Introduction
Reporting of pancreatic cystic neoplasms (PCNs) have
increased in the past decade due to the widespread use and rapid
improvement of abdominal cross-sectional imaging technologies
(1), as well as an increased
awareness of their existence. PCNs are a heterogeneous group of
tumors with serous cystic neoplasms (SCNs) being one of the most
common (1). SCNs account for ~1–2%
of pancreatic exocrine tumors (2,3),
amounting to ~20% of all cystic tumors of the pancreas (4). SCNs have four morphologic patterns:
Microcystic (honeycomb), macrocystic (oligocystic), mixed
(polycystic) and solid types (5).
Unlike the first three types, the solid type is composed of small
acini with glandular spaces (6)
which cannot be observed under a microscope. Solid type tumors
present as a mass on radiological images with overlapping features,
such as the presence of enhancement, resemble those of other solid
tumors (3,4). Solid serous cystadenomas (SSCs) of the
pancreas were first described by Perez-Ordonez et al
(7) and are reported to be the
rarest type of SCNs (2,3,8),
accounting for only 3% (5). Correct
preoperative diagnosis is required as the management of the
conditions differs considerably. SSCs are benign and are managed
conservatively (6). Surgery is only
recommended for symptoms related to the compression of adjacent
organs (9) or an uncertain diagnosis
following complete workup (10).
Solid tumors require surgery if they are symptomatic, malignant or
display potential malignancy. The aim of the current study was to
analyze the radiological results of patients with SSCs affirmed by
pathology and to summarize the significant features to assist
future clinical diagnosis and interventions.
Materials and methods
Subjects
A total of 132 patients with pathologically
confirmed SCNs that had undergone surgical treatment at The
Zhejiang University School of Medicine, Second Affiliated Hospital
(Hangzhou, China) between January 2006 and July 2017 were reviewed
and 5 of these patients with SSCs (determined by pathological
analysis) and high-quality radiological images were retrospectively
reviewed. The present study was approved by the Institutional
Review Board of Zhejiang University School of Medicine, Second
Affiliated Hospital. All patients were female, with a mean age of
44.2 years (range, 23–69 years). They had no previous medical
history of pancreatitis or pancreatic neoplasm. Of these, 3
patients were incidentally observed to present with a pancreatic
mass without discomfort, 1 patient had upper left abdominal pain
and the other had abdominal distension and nausea. A total of 4
patients presented with normal pancreatic tumor markers, whereas 1
patient exhibited a slightly elevated carbohydrate antigen 19-9
levels (47.5 IU/ml; normal <37 IU/ml). The time interval between
the first scan and surgery was 3–40 days. All patients underwent
surgical resection and were without any evidence of recurrence
during follow-up. The clinical features of the 5 patients are
summarized in Table I.
 | Table I.Clinical aspects in the 5 cases. |
Table I.
Clinical aspects in the 5 cases.
| Case | Age (years) | Sex | Symptom(s) | CA19-9 level
(IU/ml) | Preoperative
diagnosis | Surgical
procedure | Outcome |
|---|
| 1 | 69 | Female | Incidental
diagnosis | Normal | pNET | PPP with
cholecystectomy | Uneventful |
| 2 | 27 | Female | Incidental
diagnosis | Normal | SPN | LDP | Uneventful |
| 3 | 49 | Female | Left upper
abdominal pain | Normal | Pancreatic
cystadenpoma | LDP with
splenectomy | Uneventful |
| 4 | 53 | Female | Incidental
diagnosis | Normal | Pancreatic
adenocarcinoma | PPP | Uneventful |
| 5 | 23 | Female | Abdominal
distension, nausea | 47.5 | SPN | PPP | Uneventful |
Radiological examination
The preoperative computed tomography and magnetic
resonance (MR) images of all the patients were available. In
addition, 4 patients underwent magnetic resonance
cholangiopancreatography (MRCP). CT scanning was performed by
multidetector-row helical CT scanners (Somatom Definition AS;
Siemens Healthineers, Erlangen, Germany). Unenhanced images of the
upper abdomen were initially obtained using a collimation of 5 mm
with pitch 1.2 mm, tube voltage of 120 kV and a tube current of 200
mA. Depending on the patient's body weight, 80–100 ml of contrast
medium (Omnipaque 300 mg/ml; GE Healthcare, Chicago, IL, USA) was
administered intravenously at an injection rate of 3.5–4.0 ml/sec.
A three-phase contrast study was performed with 1.25 mm collimation
through the pancreas while patients held their breath. Images were
obtained at the arterial, portal venous and equilibrium phases (25,
60 and 100 sec following injection, respectively).
