Introduction
Glioma is the most common and lethal type of
intracranial tumor, accounting for ~46% of intracranial tumors
(1). The World Health Organization
(WHO) classifies glioma into four grades according to the density
and polymorphism of the cancer cells, from grade I to IV as the
malignancy increases (2). Patients
with gliomas of the low and high grades have significantly
different outcomes (3). Glioblastoma
multiforme (GBM), defined as grade IV glioma, is the most lethal
form among all of the grades. Despite receiving standard treatment
including surgery, radiation and chemotherapy, patients with GBM
have a median survival time of 14.4 months and a five-year survival
rate of 10% (4,5). The survival time of patients varies
greatly due to the complexity of the human genome, which harbors
diverse oncogenic drivers (6,7).
Therefore, identification of specific tumor-related molecular
markers based on the pathogenesis and development of glioma may aid
individual treatment and prognosis evaluation.
Nuclear factor κβ (NF-κβ) proteins are a family of
transcription factors that play central roles in a wide range of
biological processes, including cell survival and inflammatory and
immune responses (8). The five
mammalian family members, consisting of RELA proto-oncogene NF-κβ
subunit (RelA), RELB proto-oncogene NF-κβ subunit (RelB), REL
proto-oncogene NF-κβ subunit, NF-κβ subunit 1 and 2, share a
conserved Rel homology domain that mediates dimerization and DNA
binding (9). Previous studies have
mainly focused on the canonical NF-κβ signaling pathway mediated by
RelA-containing dimers and have demonstrated its important role in
regulating cancer invasion and progression (10–12). The
noncanonical NF-κβ signaling pathway, which has been more recently
described, is mediated by RelB-containing dimers and regulates
important biological processes, including B-cell survival and
maturation, dendritic cell activation and lymphoid organogenesis
(13). Although NF-κβ pathways have
been extensively investigated, the specific roles of individual
NF-κβ proteins in tumorigenesis are not well understood. A previous
study suggested the correlation between RELB and breast cancer
(14). However, the expression
characteristics of RELB and its effect on the prognosis of patients
with glioma studied using a high-throughput sequencing method of
large clinical samples have not been reported, particularly in
Chinese patients.
In the present study, the expression levels of RELB
in glioma samples in the Chinese Glioma Genome Atlas (CGGA;
www.cgga.org.cn) database, as well as its
prognostic value, were investigated. Furthermore, the biological
functions of RELB and related microRNAs (miRNAs/miRs) were
analyzed. The current study provided a novel insight into the
development of glioma and identified RELB as a prognostic biomarker
and potential therapeutic target.
Materials and methods
Patients and samples
The CGGA database was used as the discovery set in
the present study. The establishment and management of the dataset
were described in a previous study (15). The CGGA RNA sequence dataset
consisted of 325 samples, including 109 grade II samples, 72 grade
III samples and 144 grade IV samples. Of the 144 GBM samples, 6
samples were lost to follow-up; therefore, 138 samples were
included in the survival analysis. The patients with GBM were
followed up every 3 months. A further three databases were used as
validation sets, which included The Cancer Genome Atlas RNA
sequencing database (TCGA; http://cancergenome.nih.gov), the GSE16011 mRNA
microarray database (16)
(http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE16011)
and the mRNA microarray data of the Repository for Molecular Brain
Neoplasia Data (REMBRANDT; http://caintegrator-info.nci.nih.gov/rembrandt). The
four databases were normalized.
Statistical analysis
Differences in variables between groups were
evaluated using the student's t-test or the one-way analysis of
variance (ANOVA) followed by the Holm-Sidak test. Kaplan-Meier
survival curves were generated in order to estimate survival
distributions, and the log-rank test was used to assess statistical
significance between the groups. Univariate and further
multivariate Cox regression analyses were performed to assess the
prognostic value of RELB in patients. The HR and 95% CI were
calculated. A nomogram was formulated based on the results of the
multivariate Cox regression analysis. The ‘total points’ in the
nomogram, which is the sum of the individual point value of each
clinical factor, may be used to predict patient survival time.
Receiver operating characteristic curves were constructed to
determine the predictive effects of RELB expression for diagnosis.
Gene ontology (GO) analysis of the RELB expression level-related
genes was performed using the online Database for Annotation,
Visualization and Integrated Discovery (DAVID; http://david.ncifcrf.gov). Correlations between miRNAs
and RELB were analyzed by Pearson's correlation in our CGGA miRNA
microarray database. All statistical analyses were conducted using
GraphPad Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA), SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA) or several
packages of R statistical software (version 3.2.1, http://cran.r-project.org/src/base/R-3/R-3.2.1.tar.gz),
such as ‘pheatmap’ (17), ‘circlize’
(18) and ‘rms’ (19). P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of RELB expression in
patients with glioma
In total, 325 samples containing RNA sequencing data
were collected from the CGGA. The clinical characteristics of the
patients in the CGGA dataset are summarized in Table I. The clinical characteristics of the
patients in the other three datasets are summarized in Tables II–IV. Gene expression characteristics of RELB
in CGGA database were comprehensively analyzed. The results
demonstrated that the levels of RELB expression increased with the
pathological grade of gliomas. The highest expression level was
identified in grade IV gliomas, and the lowest expression level was
exhibited by grade II gliomas (Fig.
1A). Grade I gliomas were not included in the present study as
patients with grade I gliomas have the lowest degree of malignancy
and a good prognosis. Based on the TCGA subtype classification
system (20), the mesenchymal
subtype exhibited the highest expression level of RELB, while the
neural subtype had the lowest (Fig.
