Introduction
Radical prostatectomy (RP) and low-dose-rate
prostate brachytherapy (LDR) are two widely used treatment options
for patients with T1c-T3a prostate cancer (Pca) (1). However, the optimal treatment remains a
subject of debate. Contemporary guidelines recommend that treatment
decisions should be made based on tumor features, baseline prostate
specific antigen (PSA) levels, patient age, comorbidity, life
expectancy, and quality of life (2–4).
A number of studies have investigated the
oncological outcomes of different treatments, in order to identify
the population who would most benefit from a specific treatment and
to determine which treatment is superior in terms of improving the
length or quality of the patient's life (5–7).
However, the comparison of the oncological outcomes of PR and LDR
treatments remains a challenge, due to differential definitions for
recurrence and methodological biases arising from the differences
in baseline characteristics, including age, comorbidity and cancer
risk features, such as PSA, biopsy Gleason score (8) and clinical stage (5,9,10). Therefore, results from the
aforementioned previous studies are inconclusive, yielding only
weak evidence regarding which treatment is superior. A randomized
controlled trial is the ideal approach for comparing competing
treatment modalities (11,12). However, treatment options for Pca are
diverse, and therapeutic decisions are largely based on patient
preference and physician discretion (5). Compared with candidates for RP,
patients who are offered LDR generally tend to be older and have
higher comorbidity scores and more aggressive cancer-associated
risk features, such as initial PSA, clinical stage and percentage
of positive biopsies within our clinic. Therefore, a random trial
is impractical (9,13). Attempts at randomized, prospective
trials to compare PR and LDR treatments have failed, since patients
ultimately prefer to make their own treatment decisions (14).
Therefore, to the best of our knowledge, evidence of
informed clinical decisions regarding adequate treatment for
patients with T1c-T3a PCa is lacking and the comparative
effectiveness of RP and LDR for Pca in Chinese patients has yet to
be reported. Therefore, the aim of the present study was to compare
biochemical relapse-free survival time (bRFS) in patients with
T1c-T3a Pca treated with either RP or LDR at Peking Union Medical
College Hospital, Beijing, China. Variables that may predict
differences in biochemical control, including treatment modality as
a variable, were identified using the most recent consensus
definitions of biochemical failure (bF) (15,16).
Materials and methods
Study population and data
collection
A total of 429 consecutive patients (mean age 69.41
years; range, 46–83 years) with T1c-T3a Pca treated with curative
intent were retrospectively reviewed, and all the patients were
treated at Peking Union Medical College Hospital (Beijing, China)
between January 2010 and June 2015. The Tumor-Node-Metastasis
classification system (3) for the
study population, was performed according to the standards provided
by the National Comprehensive Cancer Network (NCCN) updated in 2016
(3). T1c is defined as: A tumor
identified via needle biopsy found in one or both sides, but not
palpable; T2 is defined as: Tumor is palpable and confined within
the prostate; and T3a is defined as: extraprostatic extension tumor
(unilateral or bilateral). High risk is defined as PSA ≥20.0 ng/ml,
or a Gleason score of 8–10, or tumor stage T2c. Intermediate risk
is defined as: PSA 10–20 ng/ml, or a Gleason score of 3+4=7, or
tumor stage T2b-T2c. Low risk is defined as: PSA <10 ng/ml and a
Gleason score of ≤6 and tumor stage T1-T2a (3). The inclusion criteria were the
following: A clinical T-stage between T1c and T3a, ≥2 years
follow-up post-treatment, and no distant metastasis. Patients who
received adjuvant radiation therapy/chemotherapy and/or patients
with distant metastasis were excluded from the present study. A
total of 211 (49.2%) patients underwent RP and 218 (50.8%) patients
received LDR. The choice of treatment, LDR versus RP, was
determined by the patient and/or the doctor .Written informed
consent was obtained from all individual participants included in
the present study. Patients were informed of the benefits and
consequences of each therapeutic option.
The following variables were evaluated for all
participating patients: Medical history, physical examination,
digital rectal examination, serum PSA prior to treatment, including
initial PSA (iPSA) and pathologic grading. The pathologic grading
conformed to the 2006 update of the Gleason grading system
(8). Gleason grading reported here
is from biopsy tissue. Clinical staging was based on digital rectal
examination and specific examinations, including chest radiography,
bone scintigraphy, computerized tomography (CT)-scan and/or
magnetic resonance imaging of the pelvis.
Treatments
RP was recommended for patients who either desired
surgical treatment or were determined as optimal surgical
candidates, due to favorable clinical characteristics, such as
better cardiopulmonary function and no previous history of
abdominal surgery. Surgery was performed by a pure laparoscopic
prostatectomy, with the extent of pelvic lymph node dissection
being based upon the risk category of the patient. The procedure
was performed according to the technique described by Walsh
(17). The vesico-urethral
anastomosis was made with a running suture with the suture line
Y604 (Ethieon, USA).
Treatment with LDR was planned, so that the prostate
and proximal seminal vesicles received 145 Gy with a 5-mm margin
laterally, anteriorly, and inferiorly (18). No margin was planned superiorly,
including the bladder and posteriorly, including the rectum.