MR scanning was performed using a GE Discovery MR750
3 Tesla MRI scanner (GE Healthcare). Fat-saturated T1-weighted
images, T2-weighted images and additional contrast-enhanced
T1-weighted images were obtained in all patients in the transverse
section without breathing artifact. The contrast medium (Omniscan,
0.1 ml/kg body weight; GE Healthcare) was injected at a rate of 2
ml/sec. Gadolinium-enhanced study on T1-weighted images was first
performed at 20 sec following the initiation of intravenous
contrast administration. Multiphasic images were obtained in the
portal phase at 45–52 sec, in the equilibrium phase at 75–82 sec
and in the delayed phase at 135–142 sec (slice thickness, 4 mm;
interslice gaps, 4 mm). Diffusion-weighted images (DWI) were also
acquired in all patients using the following parameters: Repetition
time/echo time, 6,000/53.0 msec; thickness, 5 mm; matrix size,
128×128; b value=0 and 800 sec/mm2. The apparent
diffusion coefficient (ADC) maps were generated on the operating
console. The MRCP examinations were performed on a 1.5 Tesla MRI
scanner (GE Healthcare). The following imaging sequences were
acquired: Axial T2 fast spin echo with fat saturation [repetition
time (TR) 6,000/echo time (TE) 63–86] with 7 mm slices and coronal
(TR 3,333.3/TE 617–705) reconstructed with 1.8 mm slices.
Image analysis
A total of 2 experienced abdominal radiologists with
no prior knowledge of the pathological outcome separately reviewed
the clinical information and imaging data, and a consensus was
reached. The following morphological features were evaluated: i)
Location of the lesion (head, neck, body or tail of pancreas); ii)
size of the lesion (maximal diameter of the tumor); iii) lesion
contour (round, ovoid, lobulated or irregular); iv) margin of the
lesion (well- or ill-defined); v) appearance of the lesion (the
density on CT, the signal intensity on MRI and the enhancement
pattern), vi) presence of a capsule; vii) central scar formation;
viii) calcification; ix) hemorrhage; x) pancreatic ductal
dilatation; xi) invasion of adjacent tissues; xii) enlarged lymph
nodes; and xiii) distant metastases.
In terms of appearance characteristics, the density
of the lesion was recorded, compared with the surrounding
pancreatic parenchyma and labeled as hyper-, iso- or
hypo-attenuating. The signal intensity of lesions on the unenhanced
T1-weighted images was compared with that of the surrounding
pancreas and graded as hypo-, iso- and hyper-intense. The signal
intensity of lesions on T2-weighted images contrasting with that of
the surrounding pancreas and spleen was described as ‘very hyper-,
hyper-, iso- and hypo-intense’ when the signal intensity of the
lesion was higher than that of the spleen, not higher than that of
the spleen but higher than that of the pancreas, similar to that of
the pancreas and lower than that of the pancreas, respectively
(11). The dynamic enhancement
pattern was evaluated in the homogeneity of the tumor (homogeneous
or heterogeneous). The regions of interest (ROIs) placed on the
lesions with the largest diameter on the ADC maps in order to cover
the entire length of the lesion were defined. The final ADC values
were expressed as the mean value mm2/s of the ROIs of
three selected slices. The presence of scars and fibrous capsules
appeared as increasing, weak and/or prolonged enhancement centrally
and peripherally, respectively. Hemorrhage was identified by the
existent areas of hyperintensity on T1-weighted images and the
corresponding hypointensity on T2-weighted images.
Pathologic analysis
A total of 2 senior pathologists investigated the
gross and microscopic specimens and reached an agreement.
Macroscopically, the 5 resected specimens revealed a solid and
well-circumscribed appearance. Histologically, hematoxylin and
eosin staining revealed that the encapsulated tumors consisted of
cuboidal or polygonal cells and dense collagenous septa. The tumor
cells had small round nuclei and clear or pale eosinophilic
cytoplasm, without cytological atypia or abnormal mitotic figures.
Neoplastic cells were sometimes arranged in small acini.
Microscopically, the surgical margins were negative with no
vascular or nerve invasion.
