Introduction
Bladder cancer is the most common tumor among
genitourinary cancers and the fourth most common cancer among males
(1). Bladder cancer has a high
incidence and mortality rate among the malignant tumors of the
genitourinary tract (2).
Furthermore, bladder cancer is more prevalent than other urinary
tract carcinomas (3). Cancer
statistical data have indicated an estimated 549,393 new cases and
199,922 mortalities from bladder cancer worldwide in 2018 (4). The majority of the newly diagnosed
tumors are superficial and may be treated by transurethral
resection (5). However, a large
number of patients have a high rate of tumor recurrence following
the first surgery (6). The
mechanisms underlying the development of bladder cancer have not
been thoroughly elucidated. Therefore, the identification of the
molecular mechanisms involved in the progression of bladder tumors
may aid the development of novel diagnostic and therapeutic
interventions. The signal transducer and activator of transcription
3 (STAT3) signaling pathway is implicated in the progression of
lung cancer (7). STAT3 is a
cytoplasmic transcription factor which is expressed in response to
a large number of cytokines and growth factors (8). A previous study suggested that STAT3
may promote the growth of tumor cells and inhibit tumor cell
apoptosis (9). STAT3 is activated by
the phosphorylation of conserved tyrosine and serine residues in
its C-terminal domains by janus kinase (JAK) proteins (9). The phosphorylation of proteins in the
JAK2/STAT3 pathway may result in the growth and proliferation of
bladder cancer cells (10). The
enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) is
a member of the polycomb group proteins (11). EZH2 serves important roles in
embryonic stem cell pluripotency and self-renewal (12,13).
Furthermore, EZH2 expression was upregulated in different types of
malignant tumors with poor prognosis, including prostate, bladder,
renal and breast carcinomas (14–17).
Previous studies revealed that EZH2 may be a marker of aggressive
urological neoplasms (18,19). However, the biological effect of EZH2
in bladder cancer and the associations between EZH2 and the
JAK2/STAT3 signaling pathway have not been elucidated. The aim of
the current study was to investigate the effects of EZH2 on bladder
carcinogenesis and to explore its mechanism in human bladder cancer
cells.
Materials and methods
Drugs and reagents
The EZH2 inhibitor UNC1999 was purchased from
Selleck Chemicals (Shanghai, China) and was dissolved in dimethyl
sulfoxide (DMSO; concentrations used in experiments, 0.1, 1, 10 and
100 µM). Antibodies against phospho-JAK2 (1:1,000 dilution; cat.
no. 3771S), JAK2 (1:1,000 dilution; cat. no. 3230S), phospho-STAT3
(1:1,000 dilution; cat. no. 9145S), STAT3 (1:1,000 dilution; cat.
no. 9139S), EZH2 (1:1,000 dilution; cat. no. 5246S) and GAPDH
(1:3,000 dilution; cat. no. 5174S), as well as horseradish
peroxidase-conjugated goat anti-rabbit and anti-mouse IgG (1:2,000
dilution; cat. nos. 7074S and 7076S), were purchased from Cell
Signaling Technology, Inc. (Boston, MA, USA).
Clinical specimens
Human bladder tumors and corresponding adjacent
normal tissues were collected from patients who underwent partial
or radical cystectomy for urothelial carcinomas of the bladder at
the Department of Urology of Renmin Hospital of Wuhan University
(Wuhan, China) between June 2015 and March 2017. The study was
approved by the Institutional Ethics Committee of Renmin Hospital
of Wuhan University. Each participant provided signed informed
consent prior to participation in the present study. Patients or
their legal surrogate decision makers provided signed informed
consent for the surgical procedures. All specimens were anonymized.
Inclusion criteria were the following: i) ≤75 years; ii)
histologically confirmed bladder cancer; iii) no severe major organ
dysfunction; and iv) no prior cancer chemotherapy. Exclusion
criteria were the following: i) ≥76 years; ii) severe major organ
dysfunction; and iii) prior cancer chemotherapy. The clinical
information of the patients, including sex, age, smoking history,
the diameter and differentiation of the tumor, lymph node
metastasis, stage of TNM, histological grade, chemotherapy received
and EZH2 expression status, were recorded. Half of the specimens
were removed and fixed in 4% paraformaldehyde at room temperature
for 1 week, followed by routine paraffin embedding, and the
remaining half were preserved in liquid nitrogen at −196°C. All of
the specimens were classified as bladder carcinoma or normal tissue
by histological identification. Tumor stage and grade were
determined according to the Union for International Cancer Control
(UICC) guidelines (20).