125I seeds were accurately introduced into preplanned
positions by a brachytherapy stepping unit MICK200 (Computerized
Medical Systems, Inc., St. Louis, MO, USA) using a standard 0.5 cm
brachytherapy template placed over the perineum. One week following
implantation, dosimetric analysis was performed by CT scan, and the
D90, which was defined as the minimum dose covering 90% of the
prostate, was obtained for each patient and ranged from 140 Gy to
155 Gy, with an average of 144 Gy.
Follow-up and study endpoints
Patients were monitored by serum PSA and digital
rectal examination monthly during the first 3 months following
treatment and every 3 months thereafter. If PSA level were stable,
routine follow-up was scheduled every 6 months for 2 years
following treatment. In cases with a rise in PSA level or patient
presenting with bone pain, a CT scan of the chest/abdomen/pelvis
along with bone scintigraphy should be performed, as recommended by
the EAU and NCCN guidelines (3,19).
Primary endpoints to determine efficacy were bRFS,
clinical relapse-free survival time (cRFS), and Pca-specific
mortality time (PCSM). bF was defined as a PSA value of ≥0.2 ng/ml
for patients who underwent RP (15)
and an increase of 2 ng/ml or >nadir PSA value (16) for patients receiving LDR. If a
patient received salvage radiotherapy or endocrine therapy, the
patient was considered as having experienced a bF. cRFS was defined
as metastases identified by medical imaging, with or without
localizing symptoms, or as biopsy-proven local recurrence. PCSM was
defined as mortality due to Pca, as noted on the death certificate
alongside the biochemical and clinical information, or the presence
of uncontrolled metastatic disease at the time the patient
succumbed.
Statistical analysis
Factors considered to influence the endpoint were
recorded for baseline analysis. The age and iPSA of the patients
are presented in Table I as the mean
(standard deviation), with number of patients in each group (n)
stated at the top of the table. Student's t-test was used to
evaluate differences in the mean of continuous variables. A
χ2 test was performed to compare ratios and Mann-Whitney
U test to compare medians. Differences between two survival curves
were evaluated by log-rank tests. Cox proportional-hazard models
were constructed to identify factors associated with bRFS. Baseline
data analysis was performed by programs the present study created
in R programming language (v.3.3.1; R Foundation for Statistical
Computing, Vienna, Austria). Survival analysis was performed with
the help of survival package (v.2.38, Therneau T) (20). P<0.05 was considered to indicate a
statistically significant difference.
 | Table I.Pretreatment characteristics for LDR
and PR groups. |
Table I.
Pretreatment characteristics for LDR
and PR groups.
| Parameters | LDR (n=218) | RP (n=211) | All (n=429) | P-value |
|---|
| Clinical
symptoms |
|
|
| 0.253 |
|
Dysuria | 112 | 121 | 233 |
|
| Health
examination | 106 | 90 | 196 |
|
| Age, years |
|
|
|
<0.001a |
| Mean
(standard deviation) | 73.41 (5.21) | 65.28 (6.49) | 69.41 (7.14) |
|
|
Median | 74 | 66 | 71 |
|
|
Range | 51–83 | 46–78 | 46–83 |
|
| Biopsy Gleason
score |
|
|
| 0.104 |
| 6 | 147 | 128 | 275 |
|
| 7
(3+4) | 34 | 46 | 80 |
|
| 7
(4+3) | 24 | 18 | 42 |
|
| 8 | 3 | 10 | 13 |
|
| 9 | 10 | 9 | 19 |
|
| Prostate volume,
ml |
|
|
| 0.092 |
|
≤30 | 120 | 98 | 218 |
|
|