Results
CT and MR images revealed 3 masses in the tail of
pancreas and 2 in the neck. The mean tumor size among the 5
patients was 2.3 cm (range, 1.5–3.2 cm; 4/5 of lesions ≤3 cm). All
cases had radiological evidence of well-circumscribed masses, which
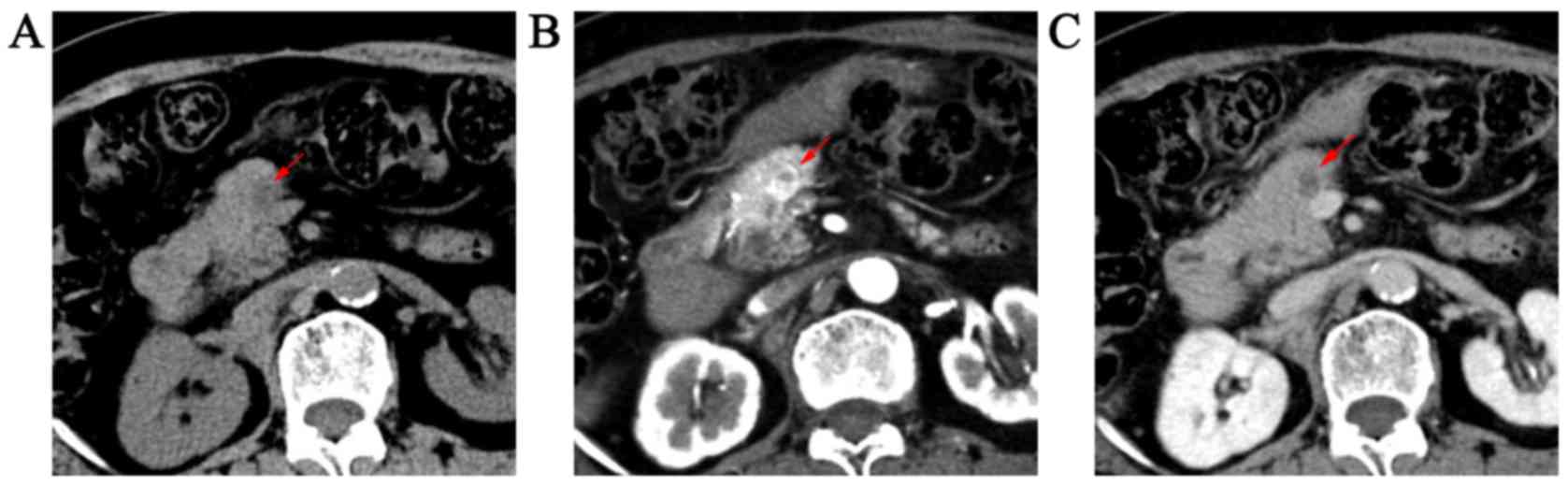
were ovoid (n=3) and lobulated (n=2). A total of 4 cases
demonstrated low-density solid masses (Fig. 1) on unenhanced CT and a high signal
intensity on T2-weighted images and isointensity on DWI (Fig. 2). Only 1 case revealed a slight
hypodense mass with central low density protruding from the
pancreatic tail on unenhanced CT and was heterogeneously
hyperintense on T2-weighted images and slightly hyperintense on
DWI. On unenhanced T1-weighted images, 5 cases revealed homogeneous
and low intensity lesions.
The 5 masses revealed two different enhancement
patterns on enhanced CT and MR imaging. Of these, 2 masses
exhibited strong and heterogeneous enhancement with a poorly
enhanced zone in the arterial phase, washout and had a homogenous
appearance, hypodensity or isodensity during portal venous and
equilibrium phases (Figs. 1B and C
and 3). Moderate and gradual
enhancement in the arterial phase, and prolonged and faintly
spotted enhancement during the portal venous and equilibrium phases
were observed in three masses. The three masses were
hypo-dense/intense relative to the surrounding normal pancreatic
tissue in all enhanced phases. Additionally, increased enhancement
of the periphery of the lesions was confirmed on the 3 phases in 4
cases, while an area of strong enhancement surrounded by a dark
thin rim was exhibited in 1 patient, which revealed the presence of
a capsule. The enhanced manifestations of lesions on MR imaging
were similar to those of the CT images.
The ADC values of the 5 lesions were
2.46×10−3, 2.18×10−3, 2.13×10−3,
2.21×10−3 and 2.53×10−3 mm2/sec,
respectively. MRCP revealed increased intensity in the four lesions
(Fig. 4), almost equivalent to that
of common bile duct and distal ductal dilation (5.5 mm) in 1 case.
No intratumoral calcification, central scar, hemorrhage, enlarged
lymph nodes, adjacent invasion or distant metastasis were observed
in all five patients. The imaging features are summarized in
Table II. All cases were
misdiagnosed.
 | Table II.Summary of the imaging features in
the five cases. |
Table II.