1B). As the somatic mutations of isocitrate dehydrogenase 1
gene (IDH1) occurred in a majority of malignant gliomas and may be
used as a prognosis indicator (7),
the correlation between RELB expression levels and IDH1 mutations
was investigated. RELB expression levels in patients harboring
wild-type IDH1 were increased compared with patients with mutant
IDH1 (Fig. 1C). The aforementioned
expression characteristics of RELB were validated in TCGA (Fig. 1D-F), GSE16011 (Fig. 1G-I) and REMBRANDT (Fig. 1J and K) datasets. These results
indicated that RELB expression was associated with the glioma grade
and that patients with the mesenchymal subtype and wild-type IDH1
have a higher expression level of RELB compared with other subtypes
and mutant IDH1.
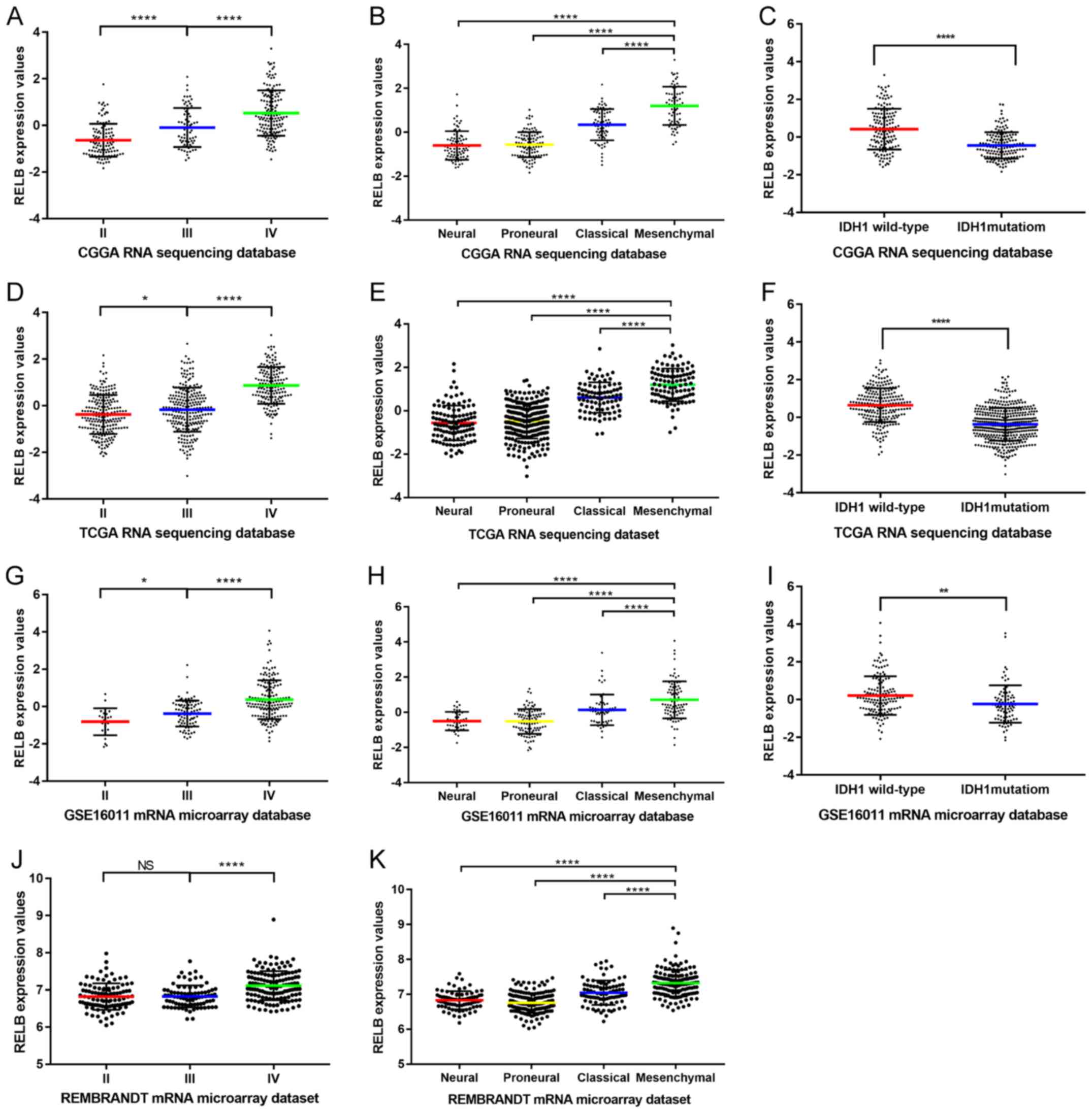 | Figure 1.RELB expression patterns in the CGGA
database and other validation datasets. (A) The expression level of
RELB in tissues from the CGGA database was positively correlated
with tumor grade. (B) RELB expression was highest in mesenchymal
subtype glioma samples from the CGGA database. (C) Patients with
wild-type IDH1 had higher levels of RELB expression compared with
those with mutant IDH1 in the CGGA database. (D) The expression
level of RELB in tissues from TCGA database was positively
correlated with tumor grade. (E) RELB expression was highest in
mesenchymal subtype glioma samples from TCGA database. (F) Patients
with wild-type IDH1 had higher levels of RELB expression compared
with those with mutant IDH1 in TCGA database. (G) The expression
level of RELB in tissues from the GSE16011 database was positively
correlated with tumor grade. (H) RELB expression was highest in
mesenchymal subtype glioma samples from the GSE16011 database. (I)
Patients with wild-type IDH1 had higher levels of RELB expression
compared with those with mutant IDH1 in the GSE16011 database. (J)
The expression level of RELB from the REMBRANDT database was
positively correlated with tumor grade. (K) RELB expression was
highest in mesenchymal subtype glioma samples from the REMBRANDT
database. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001,
as indicated. RELB, RELB proto-oncogene, NF-κβ subunit; CGGA,
Chinese Glioma Genome Atlas; TCGA, The Cancer Genome Atlas; IDH1,
isocitrate dehydrogenase 1; REMBRANDT, Repository for Molecular
Brain Neoplasia Data. |
 | Table I.Clinical and molecular
characteristics of patients in CGGA database. |
Table I.