Cell lines and cell culture
The human bladder cancer cell lines E-J and 5637
were purchased from the The Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China). E-J and 5637 cells
were authenticated by STR profiling. E-J and 5637 cells were
cultured at 37°C with 5% CO2. in RPMI 1640 medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 10% fetal bovine serum (FBS; ScienCell Research Laboratories,
Inc., San Diego, CA, USA), 0.1 mg/ml streptomycin and 100 U/ml
penicillin. The medium was replaced every 48 h.
Cell viability assay
E-J and 5637 cells were plated into 96-well plates
at a density of 5×103 cells/well. Following 24 h of
culture at 37°C, the cells were treated with different
concentrations of UNC1999 (0.1, 1, 10 and 100 µM) for different
times (24, 72 and 120 h) and subsequently incubated with 10 µl of
MTT dye/well for 4 h. Following the MTT incubation, the purple
formazan crystals were dissolved by the addition of 150 µl DMSO per
well. Cell viability was subsequently analyzed at a wavelength of
490 nm.
Apoptosis assay
E-J and 5637 cells were incubated with or without
UNC1999 (100 µM). The control group was treated with DMSO. for 72
h. The cells were collected and washed twice with PBS and
resuspended with 150 ml binding buffer (included in the Annexin
V-FITC Apoptosis Detection kit; cat. no. C1062M; Beyotime Institute
of Biotechnology). The cells were subsequently incubated with 10 µl
Annexin V-fluorescein isothiocyanate (FITC) and 5 µl propidium
iodide for 5 min at room temperature in the dark. The Annexin
V-FITC Apoptosis Detection kit was used according to the
manufacturer's protocol. Apoptotic cells were subsequently analyzed
using a flow cytometer (BD Biosciences, San Jose, CA, USA).
Analysis was performed using the BD FACSuite software (version 1.0;
BD Biosciences).
Wound healing assay
E-J and 5637 cells (5×105) were plated in
six-well plates. Following a 24 h incubation period at 37°C with 5%
CO2. wounds were made in each well using a 200 µl
pipette tip. The cells were washed three times with PBS and
incubated with UNC1999 (100 µM) at 37°C for 24 h. The wound areas
were subsequently quantified using a microscope.
Cell migration assays
A total of 5×104 E-J and 5637 cells were
plated in the upper chambers of Transwell plates (24 wells; 8 µm
pore size with polycarbonate membrane; Corning Inc., Corning, NY,
USA) in 100 µl serum-free RPMI 1640 medium. RPMI 1640 medium
containing 10% FBS and UNC1999 (100 µM) was plated in the lower
chambers. Following incubation at 37°C for 24 h, the migratory
cells were fixed with methanol at room temperature for 6 h and
stained with 0.2% crystal violet (Beyotime Institute of
Biotechnology). The numbers of stained cells in five randomly
selected fields were counted using a light microscope
(magnification, ×400; Leica Microsystems, Ltd., Milton Keynes,
UK).
Immunohistochemistry
The expression of EZH2 was assessed by
immunohistochemical staining. The bladder cancer tissues and
para-carcinoma tissues were cut into 4-µm thick sections.
Endogenous peroxidase activity was inhibited with 3% hydrogen
peroxide at 37°C for 10 min. The sections were subsequently treated
with 1:50 normal horse serum (cat. no. 16050130; Thermo Fisher
Scientific, Inc.) in tris-buffered saline for 30 min at 37°C.
Tissue sections were incubated with primary antibody directed
against EZH2 overnight at 4°C. The sections were subsequently
washed three times with PBS. The sections were incubated with a
horseradish peroxidase-labeled secondary antibody for 30 min at
20°C. The sections were incubated with 3,3′-diaminobenzidine for 2
min at 20°C. Tissues were observed under a light microscope
(magnification, ×400) and images were captured. Immunostained
sections were evaluated by two experienced pathologists under
blinded conditions.
Reverse
transcription-semi-quantitative polymerase chain reaction
(RT-qPCR)
RT-qPCR was used to assess the EZH2 mRNA level in
clinical specimens. Total RNA was extracted from the aforementioned
clinical specimens and cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). EZH2 levels were
assessed using a Roche LightCycler 480 (Roche Diagnostics GmbH,
Mannheim, Germany) and BeyoFast™ SYBR Green qPCR Mix kit (cat. no.
D7260; Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocols. GAPDH was used as an
endogenous reference gene to analyse the relative gene expression
levels. The thermocycling conditions were as follows: 1 cycle of
94°C for 3 min, followed by 35 cycles of 94°C for 5 sec, 56°C for
30 sec and 72°C for 30 sec, and a final extension at 60°C for 30
sec. The quantification cycle fluorescence value (Cq) was
calculated using SDS software (version 2.1; Applied Biosystems;
Thermo Fisher Scientific, Inc.). The expression levels were
analysed according to the 2−ΔΔCq method (21). All experiments were performed in
triplicate. The appearance of a single peak in the melting curve
implicated the specificity of the PCR products. The primer
sequences were as follows: EZH2 forward,
5′-GACCCTGACCTCTGTCTTACTT-3′, and reverse,
5′-GATGGTGCCAGGCAATAGATG-3′; and GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′, and reverse,
5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Flank xenograft model
The Institutional Animal Care and Use Committee of
Wuhan University approved the experimental protocols and supervised
the care of animals and experimental procedures. Nude mice (Balb/c
nu/nu) were purchased from the Animal Center of Wuhan University.