Summary of the imaging features in
the five cases.
|
|
|
| Radiological
findings |
|
|---|
|
|
|
|
|
|
|---|
| Case | Location | Size (cm) | Computed
tomography | Magnetic
resonance | Apparent diffusion
coefficient (mm2/sec) |
|---|
| 1 | Neck | 1.5 | Well-defined and
hypodense, wash-in and wash-out enhancement with poor enhanced
zone, enhanced capsule | Low signal on T1WI,
very high signal on T2WI, isosignal on DWI, wash-in and wash-out
enhancement with weakly enhanced zone, enhanced capsule |
2.46×10−3 |
| 2 | Tail | 3.0 | Well-defined and
slight hypodense, wash-in and wash-out enhancement with poor
enhanced zone and capsule | Low signal on T1WI,
high signal on T2WI, slight high signal on DWI, wash-in and
wash-out enhancement with poor enhanced zone and capsule |
2.18×10−3 |
| 3 | Tail | 2.0 | Well-defined and
hypodense, Lobulated change, moderate and prolonged enhancement,
enhanced capsule | Low signal on T1WI,
very high signal on T2WI, iso-signal on DWI, moderate and prolonged
enhancement, enhanced capsule |
2.13×10−3 |
| 4 | Tail | 1.8 | Well-defined and
hypodense, moderate and prolonged enhancement with obviously
enhanced capsule | Low signal on T1WI,
very high signal on T2WI, isosignal on DWI, moderate and prolonged
enhancement, enhanced capsule |
2.21×10−3 |
| 5 | Neck | 3.2 | Well-defined and
hypodense, lobulated change, moderate and prolonged enhancement,
enhanced capsule | Low signal on T1WI,
very high signal on T2WI, isosignal on DWI, moderate and prolonged
enhancement, enhanced capsule |
2.53×10−3 |
Discussion
Owing to the widespread use and rapid improvement of
cross-sectional imaging studies, there has been an increase in the
detection of PCNs (1). SSCs are
benign entities and may be misdiagnosed as pancreatic
neuroendocrine tumors (pNET), solid pseudopapillary neoplasms of
the pancreas (SPN), ductal adenocarcinomas and metastatic
carcinomas (12). Therefore,
distinguishing between SSCs and malignant types allows the
selection of an appropriate treatment strategy. Previous studies on
SSCs presenting as solid and enhanced masses are limited, with the
majority being case reports. Additionally, the radiological
features of the masses are sporadically described. Jais et
al (10) conducted an
international and multicenter study involving 2,622 SCNs and
reported that SSCs type accounted for 5% of these cases. Thus, the
aim of the current study was to analyze the radiological results of
patients with SSCs and to summarize the significant features to
improve the diagnostic accuracy.
SSCs were initially described in 1996 as solid
pancreatic tumors composed of cells that are morphologically and
histochemically indistinguishable from those of SCNs (7). Subsequent case reports supported SSCs
as an SCN subtype (8,13). Previous studies reported that SSCs
consist of serous cystadenoma cells that do not secrete fluid
(6,14) or they comprise a large number of
small cysts (3,15,16)
measuring tenths of a millimeter (13).
To the best of the authors' knowledge, only 22 SSCs
have been previously reported in detail, and the clinical and
radiologic characteristics of these cases are presented in Table III (2,3,7,8,12–26). The
demographic characteristics of the 22 cases were as follows: i)
Male and female ratio, 1:1.6; ii) mean age, 60.6 years; iii) ~50%
of lesions were located in the body of the pancreas (9/22, 45.5%);
iv) the mean size of the lesions was 2.8 cm; and v) 15/22 of the
lesions (68.2%) were ≤3 cm in diameter. SSCs were located in
different parts of the pancreas and occurred most frequently in
females in their sixth decade. Consistent with previously published
studies, all the SSCs occurred in adult females in the present
study, although the patients involved in the present study were
younger. The mean size of lesions was 2.3 cm, which was smaller
than that reported in the aforementioned studies. Of the five cases
in the present study, four of them had biomarker expression levels
(carcinoembryonic antigen, α-fetoprotein, CA19-9 and CA21-5 within
the expected range; data not shown) within the normal range, and
this was similar to previously published studies (3,8,16,18,19,21,22,23,25).
 | Table III.Summary of the characteristic imaging
findings of the 22 cases reported on public. |
Table III.