>30 | 98 | 113 | 211 |
|
| Clinical T
stage |
|
|
| 0.113 |
|
T1c | 54 | 43 | 97 |
|
|
T2a | 67 | 56 | 123 |
|
|
T2b | 21 | 38 | 59 |
|
|
T2c | 71 | 67 | 138 |
|
| T3 | 5 | 7 | 12 |
|
| iPSA, ng/ml |
|
|
| 0.067 |
| ≤4 | 9 | 3 | 12 |
|
|
4.1–10 | 61 | 89 | 150 |
|
|
>10 | 148 | 119 | 267 |
|
| Mean
(standard deviation) | 13.25 (6.63) | 12.13 (6.00) | 12.70 (6.34) |
|
| NCCN risk
category |
|
|
| 0.813 |
|
low | 49 | 53 | 102 |
|
|
intermediate | 83 | 78 | 161 |
|
|
high | 86 | 80 | 166 |
|
| Duration ADT,
months |
|
|
| <0.001 |
| 0 | 24 | 159 | 183 |
|
|
1–6 | 87 | 27 | 114 |
|
|
>6 | 107 | 25 | 132 |
|
| Follow-up time,
months |
|
|
|
<0.001a |
|
Median | 50.1 | 42.9 | 46.6 |
|
|
Range | 29–86.9 | 1–90 | 1–90 |
|
| Biochemical
recurrence |
|
|
| 0.217 |
| No | 177 | 160 | 337 |
|
|
Yes | 41 | 51 | 92 |
|
| Clinical
failure |
|
|
| 0.939 |
| No | 208 | 200 | 408 |
|
|
Yes | 10 | 11 | 21 |
|
| Patient status |
|
|
| >0.999 |
|
Alive | 214 | 208 | 422 |
|
|
Prostate cancer associated
-mortality | 4 | 1 | 5 |
|
| Other
cause-associated mortality | 0 | 2 | 2 |
|
Results
A total of 429 patients were included in the present
study, with 218 (50.8%) patients receiving LDR and 211 (49.2%)
patients that had undergone RP. The median follow-up time for PR
and LDR groups was 46.6 months. The median age of the patients was
71 years overall, with 74 and 66 years for LDR and RP,
respectively. At the last follow-up visit, 98.4% of the patients
had remained disease-free. All patients in the RP group had
received a pure laparoscopic prostatectomy. For patients receiving
LDR, the activity of 125I seeds ranged between 0.35 and
0.50 mci, and the total activity ranged between 15 to 44.5 mci,
with an average of 25.1 mci. The mean D90 for the LDR group was 144
Gy (1 standard deviation = 20.58 Gy). Neoadjuvant or adjuvant
androgen deprivation therapy (ADT) was administered to 89% of the
patients in the LDR group and to 24.6% of the patients in the RP
group. Table I presents a full
comparison of pretreatment characteristics between the LDR and PR
groups. Patients treated with LDR were older, experienced a longer
follow-up time and had a higher preponderance of combined ADT
treatment. The survival rates were expressed as point estimates
with 95% confidence intervals (CI). The bRFS at 1, 2 and 5 years
was 89.4 (95% CI, 85.5–93.6), 87.2 (95% CI, 82.8–91.7) and 79.9
(95% CI, 74.4–85.7) for LDR, and 91.0 (95% CI, 87.2–94.9), 82.8
(95% CI, 77.3–87.7) and 72.2 (95% CI, 65.0–80.3) for RP,
respectively. The log-rank test indicated that bRFS for patients
who had received a RP were lower compared with patients who had
received LDR treatment. However, this difference was not
statistically significant (P=0.077; Fig.
1A). cRFS at 1, 2 and 5 years was 99.1 (95% CI, 97.8–100), 97.7
(95% CI, 95.7–99.7) and 94.9 (95% CI, 91.9–98.1) for LDR, and 99.0
(95% CI, 97.7–100), 96.2 (95% CI, 93.6–98.8) and 94.5 (95% CI,
91.4–97.7) for RP, respectively. The log-rank test was not
significant for cRFS between RP and LDR groups (P=0.630; Fig. 1B).
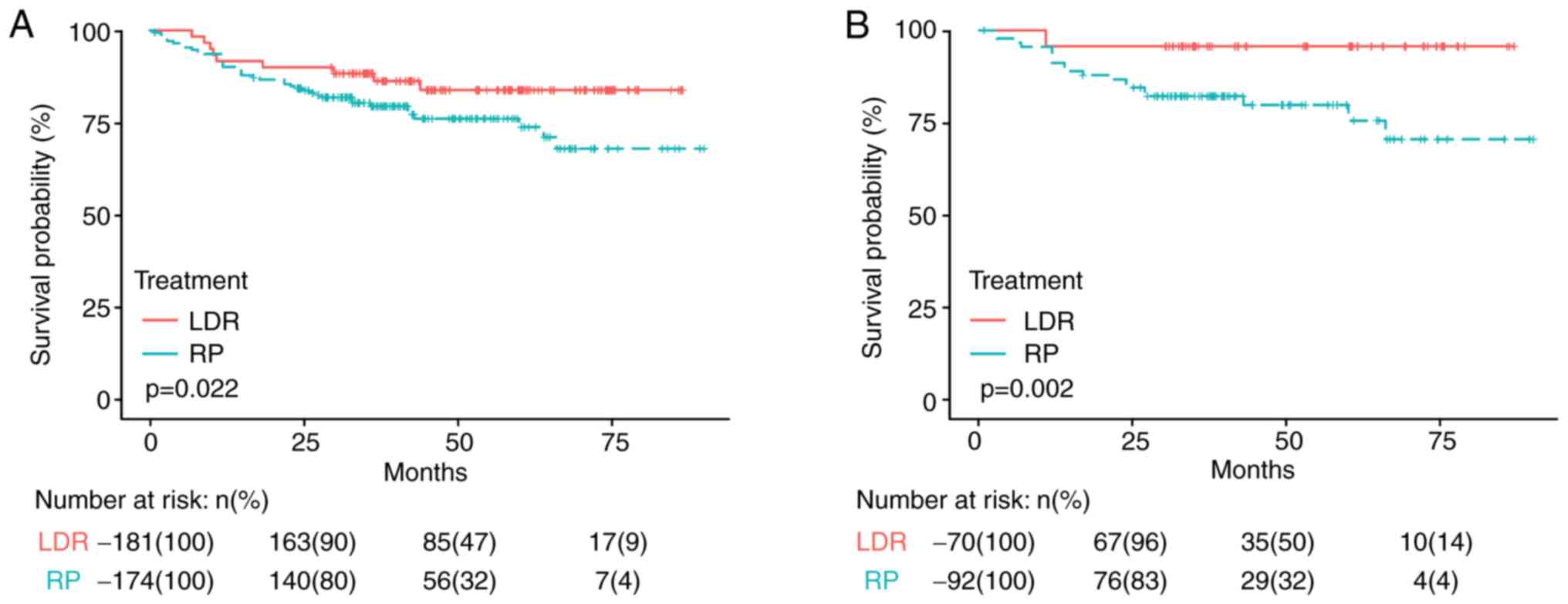
bRFS curves between LDR and RP
Log-rank test was used to compare the bRFS curves
between LDR and RP in terms of different variables according to
pretreatment characteristics. Risk of bF was significantly higher
with RP compared with LDR in the patients with a biopsy Gleason
score ≤3+4 (P=0.022; Fig. 2A) or
iPSA ≤10 ng/ml (P=0.002; Fig. 2B).