Clinical and molecular
characteristics of patients in CGGA database.
| Variable | No. of cases (n,
%) |
|---|
| Age |
|
| Age
≥60 | 289 (89) |
| Age
<60 | 36 (11) |
| Sex |
|
|
Male | 203 (62) |
|
Female | 122 (38) |
| WHO grade |
|
| II | 109 (34) |
|
III | 72 (22) |
| IV | 144 (22) |
| TCGA subtype |
|
|
Neural | 81 (25) |
|
Proneural | 102 (31) |
|
Classical | 74 (23) |
|
Mesenchymal | 68 (21) |
| IDH1 status |
|
|
Mutation | 167 (51) |
|
Wild-type | 158 (49) |
| MGMT promoter
status |
|
|
Methylated | 117 (36) |
|
Unmethylated | 139 (43) |
| NA | 69 (21) |
| Radiotherapy |
|
|
Yes | 212 (65) |
| No | 84 (26) |
| NA | 29 (9) |
| Chemotherapy |
|
|
Yes | 158 (49) |
| No | 128 (39) |
| NA | 39 (12) |
 | Table II.Clinical and molecular
characteristics of patients in the TCGA database. |
Table II.
Clinical and molecular
characteristics of patients in the TCGA database.
| Variable | No. of cases |
|---|
| WHO grade |
|
| II | 223 |
|
III | 245 |
| IV | 168 |
| TCGA subtype |
|
|
Neural | 42 |
|
Proneural | 448 |
|
Classical | 168 |
|
Mesenchymal | 41 |
| IDH1 status |
|
|
Mutation | 443 |
|
Wild-type | 246 |
 | Table IV.Clinical and molecular
characteristics of patients in the Repository for Molecular Brain
Neoplasia Data dataset. |
Table IV.
Clinical and molecular
characteristics of patients in the Repository for Molecular Brain
Neoplasia Data dataset.
| Variable | Case |
|---|
| WHO grade |
|
| II | 99 |
|
III | 84 |
| IV | 225 |
| TCGA subtype |
|
|
Neural | 47 |
|
Proneural | 140 |
|
Classical | 86 |
|
Mesenchymal | 135 |
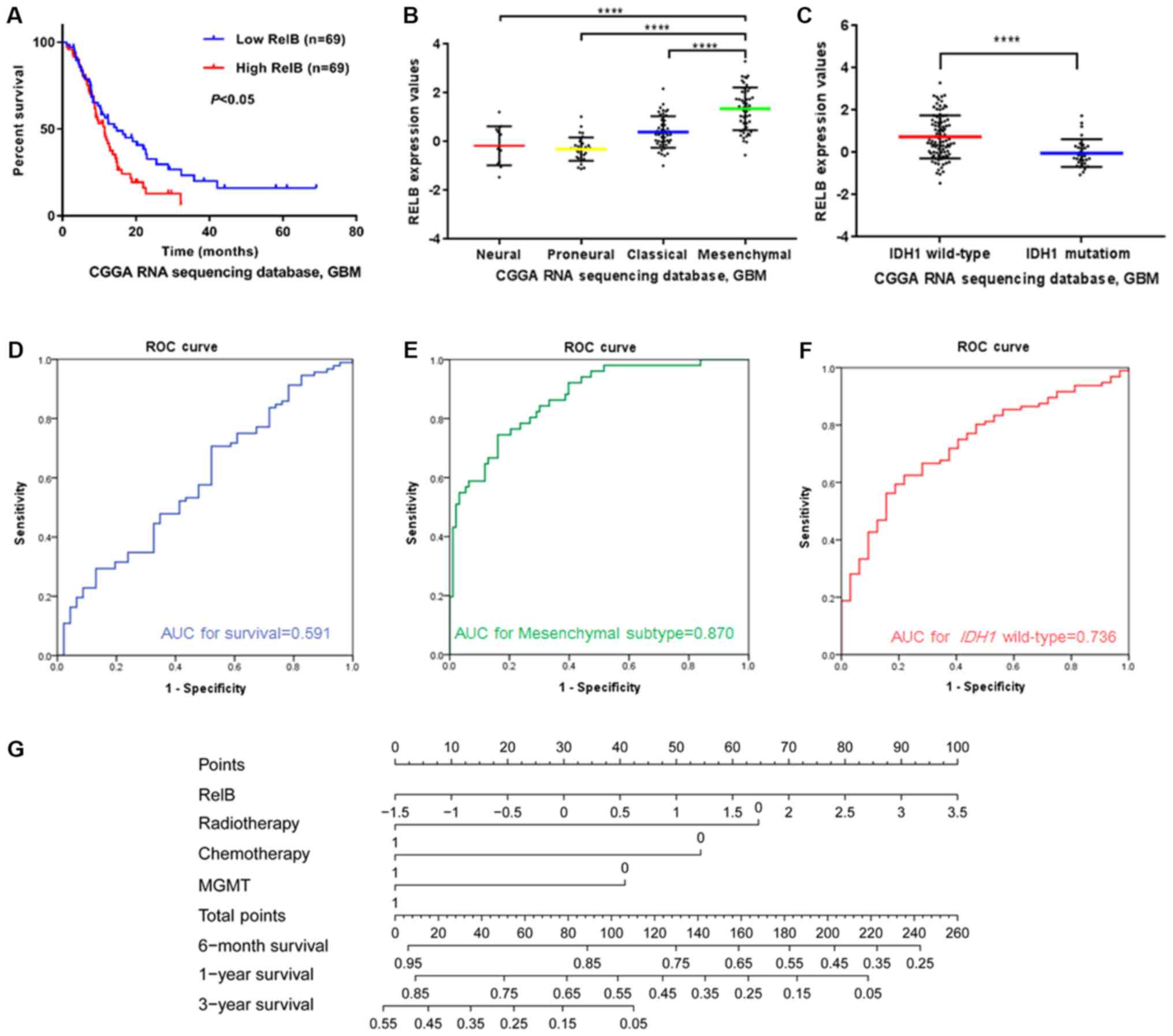
RELB is a predictive marker in
patients with GBM
The expression characteristics of RELB in patients
with GBM from the CGGA dataset were further analyzed. Of the 144
GBM samples, 6 samples were lost at follow-up; therefore, 138
samples were included in the survival analysis. Kaplan-Meier
survival analysis was used to evaluate the relationship between
RELB expression and the prognosis of patients. Half of the patients
with relatively high RELB expression had a significantly shorter
survival time than those with low RELB expression (P<0.05;