Summary of the characteristic imaging
findings of the 22 cases reported on public.
| First author,
year | Age/sex | Symptoms | Location | Size (cm) | Unenhanced CT | MRI
(T1/T2/DWI) | Enhancement
pattern | MRCP (heavy
T2) | Fibrous
capsule | Other | Preoperative
diagnosis | (Refs.) |
|---|
| Perez-Ordonez et
al, 1996 | 70/F | Diffuse abdominal
pain | Tail | 4.0 | – | – | – | – | – | – | pNET | (7) |
| Kosmahl et
al, 2004 | 50/M | None | Head | 2.5 | – | – | – | – | – | – | – | (2) |
| Yamamoto et
al, 2004 | 60/M | Epigastric
distention after meals | Uncus | 2.0 | Low | Low/High/- | Uniformly | Very high | – | – | pNET | (23) |
| Gabata et
al, 2005 | 59/F | Abdominal pain | Body | 2.0 | Low | Low/High/- | Homogeneously,
marked | Very high | – | Central scar | SSC | (16) |
| Matsumoto et
al, 2006 | 39/F | – | Body | 3.4 | Iso | – | Strongly,
uniformly | – | – | – | pNET | (25) |
| Reese et al,
2006 | 66/M | None | Head/neck | 3.5 | – | – | Intensely | – | – | Dilated pancreatic
duct, atrophy of the pancreas | pNET | (13) |
| Yamaguchi et
al, 2006 | 58/F | None | Body | 2.0 | – | – | Well-enhanced | – | – | Dilated distal
pancreatic duct | pNET | (8) |
| Sanaka et
al, 2007 | 74/M | None | Body | 1.5 | – | – | Yes | – | – | – | pNET | (14) |
| Stern et al,
2007 | 62/M | Abdominal pain | Head/body | 4.2 | – | – |
Heterogeneously | – | – | – | – | (12) |
| Lee et al,
2008 | 42/F | – | Head/neck | 0.7 | – | – | Yes | – | – | – | – | (17) |
| Casadei et
al, 2008 | 59/F | Abdominal pain | Tail | 4.0 | – | – | Marked in the early
phase | – | Yes | – | – | (18) |
| Yasuda et
al, 2011 | 72/F | None | Head | 2.0 | Low | -/High/- | Strongly | – | – | – | pNET | (15) |
| Hayashi et
al, 2012 | 74/F | – | Body | 4.2 | Low | – | Strongly,
washout | – | Yes | – | – | (26) |
|
| 57/F | – | Head | 2.1 | Low | – | Strongly,
washout |
| – | Yes | – | – |
|
| 58/F | – | Body | 3.2 | Low | – | Strongly, high in
the delayed phase | – | – | – | – | – |
| Lee et al,
2013 | 56/M | None | Body | 2.5 | – | – | Yes, until the
portal phase | – | – | – | pNET | (19) |
| Kishida et
al, 2014 | 58/M | None | Body | 2.8 | Low | Low/High/- | (CT)Increased in
the marginal zone until the late phase (MR) Marked from the edge
towards the center | Very high | – | – | pNET | (3) |
| Wu et al,
2015 | 48/M | Severe left
abdominal cramps | Head | 2.7 | – | – | The arterial-phase
enhancing | – | – | – | pNET | (20) |
|
|
| 65/F | None | Body | 2.3 | – | – | The arterial-phase
enhancing | – | – | pNET |
|
| Geramizadeh et
al, 2016 | 68/F | left upper quadrant
abdominal pain | Head | 3.0 | – | – | Yes | – | – | – | pNET | (24) |
| Katsourakis et
al, 2016 | 72/F | None | Tail | 3.0 | – | – | Strongly, rapid
washout | – | – | – | pNET | (21) |
| Hamid et al,
2017 | 53/F | Epigastric pain,
nausea and vomiting | Body | 3.0 | Low | – | Weak enhanced in
the portal phase | – | – | – | Solid tumors | (22) |
The SSCs in the present study were characterized as
well-defined solid tumors with the following features: i) Small
tumor size (≤3 cm); ii) significant high intensity on T2-weighted
images; iii) wash-in and wash-out enhancement or moderate and
prolonged enhancement; iv) high ADC value; and v) the presence of a
fibrous capsule. The tumors demonstrated marked hyperintensity on
T2-weighted images, revealing a liquid component and suggesting
they were not solid but rather of cystic nature (16). MR imaging, which is able to detect
the presence of tumors with a cystic nature (16), may be superior to CT scanning in
differentiating SSCs from solid tumors. This was supported by a
previously published case report with an exact preoperative
diagnosis of SSC (16). In the
current study, 2 masses exhibited wash-in and wash-out, which was
similar to the majority of cases in previous studies (21,26),
while 3 cases exhibited moderate, gradual and prolonged enhancement
and spotted enhancement in the latter 2 phases, similar to a SSC
case reported by Lee et al (17). The 80% marginal area was clearly
observed to demonstrate strong enhancement, and this was reported
in previously published studies (16,18,26). The
reasons for the different enhancement patterns remain unknown,
however, the high density of microvessels at the tumor margin and
the presence of a surrounding fibrous capsule may be a contributing
factor (3). It is possible that the
degree of enhancement is positively associated with the amount of
hypervascular stroma. The lesions had sharp margins and manifested
no invasion of adjacent vessels or organs. The ADC values of the 5
lesions were all >2×10−3 mm2/sec and
within the range of 2.06×10−3 to 2.86×10−3
mm2/sec as reported by Jang et al (27). Furthermore, this compares to an
optimal ADC cut-off value of 1.21×10−3
mm2/sec reported by Hayano et al that
distinguished pancreatic cancer from noncancerous tissue (28), and 1.99×10−3
mm2/sec that distinguished SSCs from pNETs (27). The ADC values in the current study
exhibited no evidence of restricted diffusion, in accordance with
their benign nature.
