Introduction
Thyroid cancer is not a rare cancer but has a
relatively slow progression in many patients. However, it may
metastasize to distant organs including lungs as disease
progression. Lung metastases of thyroid cancer are those to the
lung parenchyma, such metastases usually have multiple small
nodules in both lungs (1,2). On the contrary, however, pleural fluid
due to thyroid cancer is a rare etiology, to our knowledge, and
only 51 cases have been reported (3–19).
Pleural fluid due to thyroid cancer has been reported to be less
than 1% in all the patients with malignant pleural fluids (4,11).
Papillary thyroid cancer was the major histologic type of thyroid
cancer with pleural effusion, and other histologic types were few
(3). Thyroid cancer patients with
pleural fluid, regardless of tissue type, were long-term survivors
(3–19). In general, diagnosis of malignant
fluid is established by positive pathological findings using
specimens from pleural fluid (20).
Thoracentesis using fine needle is usually used, but in some cases
pleural biopsy or thoracoscope is performed if the appropriate
materials cannot be obtained by thoracentesis (21). As for the therapy, drainage using
thoracic tube is indicative for the patients with dyspnea,
especially those with chemotherapy-resistant malignancies (22). As for therapy, pleurodesis is usually
performed after pleural fluid drainage. However, as this is a rare
condition, standard therapy has not yet established. This case
report documents our clinical experience, we present herein a
patient with pleural fluid due to papillary thyroid cancer, and
reviewed previously reported such cases (3–19).
Materials and methods
The study was conducted in the Division of
Respiratory Medicine, Mito Medical Center, University of Tsukuba
and approved by the Ethics Committee of Division of Respiratory
Medicine, Mito Medical Center, University of Tsukuba. An informed
consent was obtained from the patient included in this study.
Clinical evaluation of the lesions followed the guideline for
diagnosis and treatment of the lung cancer (The Japan Lung Cancer
Society 2018). All cytological specimens evaluated in our study
underwent needle thoracic puncture under local anesthesia.
Cytopathological examination was performed after standard
hematoxylin and eosin staining of the specimens. Written informed
consent was obtained from the patient for publication and this
study was approved by the research ethics committee of Mito Medical
Center, University of Tsukuba-Mito Kyodo General Hospital (νo.
1660). The patient was hospitalized from March 2013 to April 2013.
Computed tomography used Aquilion 64 model manufactured by Toshiba
Medical Systems (Tokyo, Japan). Cytological specimens obtained from
pleural fluid were fixed in 10% buffered formalin, embedded in
paraffin and stained with HE method routinely for histological
examination. Immunostaining of cytokeratin 7 (CK-7), CK-19, and
thyroglobulin was performed on thyroid cancer cells obtained from
pleural fluid that were washed and fixed methanol before treatment
with serum-free protein block (Code No. X0909; DAKO Tokyo, Japan).
These cells were probed with antibodies to CK-7 (DAKO M7018; DAKO
Tokyo, Japan; 1:300), CK-19 (DAKO M0888; DAKO Tokyo, Japan; 1:100),
and thyroglobulin (Envision FLEX-Thyroglobulin, IR509; DAKO Tokyo,
Japan; 1:200). Slides were incubated with the primary antibody for
30 min at room temperature. Immunoreactivity was detected using an
indirect streptavidin-biotin method (LSAB kit; DAKO, Tokyo, Japan)
and horseradish peroxidase. Bound antibody was detected as a brown
stain.
We performed a search on PubMed using the terms
‘thyroid cancer’, ‘pleural fluid’, and ‘pleuritis’ to identify
similar cases, restricting the papers to those published in
English. The articles detected are shown in References (Reference
no. 3–19). Fifty-one patients of pleural fluid due to thyroid
cancer were confirmed. Clinicopathological features of these
patients were shown in Table I.
 | Table 1.Literature review of metastatic
pleural fluid due to thyroid cancer. |
Table 1.
Literature review of metastatic
pleural fluid due to thyroid cancer.