However, the survival time of patients who received LDR was not
significantly different from patients who received RP for any of
the following: Age, biopsy Gleason score ≥4+3, prostate volume,
iPSA >10 ng/ml, clinical T stage and NCCN risk category
(3). Comparisons of bRFS curves
between LDR and RP groups in terms of different variables are
presented in Table II.
 | Table II.Comparison of the bRFS curves between
LDR and RP groups, according to different variables using a
log-rank test. |
Table II.
Comparison of the bRFS curves between
LDR and RP groups, according to different variables using a
log-rank test.
| Variables | P-value |
|---|
| Age, years |
|
| >65 | 0.133 |
|
≤65 | 0.511 |
| Biopsy Gleason
score |
|
|
≤3+4 | 0.022a |
|
≥4+3 | 0.642 |
| Prostate volume,
ml |
|
|
>30 | 0.251 |
|
≤30 | 0.143 |
| iPSA, ng/ml |
|
|
>10 | 0.481 |
|
≤10 | 0.002a |
| Clinical T
Stage |
|
|
T1c,T2a | 0.341 |
|
T2b | 0.712 |
|
T2c,T3 | 0.132 |
| NCCN risk
category |
|
|
High-risk | 0.221 |
| Low-
and intermediate-risk | 0.079 |
Cox proportional-hazard models were constructed to
identify factors associated with bRFS, and results are presented in
Table III. With univariate
analysis of the entire cohort, clinical stage ≥T2b (P<0.001),
iPSA >10 ng/ml (P=0.004), biopsy Gleason score >3+4 (P=0.002)
and high risk according to the NCCN risk category (P<0.001) were
associated with significantly worse bRFS. On multivariate analysis
of the entire cohort, only clinical stage ≥T2b (P<0.001) was
associated with significantly worse bRFS. Treatment modality was
not predictable by multivariate analysis [hazard ratio (HR), 1.30;
95% CI, 0.80–2.12; P=0.295). However, LDR was favored over RP by
univariate analysis, but was not statistically significant (HR,
1.44; 95% CI, 0.96–2.18; P=0.080).
 | Table III.Univariate and multivariable analyses
for bRFS. |
Table III.
Univariate and multivariable analyses
for bRFS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Treatment |
| RP vs.
LDR | 0.080 | 1.44
(0.96–2.18) | 0.295 | 1.30
(0.80–2.12) |
| Age, years |
| ≤65 vs.
≥65 | 0.068 | 1.50
(0.97–2.30) | 0.497 | 1.19
(0.72–1.98) |
| Clinical T
Stage |
| ≥T2b
vs. ≤T2a |
<0.001a | 2.94
(1.88–4.61) |
<0.001a | 2.31
(1.42–3.76) |
| iPSA, ng/ml |
| >10
vs. ≤10 | 0.004a | 2.03
(1.26–3.28) | 0.077 | 1.57
(0.95–2.60) |
| Biopsy Gleason
score |
| >3+4
vs. ≤3+4 | 0.002a | 2.06
(1.30–3.23) | 0.126 | 1.45
(0.90–2.35) |
| Prostate volume,
ml |
| ≤30 vs.
>30 | 0.619 | 1.11
(0.74–1.67) | 0.763 | 1.07
(0.70–1.62) |
| NCCN risk
category |
|
High-risk vs.
intermediate/low-risk |
<0.001a | 3.63
(2.36–5.59) | – | – |
Discussion
RP, radiotherapy/brachytherapy, cryoablation and
high-intensity focused ultrasound are the common treatment methods
for T1c-T3a Pca (3). The American
Brachytherapy Society consensus guidelines suggest that
brachytherapy is a safe and efficacious procedure, acknowledged as
a standard therapy for men with localized Pca (21). The present study statistically
analyzed the data of 429 patients with T1c-T3a Pca treated at
Peking Union Medical College Hospital. The results indicated that
the bRFS rates at 5 years were 79.9 and 72.2% for LDR and RP,
respectively. The log-rank test indicated the bRFS for RP was lower
compared with LDR; however, these differences were not
statistically significant. The univariate and multivariate analysis
of the entire cohort, indicated no significant difference in bRFS
between RP and LDR. This result was consistent with recent
publications in the literature, which indicated that the bRFS rates
at 5, 10 and 15 years after surgery, external radiotherapy and
brachytherapy were similar in patients with low-risk Pca (22,23);
however, not all current publications are randomized prospective
studies, including the present study, thereby limiting the
available comparisons. In terms of tumor-associated outcomes,
Ciezki et al (24) reported
high-risk Pca treated with external beam radiation therapy (EBRT),
LDR or RP yields efficacy equivalent to cRFS, as well as a PCSM
advantage of LDR and RP over EBRT. In the present study, there was
also no statistically significant difference in the 5-year cRFS
between the two therapeutic groups. Only three patients in the RP
group died, one due to Pca and the other two due to unknown causes.