SSCs, particularly the smaller lesions, may have
been difficult to distinguish from pNETs in the previous studies
(3,7,8,13–15,19–21,23–26) and
SPNs in the current study. The majority of SSCs occur in females in
the sixth decade of life, while pNETs may develop at any age, and
small SPNs predominantly occur in females in their thirties and
forties (11). SSCs with strongly or
arterial-phase enhancement have been frequently misdiagnosed as
pNETs (3,8,15,20,21,25).
Kishida et al (3) reported
that MR images of pNETs exhibit a high intensity on the T2-weighted
image, however, this was not as high as that of a cyst. However,
hyperintensity on T2-weighted images may not be present in pNETs
(27), particularly for small-sized
lesions (29). A low radio density
on unenhanced images and a tumor density <32 Hounsfield units on
unenhanced CT images may aid in distinguishing them (27). SSCs frequently exhibit a reduced
number of low density regions when compared with the surrounding
pancreas parenchyma than pNETs (26). Hayashi et al (26) reported that the presence of a fibrous
capsule may aid the differential diagnosis of SSCs from pNETs.
Additionally, SSCs exhibit a lower density relative to the
surrounding pancreas on the delayed phase CT more frequently than
pNETs (26). This may explain the
difference in the washout rate of contrast enhancement. The gradual
and prolonged enhancement was incorrectly diagnosed as SPN in the
current study. Small (≤3 cm) SPNs demonstrate early heterogeneous
and slightly progressive fill-in enhancement and seldom appear more
enhanced than the normal pancreatic parenchyma (30). They are also initially
hypoattenuating and slightly hypoattenuating or isoattenuating
during the portal venous phase (31), which overlap with part of SSCs. Small
SPNs have two important distinguishing features: The lack of the
fibrous capsule (11,30) and hyperintensity on DWI (32). In the present study, the majority of
the SSCs displayed isointensity on DWI. Furthermore, the young age
of the patients may have led to misdiagnosis of two cases in the
current study. The tumor misdiagnosed as pancreatic adenocarcinoma
with distal ductal dilation in the present study (case 4) may be
distinguished using an increased signal intensity on T2-weighted
image and high ADC value. SSCs only detected by unenhanced MR
imaging and which appear as very hyperintense on T2-weighted
images, mimicking the oligocystic type, may be misdiagnosed as
unilocular mucinous cystic neoplasms and branch duct intraductal
papillary mucinous neoplasms (BD-IPMNs). A thick cystic wall and
curvilinear or peripheral calcification present in the cyst wall or
septa occur in mucinous cystic neoplasms (33) and are important features for a
differential diagnosis. BD-IPMNs are characterized by communication
with the main duct which is apparent on MRCP (34).
The current study had two main limitations. Firstly,
it was limited by its retrospective nature. Secondly, it included a
small number of patients with pathologically proven SSCs that
underwent surgical resection. This small number of patients was
attributed to the rarity of the subtype.
In conclusion, SSCs were frequently observed in
adult women with small (≤3 cm) lesions. Marked hyperintensity on
T2-weighted images, a high ADC value, the presence of a fibrous
capsule and enhancement patterns may well be imaging features of
SSCs.
Acknowledgements
The authors would like to thank Dr Yuan-fei Lu and
Dr Qian Zhang (The Second Affiliated Hospital of Zhejiang
University School of Medicine, Zheijang, China) for making a
significant contribution towards the revision of the
manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RSY conceived the concept and designed the study.