BALB/c nude mice (n=10; male) were used for in vivo
experiments. The mice were bred and experiments were performed in
laminar flow cabinets under specific-pathogen-free conditions in
the Laboratory Animal Center of Renmin Hospital of Wuhan
University. Nude mice were kept at the College Laboratory Animal
Center of Conventional Breeding at a temperature of 25–27°C with a
relative humidity of 45–50%, in an aseptic environment, with
adequate illumination (12-h light/dark cycle), moisture and feed.
The mice had free access to food and water. Preliminary in
vivo experiments revealed that the tumorigenic effect of E-J
cells was better than that of 5637 cells; therefore, E-J cells were
used in the in the in vivo experiments. A total of
5×106 E-J cells were inoculated in the flank region of
six-week-old nude mice (weight, 16–20 g). Two perpendicular
diameters (a, the largest; b, the smallest) of the tumor were
measured once per week with calipers for 5 weeks of monitoring.
Tumor volume (V) was calculated using the following formula: V=a ×
b2 × 0.5 (22). UNC1999
(50 mg/kg) and saline in equal volumes were injected
intraperitoneally twice per week when the mean tumor volume reached
~100 mm3. Tumor growth rate was plotted and analyzed
using GraphPad Prism software (version 5.0; GraphPad Software,
Inc., La Jolla, CA, USA).
Western blotting
E-J and 5637 cells or tumor tissues from mice were
dissociated using the Total Protein Extraction kit (Wuhan Goodbio
Technology Co., Ltd., Wuhan, China) according to the manufacturer's
instructions, and the extracted protein was examined by western
blotting. Protein concentrations were assessed using a
bicinchoninic acid assay prior to loading. A total of 40 µg
protein/lane from each sample were separated via SDS-PAGE on a 10%
gel. The separated proteins were subsequently transferred onto
nitrocellulose membranes and blocked for 2 h with 5% nonfat milk in
Tris-buffered saline and Tween-20 (TBST) buffer at room
temperature. The membranes were incubated with the primary
polyclonal antibodies against EZH2, p-JAK2, JAK2, p-STAT3, STAT3
and GAPDH overnight at 4°C. Membranes were washed three times with
TBST. Following the primary incubation, membranes were incubated
with horseradish peroxidase-labeled secondary antibodies (1:2,000).
The membranes were subsequently washed three times with TBST. The
protein bands were visualized using an enhanced chemiluminescence
detection kit (EMD Millipore, Billerica, MA, USA). The band
intensity was quantified using ImageJ software (version 2.1;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the means ± standard error of
the mean. SPSS software (version 17; SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Differences in values and
percentages among groups were compared using a paired t-test,
χ2 test, Fisher's exact test or one-way analysis of
variance followed by Dunnett's multiple comparison test. All
experiments were repeated at least three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of EZH2 in bladder
cancer
In order to investigate whether the expression level
of EZH2 was associated with the progression of bladder cancer, the
expression levels of EZH2 in bladder carcinoma tissue and adjacent
non-neoplastic parenchyma were analyzed by immunohistochemical
staining. The mean of the relative expression of EZH2 in tissues of
all paired samples was used as the cut-off value to determine high
and low expression groups. The expression levels of EZH2 were
increased in tumor tissues compared with adjacent normal tissues
(P<0.05; Fig. 1A-D). Similar
results were obtained by western blot analysis (P<0.05; Fig. 1E, F). The tumor stage and grade were
classified according to the TNM staging system of the UICC
guidelines. The level of EZH2 expression in tumor tissues was
significantly increased in the higher TNM stages (T2-T4) compared
with the lower stages (Ta-T1; Table
I). The EZH2 levels were associated with the tumor histological
stage (P=0.026) and tumor histological grade (P<0.001). These
results indicated that the expression level of EZH2 was closely
associated with the progression of bladder cancer.
 | Table I.Clinicopathological features of the
34 patients with bladder cancer and their associations with EZH2
expression. |
Table I.