A total of four patients succumbed to Pca in the LDR group.
Therefore, it was difficult to compare cancer-specific mortality
and other causes of mortality in these two groups, which would
require additional analysis with a larger group of patients over a
longer follow-up period.
Neither treatment modality has been proven superior
to the other with respect to RP and LDR; therefore, the optimal
treatment for different risk categories in Pca remains a matter of
debate (25–28). Although the study's median follow-up
time of 46.6 months was sufficient to capture a considerable number
of systemic failure events, it may have remained too brief to
achieve mortality results. Therefore, bRFS was selected as the main
evaluation criterion of curative effect. Colberg et al
(28), reported that LDR produced an
equivalent 5-year bRFS compared with RP in patients with early Pca.
Taussky et al (29), also
reported that RP and LDR treatment did not result in significantly
different outcomes at 4 years post-treatment, in patients with low-
and low-intermediate-risk Pca. However, Ferreira et al
(30) reported that the 5-year bRFS
of patients with early Pca, who had undergone brachytherapy was
significantly higher compared with those who had undergone surgery.
Furthermore, Ciezki et al (24) reported that high-risk Pca treated
with EBRT, LDR or RP yields efficacy with an improved bRFS for LDR
and EBRT compared with RP (24).
Although there are a number of published studies
evaluating a large number of low-risk Pca cases who underwent LDR,
they are notably heterogeneous, since the LDR technique employed
across various centers is different, and the methodology used when
comparing the results between RP and LDR also differs (23,31). One
such example is whether, prior to comparing results, postoperative
patients receiving salvage therapy should be excluded. It seems
that the inclusion of surgical patients who received radiation
and/or postoperative hormone therapy can skew the results in favor
of surgery, as observations from the present study. When comparing
results across different treatment modalities in the absence of
randomization, one must acknowledge the number of factors that can
influence reported outcomes. These factors include, but are not
limited to: Patient selection, definition of failure, treatment
specifics, including type of surgery and dose of radiotherapy, and
philosophy of treatment, including stepwise utilization of
modalities (surgery versus upfront combination in radiotherapeutic
approaches) (1).
In the present study, the log-rank test was employed
to compare the bRFS curves between LDR and RP under different
conditions/variables, according to pretreatment characteristics,
including patient age, biopsy Gleason score, prostate volume, iPSA,
clinical T stage and NCCN risk category. It was observed that bRFS
was significantly higher with LDR compared with RP in patients with
a biopsy Gleason score ≤3+4 or iPSA ≤10 ng/ml. In patients with
low- and intermediate-risk Pca, bRFS for patients receiving LDR was
higher compared with patients, who had undergone RP; however, the
result was not statistically significant, consistent with a recent
study (29). Ferreira et al
(30), reported 129 patients, who
had undergone either brachytherapy (64 patients) or surgery (65
patients), and when stratified according to treatment, the survival
time of patients who had undergone brachytherapy (79.70%) was
higher compared with those who had undergone surgery (44.30%). Risk
of bF was higher for surgery compared with brachytherapy (30). Taking into consideration the results
of the present study, brachytherapy may be a better option compared
with RP in patients with a biopsy Gleason score ≤3+4 or iPSA ≤10
ng/ml. In terms of the pretreatment characteristics, patients
treated with LDR were older, experienced longer follow-up time, and
had a higher preponderance of combined ADT treatment. Differences
in the definition of bF in each modality may have caused some bias
when interpreting the results. Therefore, a prospective study
comparing eligible patients is required, in order to draw a more
accurate conclusion.
There are a number of factors affecting the
prognosis of Pca, including general situation, tumor stage, tumor
grade, iPSA, age and bone scintigraphy (32–34).
Ciezki et al (24) reported
that clinical stage T3, biopsy Gleason score 8–10, higher
pretreatment PSA, shorter ADT duration and more frequent PSA
testing following therapy were all associated with a significantly
worse bRFS. Taussky et al (29), reported that younger age, higher
percentage of positive biopsies and PSA at diagnosis were
predictive of bF. In the present study, seven variables that may
affect prognosis of Pca, including patient age, clinical stage,
biopsy Gleason score, iPSA, prostate volume, NCCN risk category and
treatment modality, were analyzed. In the univariate analysis of
the entire cohort, a lower bRFS was identified in patients with
clinical stage ≥T2b, iPSA >10 ng/ml, a biopsy Gleason score
>3+4 and a high risk according to the NCCN risk category. The
treatment modality, age and prostate volume had no significant
effect on bRFS. Previous studies have reported that clinical stage
was the most dangerous factor influencing Pca prognosis (35,36). In
the multivariate analysis of the entire cohort, only clinical stage
≥T2b was associated with significantly worse bRFS, therefore, it
was an important independent prognostic factor. The bRFS of
patients with early Pca was significantly higher compared with
patients with advanced stage Pca, suggesting that early detection
and early diagnosis are key to improving the outcomes for Pca.
To evaluate the advantages of different treatment
modalities for Pca despite survival rates, it is also necessary to
evaluate other factors, including safety, complications and
treatment costs. The present study did not directly address
complication rates; however, previous reports could be referred to
(24,37,38).
Although there have been notably a limited number of direct
comparisons of RP with brachytherapy, multiple quality-of-life
studies would suggest that even in the absence of adjuvant
radiotherapy or ADT, the side effects of surgery are considerable
(24,39,40).