JYC and HYC collected cases and performed the study. YP and DS
analysed the images, and prepared figures and tables. JYC wrote the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The retrospective study was approved by The Second
Affiliated Hospital of Zhejiang University School of Medicine
Ethics Committee, and the requirement for obtaining informed
consent from all patients was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SSC
|
solid serous cystadenoma
|
|
CT
|
computed tomography
|
|
MR
|
magnetic resonance
|
|
PCN
|
pancreatic cystic neoplasm
|
|
SCN
|
serous cystic neoplasm
|
|
MRCP
|
magnetic resonance
cholangiopancreatography
|
|
DWI
|
diffusion weighted images
|
|
ADC
|
apparent diffusion coefficient
|
|
pNET
|
pancreatic neuroendocrine tumor
|
|
SPN
|
solid pseudopaillary neoplasm of
pancreas
|
|
BD-IPMN
|
branch duct intraductal papillary
mucinous neoplasm
|
References
|
1
|
Chandwani R and Allen PJ: Cystic neoplasms
of the pancreas. Annu Rev Med. 67:45–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kosmahl M, Wagner J, Peters K, Sipos B and
Klöppel GN: Serous cystic neoplasms of the pancreas: An
immunohistochemical analysis revealing alpha-inhibin,
neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol.
28:339–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kishida Y, Matsubayashi H, Okamura Y,
Uesaka K, Sasaki K, Sawai H, Imai K and Ono H: A case of solid-type
serous cystadenoma mimicking neuroendocrine tumor of the pancreas.
J Dig Dis. 15:211–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun HY, Kim SH, Kim MA, Lee JY, Han JK and
Choi BI: CT imaging spectrum of pancreatic serous tumors: Based on
new pathologic classification. Eur J Radiol. 75:e45–e55. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kimura W, Moriya T, Hirai I, Hanada K, Abe
H, Yanagisawa A, Fukushima N, Ohike N, Shimizu M, Hatori T, et al:
Multicenter study of serous cystic neoplasm of the Japan pancreas
society. Pancreas. 41:380–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Machado MC and Machado MA: Solid serous
adenoma of the pancreas: An uncommon but important entity. Eur J
Surg Oncol. 34:730–733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perez-Ordonez B, Naseem A, Lieberman PH
and Klimstra DS: Solid serous adenoma of the pancreas. The solid
variant of serous cystadenoma? Am J Surg Pathol. 20:1401–1405.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi M: Solid serous adenoma of the
pancreas: A solid variant of serous cystadenoma or a separate
disease entity? J Gastroenterol. 41:177–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
European Study Group on Cystic Tumours of
the Pancreas, . European evidence-based guidelines on pancreatic
cystic neoplasms. Gut. 67:789–804. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jais B, Rebours V, Malleo G, Salvia R,
Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM, et
al: Serous cystic neoplasm of the pancreas: A multinational study
of 2622 patients under the auspices of the International
Association of Pancreatology and European Pancreatic Club (European
Study Group on Cystic Tumors of the Pancreas). Gut. 65:305–312.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu MH, Lee JY, Kim MA, Kim SH, Lee JM, Han
JK and Choi BI: MR imaging features of small solid pseudopapillary
tumors: Retrospective differentiation from other small solid
pancreatic tumors. AJR Am J Roentgenol. 195:1324–1332. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stern JR, Frankel WL, Ellison EC and
Bloomston M: Solid serous microcystic adenoma of the pancreas.
World J Surg Oncol. 5:262007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reese SA, Traverso LW, Jacobs TW and
Longnecker DS: Solid serous adenoma of the pancreas: A rare variant
within the family of pancreatic serous cystic neoplasms. Pancreas.