Clinicopathological features of the
34 patients with bladder cancer and their associations with EZH2
expression.
|
|
| EZH2
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | No. of
patients | Low | High | P-value |
|---|
| Age (years) |
|
|
| 0.86 |
|
<60 | 12 | 4 | 8 |
|
|
>60 | 22 | 8 | 14 |
|
| Sex |
|
|
| 0.714 |
|
Male | 24 | 8 | 16 |
|
|
Female | 10 | 4 | 6 |
|
| Histological
stage |
|
|
| 0.026a |
|
Ta-T1 | 14 | 8 | 6 |
|
|
T2-T4 | 20 | 4 | 16 |
|
| Histological
grade |
|
|
|
<0.001a |
| G1 | 15 | 10 | 5 |
|
|
G2-G3 | 19 | 2 | 17 |
|
UNC1999 inhibits the proliferation and
migration of the bladder cancer cell lines E-J and 5637
The MTT, apoptosis, wound-healing and cell migration
assays were used to investigate the effects of the EZH2 inhibitor
UNC1999 on the proliferation and migration of bladder cancer cell
lines E-J and 5637 (cells in the control groups were treated with
an equal volume of DMSO; Fig. 2).
The MTT assay revealed that treatment with UNC1999 inhibited the
proliferation of the bladder cancer cell lines in a dose- and
time-dependent manner (Fig. 2A and
B). UNC1999 exhibited the greatest inhibitory effect at a final
concentration of 100 µM and an incubation period of 72 and 120 h.
The difference between the 72 and 120 h incubation periods was not
statistically significant (P>0.05). Therefore, the bladder
cancer cell lines were treated with 100 µM UNC1999 for 72 h in
subsequent experiments. Apoptosis analysis revealed that UNC1999
induced significant apoptosis in E-J and 5637 cells (Fig. 2C-E).
Cell migration is a central process in the evolution
and progression of tumors (23). The
wound healing and Transwell migration assays were used to
investigate the effects of UNC1999 on the cell migration of bladder
cancer cell lines. E-J and 5637 cells treated with UNC1999
exhibited decreased migration compared with the control group which
was treated with DMSO (P<0.05) in the wound healing assay
(Fig. 2F and G). Similar results
were obtained with the Transwell assay, where a fewer number of
cells treated with UNC1999 migrated into the lower chamber compared
with control cells (P<0.01; Fig. 2H
and I). The data obtained from the apoptosis and migration
assays suggested that UNC1999 reduced the proliferation and
migration of the bladder cancer cell lines.
EZH2 inhibition suppresses bladder
cancer via blocking of the JAK2/STAT3 signaling pathway
The JAK2/STAT3 signaling pathway controls the
invasive and aggressive phenotype of cancer cells (24). The current study investigated the
effect of EZH2 on the phosphorylation levels of JAK2 and STAT3, as
the phosphorylation is required for the activity of the JAK2/STAT3
signaling pathway. Constitutive p-JAK2 and p-STAT3 levels were
decreased in E-J and 5637 cells treated with UNC1999 compared with