Fig. 2A). Additionally, the
characteristics of RELB expression in patients with GBM were the
same as those concluded from the four independent databases. The
expression levels of RELB were higher in the mesenchymal subtype
(Fig. 2B) and IDH1 wild-type gliomas
(Fig. 2C) compared with the
corresponding control groups (P<0.01). The area under the curve
for RELB expression levels as a predictor of one-year survival,
mesenchymal subtype and IDH1 wild-type in the CGGA dataset was
0.591 (Fig. 2D), 0.870 (Fig. 2E) and 0.736 (Fig. 2F), respectively.
In order to further identify the prognostic value of
RELB in patients with GBM, the Cox proportional hazards model was
used (Table V).
O6-methylguanine-DNA methyltransferase (MGMT) promoter
methylation status is a prognostic and predictive factor for
patients with GBM (4), and was
therefore analyzed in the current study. The results of the
univariate analysis demonstrated that age, RELB expression level,
radiotherapy, chemotherapy and MGMT promoter methylation status
affected the overall survival (OS) of patients (P<0.05), while
sex was not a significant factor (P>0.05). Subsequently,
multivariate Cox proportional hazards analysis of the
aforementioned significant influencing factors was conducted. The
expression of RELB was demonstrated to be an independent effective
factor in the survival time of patients with GBM (P<0.05) and
could be used independently to predict the prognosis of patients
with GBM. In order to facilitate the utilization of RELB
expression, different nomograms of survival time were plotted that
incorporated the RELB expression level and the aforementioned
clinical information (Fig. 2G). The
results showed that RELB expression contributed the most risk
points (range, 0–100), whereas the other clinical information had
smaller contributions.
 | Table V.Cox regression analysis of clinical
parameters in patients with glioblastoma multiforme. |
Table V.
Cox regression analysis of clinical
parameters in patients with glioblastoma multiforme.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 1.227 | 0.795–1.893 | 0.355 | – | – | – |
| Age (≥60 vs.