Buron et al (40), reported
that impotence and urinary incontinence were more pronounced
following RP, whereas urinary frequency, urgency and urination pain
were more frequent following LDR. Mean societal costs did not
differ between LDR and RP regardless of the period. The same
conclusion has been reported in other previous studies (41,42).
Giberti et al (43), reported
18% incontinence in patients who underwent surgery in a study of
174 patients completing a 5-year assessment. In addition,
incontinence was sufficiently severe in these patients that 5% of
them required corrective surgery compared with none of the patients
receiving brachytherapy (43).
Strictures were more common following surgery, observed in 6.5% of
the cases compared with 2% following brachytherapy. Symptoms of
irritation remained common at 1 year in the brachytherapy group
(20%) compared with 5% in patients who underwent surgery, and by 5
years, there was no difference in potency of 65 and 68%, in the
brachytherapy and surgery group, respectively. The latest study
reported that the 10-year cumulative incidence of grade 3
genitourinary toxicity was 7.2 for LDR and 16.4% for RP, while the
10-year cumulative incidence of grade 3 gastrointestinal toxicity
was 1.1 and 1.0% for LDR and RP, respectively (24).
The present study presents with a number of
limitations. The baseline characteristics of the two groups did not
completely match, which was inevitable due to random grouping in a
retrospective study. In addition, the aim of the present study was
to provide a guide to aid clinical decision-making at diagnosis.
Therefore, duration of ADT following initial treatment, which may
contribute to survival, was not adjusted. However, in previous
studies using models adjusted for risk, ADT was not reported to be
an independent predictor (13,44). The
definition of PSA recurrence was different for the RP versus the
LDR group. All patients with T1c-T3a Pca were included in the
present study, comprising high-, intermediate- and low-risk Pca,
resulting in a very heterogeneous sampling of patients.
Furthermore, the follow-up period was relatively short compared
with previous similar studies (1,10,22,24,29,45).
In the present study, a limited number of patients died; therefore,
whether higher bRFS rates observed in patients could translate into
superior oncological endpoints requires further investigation. A
longer observational period is required for a meaningful comparison
of overall survival time. The present study also did not
investigate differences in adverse events and quality of life,
which are crucial clinical endpoints; however, this was not the
focus of the present study.
Therefore, taking into consideration the results in
this study and the aforementioned literature, it can be concluded
that LDR, with or without androgen deprivation, is the optimal
treatment option for patients with T1c-T3a Pca, producing
equivalent bRFS and cRFS rates compared with RP. Clinical T stage
≥T2b was an independent predictor for worse bRFS. A longer
follow-up may be necessary to detect a difference in biochemical
outcome between these two treatments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ, WY and HL conceived the study and its design.
ZZ, YZ and FZ acquired the data. ZZ and ZJ analyzed and interpreted
the data and drafted the manuscript. WY, FZ, ZJ and HL performed
critical revision of the manuscript. WY supervised the study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards of the institutional and/or national research committee
and with the 1975 Declaration of Helsinki and its later amendments
or comparable ethical standards. The present study was approved by
the Institutional Review Board of Peking Union Medical College
Hospital (Beijing, China; protocol no. S-K710). Written informed
consent was obtained from all individual participants included in
the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADT
|
androgen deprivation therapy
|
|
bF
|
biochemical failure
|
|
bRFS
|
biochemical relapse-free survival
time
|
|
cRFS
|
clinical relapse-free survival
time
|
|
EBRT
|
external beam radiation therapy
|
|
iPSA
|
initial prostate-specific antigen
|
|
LDR
|
low-dose-rate brachytherapy
|
|
Pca
|
prostate cancer
|
|
PCSM
|
prostate cancer-specific mortality
time
|
|
RP
|
radical prostatectomy
|
References
|
1
|
Crook J: Long-term oncologic outcomes of
radical prostatectomy compared with brachytherapy-based approaches
for intermediate- and high-risk prostate cancer. Brachytherapy.
14:142–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1:
Screening, Diagnosis, and Local Treatment with Curative Intent. Eur
Urol. 71:618–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohler JL, Armstrong AJ, Bahnson RR,
D'Amico AV, Davis BJ, Eastham JA, Enke CA, Farrington TA, Higano
CS, Horwitz EM, et al: Prostate cancer, Version 1.2106. J Natl
Compr Canc Netw. 14:19–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gómez-Veiga F, Rodríguez-Antolín A, Miñana
B, Hernández C, Suárez JF, Fernández-Gómez JM, Unda M, Burgos J,
Alcaraz A, Rodríguez P, et al GESCAP, : Diagnosis and treatment for
clinically localized prostate cancer. Adherence to the European
Association of Urology clinical guidelines in a nationwide
population-based study - GESCAP group. Actas Urol Esp. 41:359–367.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wallis CJD, Glaser A, Hu JC, Huland H,
Lawrentschuk N, Moon D, Murphy DG, Nguyen PL, Resnick MJ and Nam
RK: Survival and Complications Following Surgery and Radiation for
Localized Prostate Cancer: An International Collaborative Review.
Eur Urol. 73:11–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nepple KG, Stephenson AJ, Kallogjeri D,
Michalski J, Grubb RL III, Strope SA, Haslag-Minoff J, Piccirillo
JF, Ciezki JP, Klein EA, et al: Mortality after prostate cancer
treatment with radical prostatectomy, external-beam radiation
therapy, or brachytherapy in men without comorbidity. Eur Urol.