controls (P<0.05; Fig. 3). These
data suggested that inhibition of EZH2 may regulate the activation
of the JAK2/STAT3 signaling pathway in bladder cancer cell
lines.
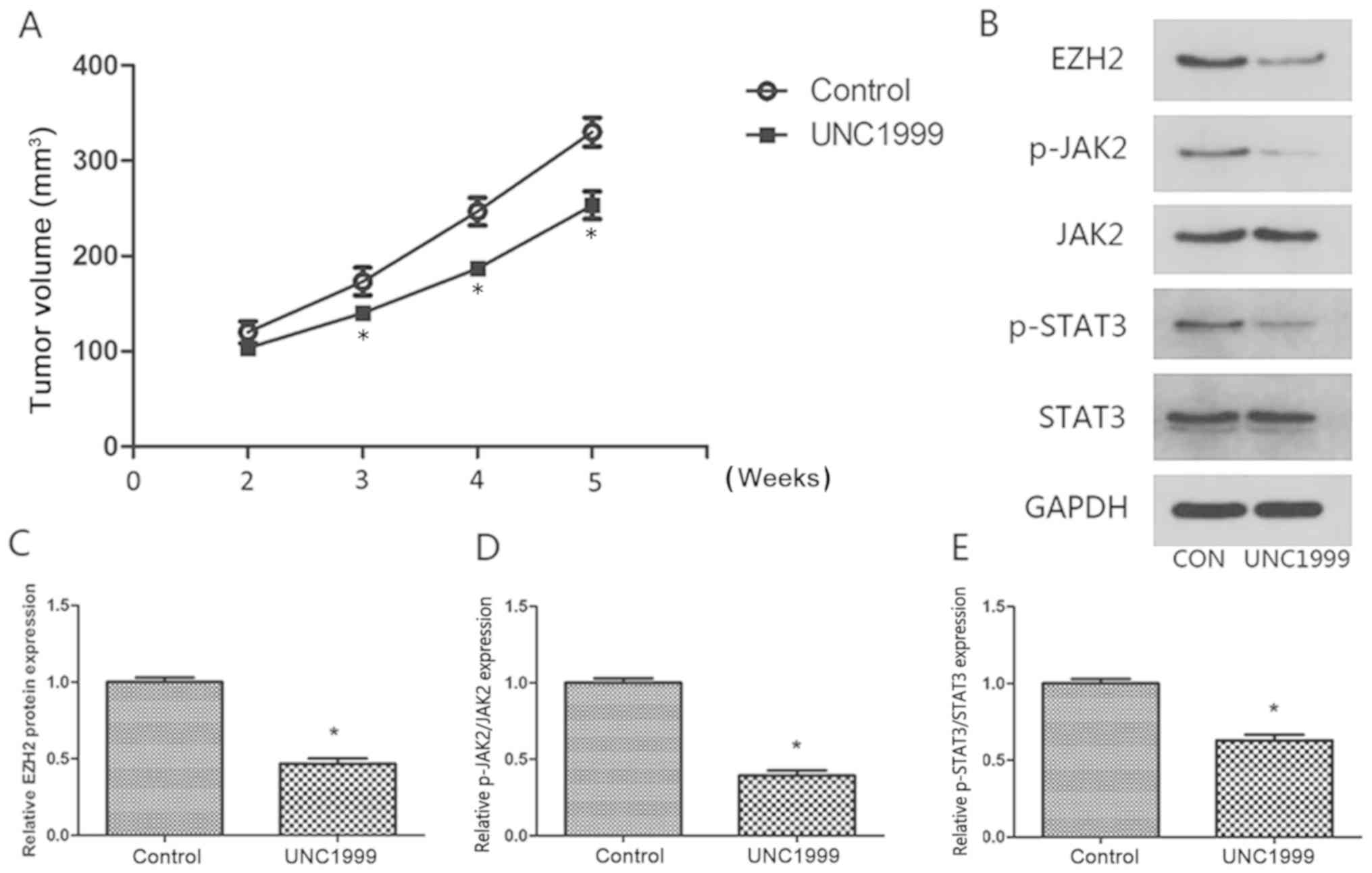
UNC1999 inhibits bladder cancer growth
in vivo
To further explore the antitumor activity of UNC1999
in vivo, E-J tumor xenografts were analyzed. UNC1999
exhibited significant antitumor activity in nude mice bearing E-J
tumor xenografts at a dose of 50 mg/kg (P<0.05; Fig. 4A). In order to examine the mechanism
underlying the inhibition of tumor growth by UNC1999 in
vivo, the expression levels of JAK2 and STAT3 were measured
using western blotting. The expression levels of JAK2 and STAT3
were significantly decreased in the tumors treated with UNC1999
compared with the control tumors (P<0.05; Fig. 4B-E). These results suggested that
UNC1999 inhibited tumor growth in vivo by inhibiting EZH2
and subsequent inhibition of the JAK2/STAT3 signaling pathway.
Discussion
Bladder cancer is a common cause of
cancer-associated mortality worldwide (25). The majority of patients with bladder
cancer are diagnosed at an advanced stage of the disease due to a
lack of disease-specific makers in the early stages (26). Surgery and chemotherapy are currently
the most effective treatments for bladder cancer; however, the
prognosis remains poor in the advanced stages and the overall
survival rate is unsatisfactory (27). A number of oncogenes have been
identified in recent years, but the molecular mechanisms underlying
tumorigenesis of bladder cancer remain unknown (28). Therefore, the identification of novel
molecular targets in bladder cancer may improve the diagnosis and
prognosis of the disease.
EZH2 is a member of the polycomb repressive complex
2. EZHZ, together with embryonic ectoderm development and S UZ12
polycomb repressive complex 2 subunit, catalyze the di- and
tri-methylation of histone H3 lysine 27 (H3K27), which is essential
for embryonic stem cell pluripotency and self-renewal (29). EZH2 is an adhesion protein expressed
in a number of organs and tissues, and it can promote cancer
metastasis (30). EZH2 has been
reported to be a marker of the aggressive stages of prostate cancer
(31). The ectopic overexpression of
EZH2 can lead to the transformation of normal prostatic cells and
the canceration of breast epithelium (32). EZH2 upregulation is a potential tumor
biomarker and contributes to tumor progression; therefore, it is
deemed to be an oncogene (33). A
previous study suggested that EZH2 inhibition by small interfering
RNA may decrease cancer cell proliferation, induce cancer cell
apoptosis in vitro and decrease breast xenograft growth
in vivo (34). Taken
together, EZH2 may be a novel target for drug development for
different types of cancer.