<60) | 1.722 | 1.041–2.850 | 0.034a | 0.930 | 0.494–1.750 | 0.822 |
| RELB expression
(high vs. low) | 1.265 | 1.043–1.533 | 0.017a | 1.332 | 1.005–1.766 | 0.046a |
| Radiotherapy (yes
vs. no) | 0.412 | 0.259–0.653 |
<0.001a | 0.404 | 0.243–0.674 |
<0.001a |
| Chemotherapy (yes
vs. no) | 0.336 | 0.214–0.528 |
<0.001a | 0.466 | 0.282–0.768 | 0.003a |
| MGMT promoter
status (methylated vs. unmethylated) | 0.564 | 0.364–0.872 | 0.010a | 0.572 | 0.352–0.931 | 0.024a |
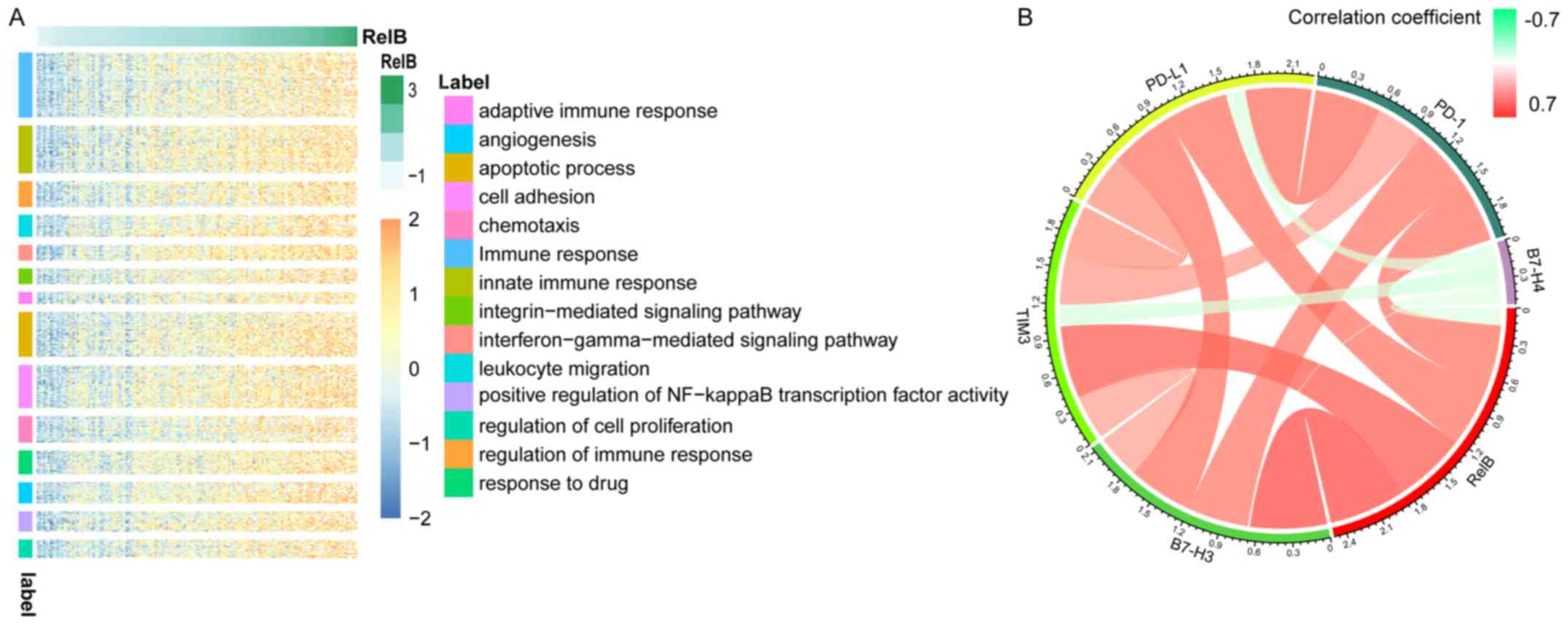
RELB-related biological processes in
patients with GBM
In order to investigate the biological processes
associated with RELB expression in patients with GBM, Pearson's
correlation analysis was performed between RELB expression and
other genes in the dataset. In total, 766 significant positively
correlated genes (r>0.5) were identified and used for subsequent
GO analysis using the DAVID website. The genes that positively
correlated with RELB expression were involved in the ‘immune
response’, ‘cell activation’, ‘apoptotic process’ and ‘cell
adhesion’ (Fig. 3A). The association
between RELB and immune checkpoint genes in glioma was analyzed and
that PD-1, B7-H3, TIM3 and PD-L1 were positively correlated with
RELB expression (Fig. 3B) (21).
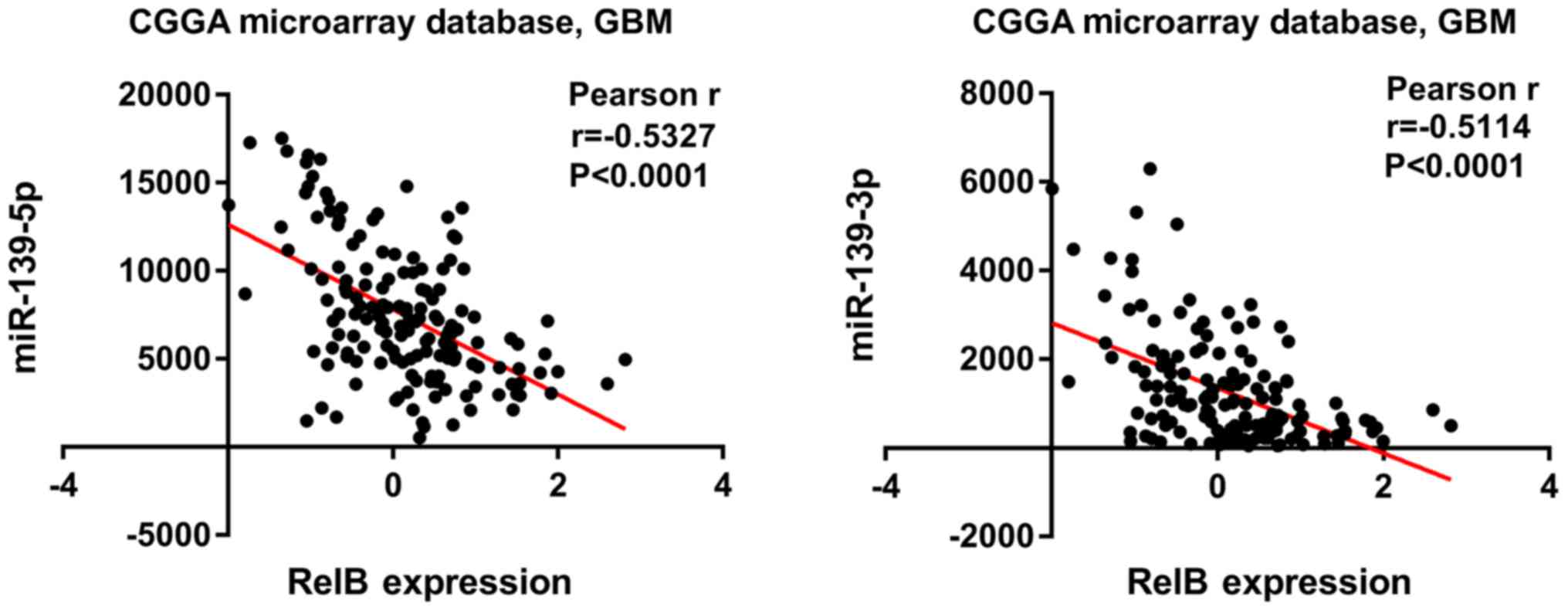
Correlation between RELB expression
and miRNA levels in patients with GBM
In order to investigate the correlation between RELB
and miRNA, 829 candidate miRNAs were analyzed in patients with GBM
from the CGGA dataset. The results showed that an increased RELB
expression was associated with the decreased expression of
miR-139-5p and miR-139-3p (P<0.0001), which were the most
negatively correlated with RELB expression (Fig. 4).