33:96–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanaka MR, Kowalski TE, Brotz C, Yeo CJ,
McCue P and Palazzo J: Solid serous adenoma of the pancreas: A rare
form of serous cystadenoma. Dig Dis Sci. 52:3154–3156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yasuda A, Sawai H, Ochi N, Matsuo Y, Okada
Y and Takeyama H: Solid variant of serous cystadenoma of the
pancreas. Arch Med Sci. 7:353–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gabata T, Terayama N, Yamashiro M,
Takamatsu S, Yoshida K, Matsui O, Usukura M, Takeshita M and Minato
H: Solid serous cystadenoma of the pancreas: MR imaging with
pathologic correlation. Abdom Imaging. 30:605–609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SE, Kwon Y, Jang JY, Kim YH, Hwang DW,
Kim MA, Kim SH and Kim SW: The morphological classification of a
serous cystic tumor (SCT) of the pancreas and evaluation of the
preoperative diagnostic accuracy of computed tomography. Ann Surg
Oncol. 15:2089–2095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Casadei R, D'Ambra M, Pezzilli R, Ricci C,
Calculli L, Lega S, Antonacci N, Monari F and Minni F: Solid serous
microcystic tumor of the pancreas. JOP. 9:538–540. 2008.PubMed/NCBI
|
|
19
|
Lee SD, Han SS and Hong EK: Solid serous
cystic neoplasm of the pancreas with invasive growth. J
Hepatobiliary Pancreat Sci. 20:454–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu W, Hong X, Li J, Dai M, Wang W, Tong A,
Zhu Z, Dai H and Zhao Y: Solid serous cystadenoma of the pancreas:
A case report of 2 patients revealing vimentin, β-catenin, α-1
antitrypsin, and α-1 antichymotrypsin as new immunohistochemistry
staining markers. Medicine (Baltimore). 94:e6442015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katsourakis A, Dimitriou I, Noussios G,
Chatzis I and Chatzitheoclitos E: Solid serous adenoma of the
pancreas: A case report and review of the literature. Case Rep
Surg. 2016:37302492016.PubMed/NCBI
|
|
22
|
Hamid M, Tbouda M, Majbar AM, Raiss M and
Ahallat M: Pancreatic solid serous cystadenoma treated by
laparoscopy: Presentation of a new case report and review of the
literature. Int J Surg Case Rep. 40:97–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto T, Takahashi N, Yamaguchi T and
Imamura Y: A case of solid variant type of pancreatic serous
cystadenoma mimicking islet cell tumor. Clin Imaging. 28:49–51.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geramizadeh B, Dabbaghmanesh MH,
Nikeghbalian S and Soleimani N: Solid serous adenoma of pancreas,
misdiagnosed as neuroendocrine tumor, a rare case report and review
of the literature. J Gastrointest Cancer. 47:462–465. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsumoto M, Iguchi M, Ohtsuki Y, Kimura
M, Watanabe R, Watanabe N, Okada Y, Kurabayashi A, Takahashi T and
Furihata M: A case of solid serous adenoma of the pancreas
ultrastructurally harbouring ribosome-lamella complexes. Pathology.
38:361–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi K, Fujimitsu R, Ida M, Sakamoto K,
Higashihara H, Hamada Y and Yoshimitsu K: CT differentiation of
solid serous cystadenoma vs endocrine tumor of the pancreas. Eur J
Radiol. 81:e203–e208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang KM, Kim SH, Song KD, Kim YK, Lee SJ
and Choi D: Differentiation of solid-type serous cystic neoplasm
from neuroendocrine tumour in the pancreas: Value of abdominal MRI
with diffusion-weighted imaging in comparison with MDCT. Clin
Radiol. 70:153–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayano K, Miura F, Amano H, Toyota N, Wada
K, Kato K, Sano K, Takeshita K, Aoyagi T, Shuto K, et al:
Correlation of apparent diffusion coefficient measured by
diffusion-weighted MRI and clinicopathologic features in pancreatic
cancer patients. J Hepatobiliary Pancreat Sci. 20:243–248. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bakir B, Salmaslioğlu A, Poyanlı A,
Rozanes I and Acunas B: Diffusion weighted MR imaging of pancreatic
islet cell tumors. Eur J Radiol. 74:214–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao X, Ji Y, Zeng M, Rao S and Yang B:
Solid pseudopapillary tumor of the pancreas: Cross-sectional
imaging and pathologic correlation. Pancreas. 39:486–491. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baek JH, Lee JM, Kim SH, Kim SJ, Kim SH,
Lee JY, Han JK and Choi BI: Small (<or=3 cm) solid
pseudopapillary tumors of the pancreas at multiphasic multidetector
CT. Radiology. 257:97–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jang KM, Kim SH, Kim YK, Park MJ, Lee MH,
Hwang J and Rhim H: Imaging features of small (≤3 cm) pancreatic
solid tumors on gadoxetic-acid-enhanced MR imaging and
diffusion-weighted imaging: An initial experience. Magn Reson
Imaging. 30:916–925. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Javadi S, Menias CO, Korivi BR, Shaaban
AM, Patnana M, Alhalabi K and Elsayes KM: Pancreatic calcifications
and calcified pancreatic masses: Pattern recognition approach on
CT. AJR Am J Roentgenol. 209:77–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahani DV, Kadavigere R, Blake M,
Fernandez-Del Castillo C, Lauwers GY and Hahn PF: Intraductal
papillary mucinous neoplasm of pancreas: Multi-detector row CT with
2D curved reformations-correlation with MRCP. Radiology.
238:560–569. 2006. View Article : Google Scholar : PubMed/NCBI
|