UNC1999 is a novel
S-adenosyl-l-methionine-competitive EZH2 inhibitor that has
demonstrated efficacy in different types of cancer, including
leukemia, colon cancer and multiple myeloma (35). The results obtained in the present
study indicated that the EZH2 inhibitor UNC1999 may reduce cancer
cell proliferation and migration, induce bladder cancer cell
apoptosis and contribute to the regression of bladder tumor
xenografts in mice. Additionally, the present study revealed that
EZH2 may be upregulated in bladder cancer and suggested that
pharmacological inhibition of EZH2 may be a novel therapeutic
strategy in bladder cancer. The increase of intratumoral EZH2
expression, which is associated with bladder cancer progression and
poor prognosis, is indicated as an independent prognostic factor
for the overall survival time in clinical patients (15,36,37). Lee
et al (36) revealed a
predictive value of high expression levels of EZH2 for prognosis in
bladder tumors, and that the E2F transcription factor 1-EZH2-SUZ12
polycomb repressive complex 2 subunit-driven transcriptional events
may regulate cancer aggressiveness and chemoresistance. This
highlights the potential applications for intratumoral EZH2
immunostaining in future clinical prognostic stratification and
therapeutic interventions.
JAK2 is a vital hormone signaling and intracellular
mediator of cytokines (38). JAK2
can activate a series of downstream signaling pathways, including
the STAT cascade (39,40). STAT3, a member of the STAT family,
may be activated by phosphorylation at the tyrosine residue 705 to
regulate the release of growth factors and cytokines in gastric
cancer (41). The activation of the
JAK2/STAT3 signaling pathway was associated with the growth,
migration and metastasis of lung cancer cells (42). Lei et al (43) revealed that the activation of the
JAK2/STAT3 signaling pathway may promote the proliferation and
migration of cancer cells as well as inhibit apoptosis in renal
cell carcinoma. The inhibition of JAK2/STAT3 phosphorylation in
pancreatic cancer may reduce the proliferation of pancreatic cancer
cells in vivo and in vitro (44). In the current study, the western
blotting results revealed that the phosphorylation of the
JAK2/STAT3 signaling pathway was associated with the proliferation
of bladder cancer cells, and the downregulation of EZH2 expression
levels was associated with low expression levels of p-JAK2 in
vivo and in vitro. These results suggested that EZH2
activity may serve an important role in bladder cancer
development.
In summary, the present study demonstrated that
UNC1999 decreased the proliferation, and migration of bladder
cancer cells and increased apoptosis by inhibiting the activity of
EZH2. Furthermore, the inhibition of EZH2 activity was associated
with inhibition of the JAK2/STAT3 signaling pathway. Thus, EZH2 may
be a potential therapeutic target for the treatment of patients
with bladder cancer.
The present study had a number of limitations.
UNC1999 is the first orally bioavailable inhibitor that has high
in vitro potency for wild-type and mutant EZH2 as well as
EZH1. EZH1 is a H3K27 methyltransferase that shares 96% sequence
identity with EZH2 in their respective catalytic domains (45). To the best of our knowledge, there
are no previously published studies suggesting that EZH1 may be
associated with tumor progression. Additionally, the current study
did not investigate the mechanism of EZH1 in bladder cancer. Future
experiments knocking out or silencing EZH2 may remove the
potentially confounding results obtained by inhibiting EZH1. The
results of these studies may be compared with the results obtained
with or without UNC1999 inhibition. The current study suggested
that the JAK2/STAT3 signaling pathway may serve a key role in the
in the carcinogenic mechanism of EZH2 in bladder cancer. However,
it is likely that several other pathways may be involved in the
progression of bladder cancer. Long non-coding RNA, microRNA,
circular RNA and exosomes may also be implicated. Future studies
investigating other possible mechanisms underlying the progression
of bladder cancer are required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets produced during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
ZC, JG and LW conceived and designed the
experiments. ZC and YD performed the experiments. XL, HC, MW, XW
and XDW analyzed the data, contributed to the interpretation of
results obtained and wrote the manuscript. JG edited the final
draft of the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Renmin Hospital of Wuhan University (Wuhan, China),
and written informed consent was obtained from all participants.
The Institutional Animal Care and Use Committee of Wuhan University
approved the experimental protocols and supervised the care of
animals and experimental procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Z, Zhang G and Kong C: Targeted
inhibition of polo-like kinase 1 by a novel small-molecule
inhibitor induces mitotic catastrophe and apoptosis in human
bladder cancer cells. J Cell Mol Med. 21:758–767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan K, Zhang C, Feng J, Hou L, Yan L, Zhou
Z, Liu Z, Liu C, Fan Y, Zheng B and Xu Z: Induction of G1 cell
cycle arrest and apoptosis by berberine in bladder cancer cells.
