jackman A, Haupt Y, Rosin-Arbesfeld R and Sherman L: HPV16 E6 and
E6AP differentially cooperate to stimulate or augment Wnt
signaling. Virology 468–470. 510–523. 2014. View Article : Google Scholar
|
|
68
|
Ghittoni R, Accardi R, Chiocca S and
Tommasino M: Role of human papillomaviruses in carcinogenesis.
Ecancermedicalscience. 9:5262015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yeo-Teh NSL, Ito Y and Jha S: High-risk
human papillomaviral oncogenes E6 and E7 target key cellular
pathways to achieve oncogenesis. Int J Mol Sci. 19(pii): E17062018.
View Article : Google Scholar : PubMed/NCBI
|