64:372–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wallis CJD, Saskin R, Choo R, Herschorn S,
Kodama RT, Satkunasivam R, Shah PS, Danjoux C and Nam RK: Surgery
Versus Radiotherapy for Clinically-localized Prostate Cancer: A
Systematic Review and Meta-analysis. Eur Urol. 70:21–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: Update on the Gleason grading system for prostate
cancer: Results of an international consensus conference of
urologic pathologists. Adv Anat Pathol. 13:57–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kibel AS, Ciezki JP, Klein EA, Reddy CA,
Lubahn JD, Haslag-Minoff J, Deasy JO, Michalski JM, Kallogjeri D,
Piccirillo JF, et al: Survival among men with clinically localized
prostate cancer treated with radical prostatectomy or radiation
therapy in the prostate specific antigen era. J Urol.
187:1259–1265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sooriakumaran P1, Nyberg T, Akre O,
Haendler L, Heus I, Olsson M, Carlsson S, Roobol MJ, Steineck G and
Wiklund P: Comparative effectiveness of radical prostatectomy and
radiotherapy in prostate cancer: observational study of mortality
outcomes. BMJ. 26:348:g15022014.
|
|
11
|
Concato J, Shah N and Horwitz RI:
Randomized, controlled trials, observational studies, and the
hierarchy of research designs. N Engl J Med. 342:1887–1892. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Donovan JL, Lane JA, Peters TJ, Brindle L,
Salter E, Gillatt D, Powell P, Bollina P, Neal DE and Hamdy FC;
ProtecT Study Group, : Development of a complex intervention
improved randomization and informed consent in a randomized
controlled trial. J Clin Epidemiol. 62:29–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cooperberg MR, Vickers AJ, Broering JM and
Carroll PR: Comparative risk-adjusted mortality outcomes after
primary surgery, radiotherapy, or androgen-deprivation therapy for
localized prostate cancer. Cancer. 116:5226–5234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wallace K, Fleshner N, Jewett M, Basiuk J
and Crook J: Impact of a multi-disciplinary patient education
session on accrual to a difficult clinical trial: The Toronto
experience with the surgical prostatectomy versus interstitial
radiation intervention trial. J Clin Oncol. 24:4158–4162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cookson MS, Aus G, Burnett AL,
Canby-Hagino ED, D'Amico AV, Dmochowski RR, Eton DT, Forman JD,
Goldenberg SL, Hernandez J, et al: Variation in the definition of
biochemical recurrence in patients treated for localized prostate
cancer: The American Urological Association Prostate Guidelines for
Localized Prostate Cancer Update Panel report and recommendations
for a standard in the reporting of surgical outcomes. J Urol.
177:540–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roach M III, Hanks G, Thames H Jr,
Schellhammer P, Shipley WU, Sokol GH and Sandler H: Defining
biochemical failure following radiotherapy with or without hormonal
therapy in men with clinically localized prostate cancer:
Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int
J Radiat Oncol Biol Phys. 65:965–974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walsh PC: Anatomic radical prostatectomy:
Evolution of the surgical technique. J Urol. 160:2418–2424. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Merrick GS, Butler WM, Dorsey AT, Lief JH
and Benson ML: Seed fixity in the prostate/periprostatic region
following brachytherapy. Int J Radiat Oncol Biol Phys. 46:215–220.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al European Association of Urology, : EAU guidelines
on prostate cancer. part 1: Screening, diagnosis, and local
treatment with curative intent-update 2013. Eur Urol. 65:124–137.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Therneau TM and Grambsch PM: Modeling
Survival Data: Extending the Cox Model. Springer; New York, NY:
2000, View Article : Google Scholar
|
|
21
|
Hannoun-Lévi JM: Brachytherapy for
prostate cancer: Present and future. Cancer Radiother. 21:469–472.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prada PJ, González H, Fernández J, Jiménez
I, Iglesias A and Romo I: Biochemical outcome after high-dose-rate
intensity modulated brachytherapy with external beam radiotherapy:
12 years of experience. BJU Int. 109:1787–1793. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kupelian PA, Potters L, Khuntia D, Ciezki
JP, Reddy CA, Reuther AM, Carlson TP and Klein EA: Radical
prostatectomy, external beam radiotherapy <72 Gy, external beam
radiotherapy > or =72 Gy, permanent seed implantation, or
combined seeds/external beam radiotherapy for stage T1-T2 prostate
cancer. Int J Radiat Oncol Biol Phys. 58:25–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ciezki JP, Weller M, Reddy CA, Kittel J,
Singh H, Tendulkar R, Stephans KL, Ulchaker J, Angermeier K,
Stephenson A, et al: A comparison between low-dose-rate
brachytherapy with or without androgen deprivation, external beam
radiation therapy with or without androgen deprivation, and radical
prostatectomy with or without adjuvant or salvage radiation therapy
for high-risk prostate cancer. Int J Radiat Oncol Biol Phys.
97:962–975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciezki JP: High-risk prostate cancer in
the modern era: Does a single standard of care exist? Int J Radiat
Oncol Biol Phys. 87:440–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Amico AV, Whittington R, Malkowicz SB,
Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA,
Kaplan I, Beard CJ, et al: Biochemical outcome after radical
prostatectomy, external beam radiation therapy, or interstitial
radiation therapy for clinically localized prostate cancer. JAMA.