Eur J Pharmacol. 661:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daneshmand S, Patel S, Lotan Y, Pohar K,
Trabulsi E, Woods M, Downs T, Huang W, Jones J, O'Donnell M, et al:
Efficacy and safety of blue light flexible cystoscopy with
hexaminolevulinate in the surveillance of bladder cancer: A phase
III, comparative, multicenter study. J Urol. 199:1158–1165. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun J, Yu M, Lu Y, Thakur C, Chen B, Qiu
P, Zhao H and Chen F: Carcinogenic metalloid arsenic induces
expression of mdig oncogene through JNK and STAT3 activation.
Cancer Lett. 346:257–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Devarajan E and Huang S: STAT3 as a
central regulator of tumor metastases. Curr Mol Med. 9:626–633.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wagner KU and Schmidt JW: The two faces of
Janus kinases and their respective STATs in mammary gland
development and cancer. J Carcinog. 10:322011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu W, Zhang E, Song L, Tu L, Wang Z, Tian
F, Aikenmu K, Chu G and Zhao J: Long noncoding RNA HOXD-AS1
aggravates osteosarcoma carcinogenesis through epigenetically
inhibiting p57 via EZH2. Biomed Pharmacother. 106:890–895. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pasini D, Bracken AP, Hansen JB, Capillo M
and Helin K: The polycomb group protein Suz12 is required for
embryonic stem cell differentiation. Mol Cell Biol. 27:3769–3779.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chamberlain SJ, Yee D and Magnuson T:
Polycomb repressive complex 2 is dispensable for maintenance of
embryonic stem cell pluripotency. Stem Cells. 26:1496–1505. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Labbe DP, Sweeney CJ, Brown M, Galbo P,
Rosario S, Wadosky KM, Ku SY, Sjöström M, Alshalalfa M, Erho N, et
al: TOP2A and EZH2 provide early detection of an aggressive
prostate cancer subgroup. Clin Cancer Res. 23:7072–7083. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X,
Wang M, Huang C, Wang L, Zeng F and Jiang G: LncRNA SPRY4-IT1
sponges miR-101-3p to promote proliferation and metastasis of
bladder cancer cells through up-regulating EZH2. Cancer Lett.
388:281–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Chen Y, Geng H, Qi C, Liu Y and
Yue D: Overexpression of YB1 and EZH2 are associated with cancer
metastasis and poor prognosis in renal cell carcinomas. Tumour
Biol. 36:7159–7166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reijm EA, Timmermans AM, Look MP,
Meijer-van GM, Stobbe CK, van Deurzen CH, Martens JW, Sleijfer S,
Foekens JA, Berns PM and Jansen MP: High protein expression of EZH2
is related to unfavorable outcome to tamoxifen in metastatic breast
cancer. Ann Oncol. 25:2185–2190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen H, Morrison CD, Zhang J, Underwood W
III, Yang N, Frangou C, Eng K, Head K, Bollag RJ, Kavuri SK, et al:
6p22.3 amplification as a biomarker and potential therapeutic
target of advanced stage bladder cancer. Oncotarget. 4:2124–2134.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Wang Y, Qiu J, Li Q, Yuan C, Zhang
W, Wang D, Ye J, Jiang H, Yang J and Cheng J: The polycomb group
protein EZH2 is a novel therapeutic target in tongue cancer.
Oncotarget. 4:2532–2549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magers MJ, Lopez-Beltran A, Montironi R,
Williamson SR, Kaimakliotis HZ and Cheng L: Staging of bladder
cancer. Histopathology. 74:112–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2008.
View Article : Google Scholar
|
|
22
|
Moon JH, Hong SW, Kim JE, Shin JS, Kim JS,
Jung SA, Ha SH, Lee S, Kim J, Lee DH, et al: Targeting β-catenin
overcomes MEK inhibition resistance in colon cancer with KRAS and
PIK3CA mutations. Br J Cancer. 4–April;2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Weihs D and Vermolen FJ: A model
for cell migration in non-isotropic fibrin networks with an
application to pancreatic tumor islets. Biomech Model Mechanobiol.
17:367–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao JM, Cheng W, He XG, Liu YL, Wang FF
and Gao YF: Long non-coding RNA PICART1 suppresses proliferation
and promotes apoptosis in lung cancer cells by inhibiting
JAK2/STAT3 signaling. Neoplasma. 65:779–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dehayni Y, Tetou M, Khdach Y, Janane A,
Alami M and Ameur A: Prognostic of older age for patients with
invasive-muscle-bladder cancer and treated by radical cystectomy.