Discussion
GBM is the most common and lethal type of brain
tumor, in which cancer cells penetrate the adjacent normal tissues
with no definite range. Current therapeutic approaches, including
surgery, radiotherapy and chemotherapy, do not achieve satisfactory
results (22). Genome differences in
patients make a difference in prognosis. Therefore, it is necessary
to identify effective and differential molecular markers in order
to assist with the accurate prognosis of patients. An in-depth
study of cancer development revealed that NF-κβ transcription
factors participate in a wide variety of biological processes,
including inflammation, apoptosis and proliferation (23). Therefore, clarifying the individual
role of key NF-κβ subunits in cancer may aid the development of
novel therapeutic agents.
The present study focused on RELB, a member of the
alternative NF-κβ signaling pathway, which was initially identified
as a regulator of the adaptive immune response (24). A previous study revealed that
abnormal activity of RELB serves a role in the development of solid
tumors and hematopoietic malignancies (25). In breast cancer, high RELB expression
was demonstrated to confer more highly invasive phenotypes
(26), and inhibition of RELB
decreased proliferation (27). In
prostate cancer, high RELB expression was observed to enhance cell
growth and exert a radioprotective role in cancer cells (28,29).
According to these studies, RELB exerts a tumor-supportive role in
a several types of cancer. In glioma cells, RELB promoted cell
survival and invasion (30,31). However, these studies were conducted
in cell lines or animal models and not based on human clinical
specimens. In the present study, the pathological and biological
role of RELB in glioma was investigated in a large number of
Chinese patients.
In the present study, RELB expression was identified
to be upregulated in higher stage gliomas, mesenchymal subtypes and
IDH1 wild-type gliomas in four independent databases, indicating
the association of RELB expression levels with oncological
biological processes. Based on RNA-sequencing analysis of patients
with GBM, RELB may serve as an indicator of mesenchymal subtype and
IDH1 wild-type gliomas. Moreover, high expression of RELB predicted
a significantly shorter survival time for patients with glioma. The
independent prognostic value of the RELB expression level was
observed via multivariate analysis (P<0.05).
RELB is involved in regulating the biological
activities of cancer cells. Cormier et al (32) reported that RELB activation was
important for promoting the survival of multiple myeloma cells
through the upregulation of anti-apoptotic proteins. Ge et
al (33) revealed that RELB was
associated with the levels of certain key regulators in
endometrioid adenocarcinoma, and that high RELB expression levels
may lead to endometrial cell tumorigenicity. In order to elucidate
the role of RELB in the progression of glioma, biological
functional annotation of RELB-related genes was performed in the
present study. The GO analysis showed that RELB was mainly related
to ‘apoptotic processes’ and ‘cell adhesion’ in patients with
glioma. High expression of RELB may increase the adhesion, invasion
and proliferation of cancer cells, inhibit apoptosis of cells and
lead to the progression of glioma. In addition, the results also
demonstrated an association between RELB and the immune response in
patients. The findings of the present study are consistent with the
results of previous studies (32,33). The
NF-κβ family regulates a number of processes, ranging from the
development and survival of lymphocytes and lymphoid organs to the
control of immune responses and malignant transformation (34,35).
RELB is involved in dendritic cell maturation and immune tolerance
to inflammation (36,37). The GO term analysis results of the
present study identified that RELB regulates the immune process in
patients with glioma, including the immune response, the
interferon-gamma-mediated signaling pathway and leukocyte
migration. The expression levels of PD-1, PD-L1, TIM3 and B7-H3
were positively correlated with the expression level of RELB in the
present study, suggesting that RELB may be associated with immune
checkpoints (38).
miRNAs are small non-coding RNA molecules that
regulate a large variety of biological processes in
sequence-specific manners (39).
miRNAs destabilize the target mRNAs or suppress their translation
in order to regulate gene expression (40). Aberrantly expressed miRNAs were
demonstrated to serve as oncogenes or tumor suppressors in cancer
(41). The present study identified
a negative correlation between the expression level of RELB and
that of miR-139-3p and miR-139-5p. miR-139-3p and miR-139-5p are
derived from pre-miR-139 and serve roles in the development of
cancer. Sun et al (42)
reported that ectopic expression of miR-139-5p significantly
suppressed cell growth and metastasis through inhibition of cyclin
D1 and matrix metalloproteinases in non-small cell lung cancer.