280:969–974. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein EA, Ciezki J, Kupelian PA and
Mahadevan A: Outcomes for intermediate risk prostate cancer: Are
there advantages for surgery, external radiation, or brachytherapy?
Urol Oncol. 27:67–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colberg JW, Decker RH, Khan AM, McKeon A,
Wilson LD and Peschel RE: Surgery versus implant for early prostate
cancer: Results from a single institution, 1992–2005. Cancer J.
13:229–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taussky D, Ouellet V, Delouya G and Saad
F: A comparative study of radical prostatectomy and permanent seed
brachytherapy for low- and intermediate-risk prostate cancer. Can
Urol Assoc J. 10:246–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferreira AS, Guerra MR, Lopes HE, Lima UT,
Vasconcelos YA and Teixeira MT: Brachytherapy and radical
prostatectomy in patients with early prostate cancer. Rev Assoc Med
Bras. 61:431–439. 2015.doi.org/10.1590/1806-9282.61.05.431.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holmberg L, Bill-Axelson A, Helgesen F,
Salo JO, Folmerz P, Häggman M, Andersson SO, Spångberg A, Busch C,
Nordling S, et al Scandinavian Prostatic Cancer Group Study Number
4, : A randomized trial comparing radical prostatectomy with
watchful waiting in early prostate cancer. N Engl J Med.
347:781–789. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matzkin H, Perito PE and Soloway MS:
Prognostic factors in metastatic prostate cancer. Cancer. 72
(Suppl):3788–3792. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindberg C, Davidsson T, Gudjónsson S,
Hilmarsson R, Liedberg F and Bratt O: Extended pelvic
lymphadenectomy for prostate cancer: Will the previously reported
benefits be reproduced in hospitals with lower surgical volumes?
Scand J Urol Nephrol. 43:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakashima J, Kikuchi E, Miyajima A,
Nakagawa K, Oya M, Ohigashi T and Murai M: Simple stratification of
survival using bone scan and serum C-reactive protein in prostate
cancer patients with metastases. Urol Int. 80:129–133. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joly F and Henry-Amar M: Prognostic
factors of localised, locally advanced or metastatic prostate
cancer. Bull Cancer. 94 (Suppl):35–43. 2007.
|
|
36
|
Kakehi Y: Watchful waiting as a treatment
option for localized prostate cancer in the PSA era. Jpn J Clin
Oncol. 33:1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leong N, Pai HH, Morris WJ, Keyes M,
Pickles T, Tyldesley S and Wu J: British Columbia Cancer Agency:
Rectal ulcers and rectoprostatic fistulas after (125)I low dose
rate brachytherapy. J Urol. 195:1811–1816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jarosek SL, Virnig BA, Chu H and Elliott
SP: Propensity-weighted long-term risk of urinary adverse events
after prostate cancer surgery, radiation, or both. Eur Urol.
67:273–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pardo Y, Guedea F, Aguiló F, Fernández P,
Macías V, Mariño A, Hervás A, Herruzo I, Ortiz MJ, Ponce de León J,
et al: Quality-of-life impact of primary treatments for localized
prostate cancer in patients without hormonal treatment. J Clin
Oncol. 28:4687–4696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Buron C, Le Vu B, Cosset JM, Pommier P,
Peiffert D, Delannes M, Flam T, Guerif S, Salem N, Chauveinc L, et
al: Brachytherapy versus prostatectomy in localized prostate
cancer: Results of a French multicenter prospective medico-economic
study. Int J Radiat Oncol Biol Phys. 67:812–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lardas M, Liew M, van den Bergh RC, De
Santis M, Bellmunt J, Van den Broeck T, Cornford P, Cumberbatch MG,
Fossati N, Gross T, et al: Quality of Life Outcomes after Primary
Treatment for Clinically Localised Prostate Cancer: A Systematic
Review. Eur Urol. 72:869–885. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen RC, Basak R, Meyer AM, Kuo TM,
Carpenter WR, Agans RP, Broughman JR, Reeve BB, Nielsen ME, Usinger
DS, et al: Association between choice of radical prostatectomy,
external beam radiotherapy, brachytherapy, or active surveillance
and patient-reported quality of life among men with localized
prostate cancer. JAMA. 317:1141–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Giberti C, Chiono L, Gallo F, Schenone M
and Gastaldi E: Radical retropubic prostatectomy versus
brachytherapy for low-risk prostatic cancer: A prospective study.
World J Urol. 27:607–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cooperberg MR, Grossfeld GD, Lubeck DP and
Carroll PR: National practice patterns and time trends in androgen
ablation for localized prostate cancer. J Natl Cancer Inst.
95:981–989. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koo KC, Cho JS, Bang WJ, Lee SH, Cho SY,
Kim SI, Kim SJ, Rha KH, Hong SJ and Chung BH: Cancer-specific
mortality among Korean men with localized or locally advanced
prostate cancer treated with radical prostatectomy versus
radiotherapy: A multi-center study using propensity scoring and
competing risk regression analyses. Cancer Res Treat. 50:129–137.
2018. View Article : Google Scholar : PubMed/NCBI
|