Prog Urol. 28:166–172. 2018.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Racioppi M, D'Agostino D, Totaro A, Pinto
F, Sacco E, D'Addessi A, Marangi F, Palermo G and Bassi PF: Value
of current chemotherapy and surgery in advanced and metastatic
bladder cancer. Urol Int. 88:249–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hammerle M, Gutschner T, Uckelmann H,
Ozgur S, Fiskin E, Gross M, Skawran B, Geffers R, Longerich T,
Breuhahn K, et al: Posttranscriptional destabilization of the
liver-specific long noncoding RNA HULC by the IGF2 mRNA-binding
protein 1 (IGF2BP1). Hepatology. 58:1703–1712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muller J, Hart CM, Francis NJ, Vargas ML,
Sengupta A, Wild B, Miller EL, O'Connor MB, Kingston RE and Simon
JA: Histone methyltransferase activity of a drosophila polycomb
group repressor complex. Cell. 111:197–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shahabipour F, Caraglia M, Majeed M,
Derosa G, Maffioli P and Sahebkar A: Naturally occurring
anti-cancer agents targeting EZH2. Cancer Lett. 400:325–335. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karanikolas BD, Figueiredo ML and Wu L:
Polycomb group protein enhancer of zeste 2 is an oncogene that
promotes the neoplastic transformation of a benign prostatic
epithelial cell line. Mol Cancer Res. 7:1456–1465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto I, Nosho K, Kanno S, Igarashi H,
Kurihara H, Ishigami K, Ishiguro K, Mitsuhashi K, Maruyama R, Koide
H, et al: EZH2 expression is a prognostic biomarker in patients
with colorectal cancer treated with anti-EGFR therapeutics.
Oncotarget. 8:17810–17818. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gonzalez ME, Li X, Toy K, DuPrie M,
Ventura AC, Banerjee M, Ljungman M, Merajver SD and Kleer CG:
Downregulation of EZH2 decreases growth of estrogen
receptor-negative invasive breast carcinoma and requires BRCA1.
Oncogene. 28:843–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu B, On DM, Ma A, Parton T, Konze KD,
Pattenden SG, Allison DF, Cai L, Rockowitz S, Liu S, et al:
Selective inhibition of EZH2 and EZH1 enzymatic activity by a small
molecule suppresses MLL-rearranged leukemia. Blood. 125:346–357.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SR, Roh YG, Kim SK, Lee JS, Seol SY,
Lee HH, Kim WT, Kim WJ, Heo J, Cha HJ, et al: Activation of EZH2
and SUZ12 regulated by E2F1 predicts the disease progression and
aggressive characteristics of bladder cancer. Clin Cancer Res.
21:5391–5403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Zhong G, He W, Yu H, Huang J and
Lin T: lncRNA Up-regulated in nonmuscle invasive bladder cancer
facilitates tumor growth and acts as a negative prognostic factor
of recurrence. J Urol. 196:1270–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu B, Chen X, Tan J and Xu X: Effect of
AG490 on JAK2/STAT3 signaling pathway in human retinoblastoma
HXO-RB44 cell lines. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
43:1061–1067. 2018.(In Chinese). PubMed/NCBI
|
|
39
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Muller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zheng L, Chen J, Zhou Z and He Z:
Knockdown of long non-coding RNA HOXD-AS1 inhibits gastric cancer
cell growth via inactivating the JAK2/STAT3 pathway. Tumour Biol.
39:10104283177053352017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim MJ, Nam HJ, Kim HP, Han SW, Im SA, Kim
TY, Oh DY and Bang YJ: OPB-31121, a novel small molecular
inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an
antitumor activity in gastric cancer cells. Cancer Lett.
335:145–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song Y, Kong L, Sun B, Gao L, Chu P, Ahsan
A, Qaed E, Lin Y, Peng J, Ma X, et al: Induction of autophagy by an
oleanolic acid derivative, SZC017, promotes ROS-dependent apoptosis
through Akt and JAK2/STAT3 signaling pathway in human lung cancer
cells. Cell Biol Int. 41:1367–1378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lei J, Xiao JH, Zhang SH, Liu ZQ, Huang K,
Luo ZP, Xiao XL and Hong ZD: Non-coding RNA 886 promotes renal cell
carcinoma growth and metastasis through the Janus kinase 2/signal
transducer and activator of transcription 3 signaling pathway. Mol
Med Rep. 16:4273–4278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Z, Wang F, Du C, Guo H, Ma L, Liu X,
Kornmann M, Tian X and Yang Y: BRM/SMARCA2 promotes the
proliferation and chemoresistance of pancreatic cancer cells by
targeting JAK2/STAT3 signaling. Cancer Lett. 402:213–224. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang X, Li F, Konze KD, Meslamani J, Ma A,
Brown PJ, Zhou MM, Arrowsmith CH, Kaniskan HÜ, Vedadi M and Jin J:
Structure-activity relationship studies for enhancer of zeste
homologue 2 (EZH2) and enhancer of zeste homologue 1 (EZH1)
inhibitors. J Med Chem. 59:7617–7633. 2016. View Article : Google Scholar : PubMed/NCBI
|