Additionally, previous studies indicated that miR-139-5p has
antitumor effects in several types of cancer (43,44).
Previous studies revealed that miR-139-3p was involved in the
carcinogenesis and development of various types of cancer (45,46).
Huang et al (47)
investigated the mechanism of miR-139-3p in the progression of
cervical cancer and revealed that miR-139-3p inhibited cell
proliferation and induced cell apoptosis through downregulation of
NIN1 (RPN12) binding protein 1 homolog expression. As miR-139-5p
and miR-139-3p have demonstrated anticancer effects, their
downregulation may contribute to the progression of cancer. This is
consistent with the results of the analysis of patients with glioma
in the present study. Decreased expression of miR-139-5p and
miR-139-3p may result in the upregulation of RELB, which, based on
the GO term analysis results, may subsequently induce the
proliferation, migration and progression of glioma cells and lead
to a poor prognosis in patients. A limitation of the current study
was that the effects of RELB were not demonstrated experimentally.
Future studies are required to investigate the effects of the
expression levels of RELB in vitro and in vivo. The
RELB related pathway which take part in the progression of glioma
was also interesting. It is a further investigation.
In summary, the present study identified the
expression patterns and biological functions of RELB in patients
with glioma. RELB expression levels are increased in patients with
the mesenchymal subtype and wild-type IDH1, resulting in a shorter
OS. RELB may therefore be used as an independent prognostic
indicator in patients with glioma. In addition, the activity of
RELB was found to be associated with the immune response, apoptosis
and cell adhesion of cancer cells. Furthermore, the expression
levels of RELB displayed a negative correlation with miR-139-5p and
miR-139-3p. The results obtained in the current study suggested
that RELB may be a novel and promising prognostic marker or
therapeutic target for patients with glioma.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Nature Science Foundation of China (grant nos. 81802994, 81502495
and 81702460).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH and FZ designed the experiments. FZ, KW and RH
analyzed the data and contributed to the analytical tools. YZ and
YL analyzed the data and reviewed the literature. FZ and KW wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN: Molecular pathology of malignant
gliomas. Annu Rev Pathol. 1:97–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang T, Mao Y, Ma W, Mao Q, You Y, Yang
X, Jiang C, Kang C, Li X, Chen L, et al: CGCG clinical practice
guidelines for the management of adult diffuse gliomas. Cancer
Lett. 375:263–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang W, Zhang J, Hoadley K, Kushwaha D,
Ramakrishnan V, Li S, Kang C, You Y, Jiang C, Song SW, et al:
miR-181d: A predictive glioblastoma biomarker that downregulates
MGMT expression. Neuro Oncol. 14:712–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao ZS, Chen HM, Yang MY, Zhang CB, Yu K,
Ye WL, Hu BQ, Yan W, Zhang W, Akers J, et al: RNA-seq of 272
gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in
secondary glioblastomas. Genome Res. 24:1765–1773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: Remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baud V and Collares D: Post-translational
modifications of RelB NF-κB subunit and associated functions.
Cells. 5(pii): E222016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhat KPL, Balasubramaniyan V, Vaillant B,
Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L,
James JD, Goodman LD, et al: Mesenchymal differentiation mediated
by NF-κB promotes radiation resistance in glioblastoma. Cancer
Cell. 24:331–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raychaudhuri B, Han Y, Lu T and Vogelbaum
MA: Aberrant constitutive activation of nuclear factor kappaB in
glioblastoma multiforme drives invasive phenotype. J Neurooncol.
85:39–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lopez-Guerra M and Colomer D: NF-kappaB as
a therapeutic target in chronic lymphocytic leukemia. Expert Opin
Ther Targets. 14:275–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun SC: Non-canonical NF-κB signaling
pathway. Cell Res. 21:71–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Belguise K, Kersual N, Kirsch KH,
Mineva ND, Galtier F, Chalbos D and Sonenshein GE: Oestrogen
signalling inhibits invasive phenotype by repressing RelB and its
target BCL2. Nat Cell Biol. 9:470–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu H, Wang Z, Liu Y, Zhang C, Li M, Zhang
W, Wang K, Cai J, Cheng W, Huang H and Jiang T: Genome-wide
transcriptional analyses of Chinese patients reveal cell migration
is attenuated in IDH1-mutant glioblastomas. Cancer Lett.
357:566–574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Zhang W, Chen D, Lv Y, Zheng J,
Lilljebjörn H, Ran L, Bao Z, Soneson C, Sjögren HO, et al: A glioma
classification scheme based on coexpression modules of EGFR and
PDGFRA. Proc Natl Acad Sci USA. 111:3538–3543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Zhang C, Liu X, Wang Z, Sun L, Li
G, Liang J, Hu H, Liu Y, Zhang W and Jiang T: Molecular and
clinical characterization of PD-L1 expression at transcriptional
level via 976 samples of brain glioma. Oncoimmunology.
5:e11963102016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu Z, Gu L, Eils R, Schlesner M and Brors
B: Circlize implements and enhances circular visualization in R.
Bioinformatics. 30:2811–2812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang ZL, Zhao LJ, Chai L, Zhou SH, Wang
F, Wei Y, Xu YP and Zhao P: Seven LncRNA-mRNA based risk score
predicts the survival of head and neck squamous cell carcinoma. Sci
Rep. 7:3092017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verhaak RG, Hoadley KA, Purdom E, Wang V,
Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al:
Integrated genomic analysis identifies clinically relevant subtypes
of glioblastoma characterized by abnormalities in PDGFRA, IDH1,
EGFR, and NF1. Cancer Cell. 17:98–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JY and Nam JH: Progestins in the
fertility-sparing treatment and retreatment of patients with
primary and recurrent endometrial cancer. Oncologist. 20:270–278.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burkly L, Hession C, Ogata L, Reilly C,
Marconi LA, Olson D, Tizard R, Cate R and Lo D: Expression of relB
is required for the development of thymic medulla and dendritic
cells. Nature. 373:531–536. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baud V and Jacque E: The alternative NF-kB
activation pathway and cancer: Friend or foe? Med Sci (Paris).
24:1083–1088. 2008.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mineva ND, Wang X, Yang S, Ying H, Xiao
ZX, Holick MF and Sonenshein GE: Inhibition of RelB by
1,25-dihydroxyvitamin D3 promotes sensitivity of breast cancer
cells to radiation. J Cell Physiol. 220:593–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Demicco EG, Kavanagh KT, Romieu-Mourez R,
Wang X, Shin SR, Landesman-Bollag E, Seldin DC and Sonenshein GE:
RelB/p52 NF-kappaB complexes rescue an early delay in mammary gland
development in transgenic mice with targeted superrepressor
IkappaB-alpha expression and promote carcinogenesis of the mammary
gland. Mol Cell Biol. 25:10136–10147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Josson S, Fang F, Oberley TD, St
Clair DK, Wan XS, Sun Y, Bakthavatchalu V, Muthuswamy A and St
Clair WH: RelB enhances prostate cancer growth: Implications for
the role of the nuclear factor-kappaB alternative pathway in
tumorigenicity. Cancer Res. 69:3267–3271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Josson S, Xu Y, Fang F, Dhar SK, St Clair
DK and St Clair WH: RelB regulates manganese superoxide dismutase
gene and resistance to ionizing radiation of prostate cancer cells.
Oncogene. 25:1554–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DW, Ramakrishnan D, Valenta J, Parney
IF, Bayless KJ and Sitcheran R: The NF-κB RelB protein is an
oncogenic driver of mesenchymal glioma. PLoS One. 8:e574892013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cherry EM, Lee DW, Jung JU and Sitcheran
R: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK)
promotes glioma cell invasion through induction of NF-κB-inducing
kinase (NIK) and noncanonical NF-κB signaling. Mol Cancer.
14:92015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cormier F, Monjanel H, Fabre C, Billot K,
Sapharikas E, Chereau F, Bordereaux D, Molina TJ, Avet-Loiseau H
and Baud V: Frequent engagement of RelB activation is critical for
cell survival in multiple myeloma. PLoS One. 8:e591272013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ge QL, Liu SH, Ai ZH, Tao MF, Ma L, Wen
SY, Dai M, Liu F, Liu HS, Jiang RZ, et al: RelB/NF-κB links cell
cycle transition and apoptosis to endometrioid adenocarcinoma
tumorigenesis. Cell Death Dis. 7:e24022016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: Critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vogel CF, Wu D, Goth SR, Baek J, Lollies
A, Domhardt R, Grindel A and Pessah IN: Aryl hydrocarbon receptor
signaling regulates NF-κB RelB activation during dendritic-cell
differentiation. Immunol Cell Biol. 91:568–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu HC, Qiu T, Liu XH, Dong WC, Weng XD,
Hu CH, Kuang YL, Gao RH, Dan C and Tao T: Tolerogenic dendritic
cells generated by RelB silencing using shRNA prevent acute
rejection. Cell Immunol. 274:12–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu L, Bai Y and Wang Z: Elevated T cell
activation score is associated with improved survival of breast
cancer. Breast Cancer Res Treat. 164:689–696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun C, Sang M, Li S, Sun X, Yang C, Xi Y,
Wang L, Zhang F, Bi Y, Fu Y and Li D: Hsa-miR-139-5p inhibits
proliferation and causes apoptosis associated with down-regulation
of c-Met. Oncotarget. 6:39756–39792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wong CC, Wong CM, Tung EK, Au SL, Lee JM,
Poon RT, Man K and Ng IO: The microRNA miR-139 suppresses
metastasis and progression of hepatocellular carcinoma by
down-regulating Rho-kinase 2. Gastroenterology. 140:322–331. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang K, Jin J, Ma T and Zhai H: miR-139-5p
inhibits the tumorigenesis and progression of oral squamous
carcinoma cells by targeting HOXA9. J Cell Mol Med. 21:3730–3740.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Duan B, Dong Y, He C, Zhou H, Sheng
H, Gao H and Zhang X: MicroRNA-139-3p indicates a poor prognosis of
colon cancer. Int J Clin Exp Pathol. 7:8046–8052. 2014.PubMed/NCBI
|
|
46
|
Wang M, Wen TF, He LH, Li C, Zhu WJ and
Trishul NM: A six-microRNA set as prognostic indicators for bile
duct cancer. Int J Clin Exp Med. 8:17261–17270. 2015.PubMed/NCBI
|
|
47
|
Huang P, Xi J and Liu S: miR-139-3p
induces cell apoptosis and inhibits metastasis of cervical cancer
by targeting NOB1. Biomed Pharmacother. 83:850–856. 2016.
View Article : Google Scholar : PubMed/NCBI
|