| Author, year | No. of Patients | Age/gender | Type | Diagnosis-metastasis
(yr) | Metastasis | Therapy | Survival (mo) | (Refs.) |
|---|
| Hyman 1979 | 1 | 76/F | Pap | – | Lung | Supportive care | 2 | (3) |
| Vassilopoulou
1994 | 10 |
46–75 | Pap | 0–12 | Lung | RI, pleurodesis | 1–20 | (4) |
| Vernon 1992 | 1 | 50/F | Pap | 3 | Lung, bone | Thoracentesisnd | 5 | (5) |
| Kovacs 1994 | 1 | 68/M | Pap | 0 | Pericardium, LN
supportive care | nd | 6 | (6) |
| Nomori 1997 | 1 | 68/F | Pap | 1 | Lung | Surgery | 8 | (7) |
| Siddaraju 2007 | 1 | 46/M | Pap | 0 | LN | nd | nd | (8) |
| Hsu 2009 | 1 | 77/F | Hür | 0 | – | Thoracentesis | 6 | (9) |
| Jeon 2011 | 4 | 51–74 M2F2 | All pap | 2–9 | Bone, brain, liver
pleurodesis | 6–21 | 10 | (10) |
| Olsen 2013 | 6 | 47-80, M3F3 | 4 pap, 2ATC | 0–12 | Bone | nd | 12 | (11) |
| Rosenstengel
2013 | 1 | 71/M | Pap | 11 | Lung | Pleurodesis | nd | (12) |
| Abe 2014 | 1 | 61/M | Pap | 10 | Lung | Supportive care | 6 | (13) |
| Sakamoto 2015 | 3 | 49/F 81/F 50/F | All pap | 2983 | Lung, liver,
bone | nd | 12 | (14) |
| Vyas 2016 | 5 | 39–78 M3F2 | All pap | 1–34 | nd | nd | 4–14 | (15) |
| Tomoda 2016 | 18 | 22–79 M3F15 | 16 pap, 2 foll | 0–18 | Bone, brain,
liver | Pleurodesis | 1–3 | (16) |
| Kim 2017 | 1 | 61/F | Pap | 19 | None | Chemotherapy | 4 | (17) |
| Kosmas 2017 | 1 | 69/F | Pap | 20 | None | Supportive
care | 4 | (18) |
| Uchida 2019 | 1 | 73/F | Poorly
differentiated | 0 | Lung | Lenvatinib | 13 | (19) |
Case report
A 91-year-old male presented to the hospital with a
1-mo history of dyspnea on effort, which had worsened progressively
over the previous few days. He had a 68-year history of
tuberculaous pleuritis of the left and a 6-year history of
surgically treated thyroid cancer. Physical examination showed that
he was afebrile and had poor performance status. Neck examination
showed well-healed cervical scars without adenopathy and chest
examination showed dullness on the right side occupying one third
of the chest. The peripheral blood test did not show leukocytosis
and elevated levels of C-reactive protein, carcinoembryonic
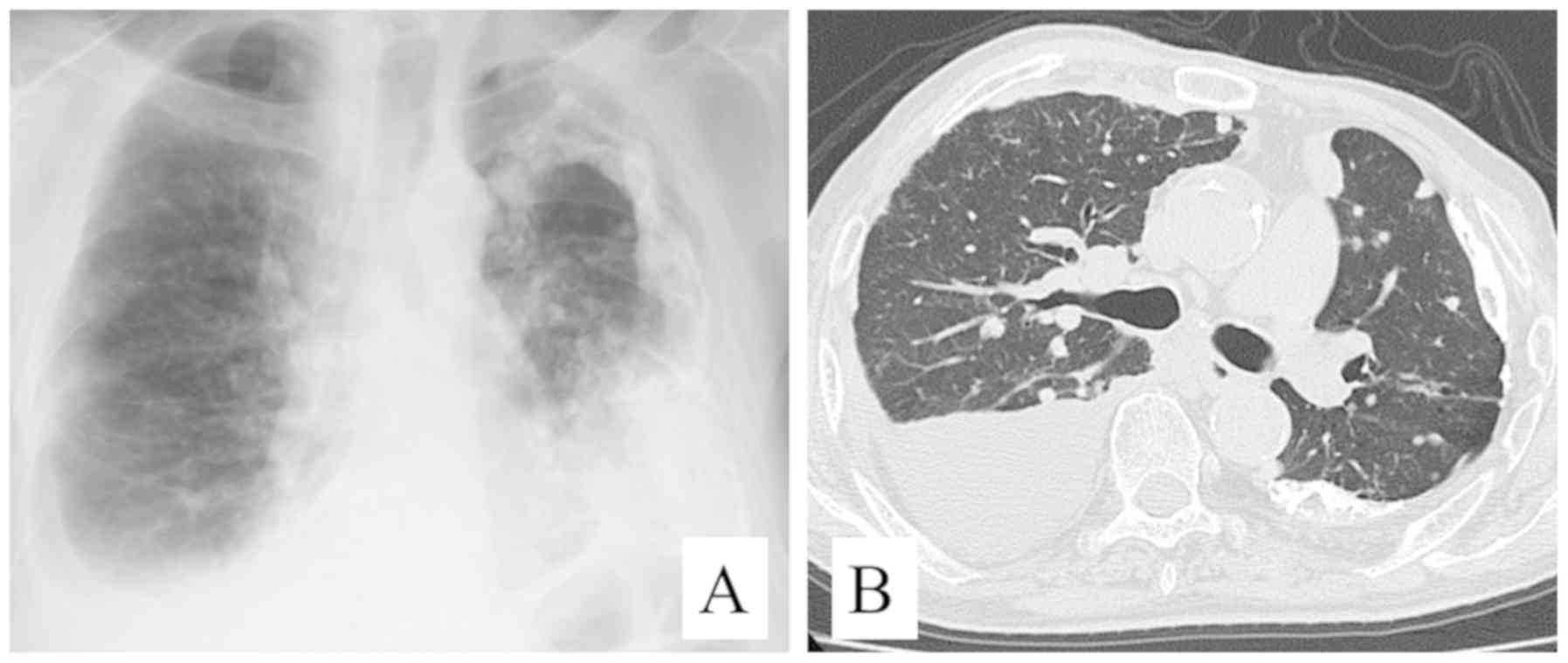
antigen, and CA19-9. A chest radiograph showed thickening and
calcification of the left pleura, and small nodules in both lungs
with pleural fluid in the right. Chest CT revealed massive right
pleural fluid causing atelectasis of the right lung and shift of
the mediastinum to the left side. Chest radiograph and chest CT
scan also showed thickening and calcification of the left pleura,
and well-circumscribed nodules measuring up to 15 mm in both lungs
(Fig. 1). Primary or metastatic
pulmonary cancer with pleural fluid, or recurrence of tuberculosis
was highly suspected. Thoracentesis of pleural fluid was performed
to alleviate severe dyspnea, yielding a serous fluid with malignant
cells on cytological examination. Cancer cells, which had
intra-nuclear inclusion body, were found in pleural fluid, and
immunocytochemical staining of the cells was positive for
cytokeratin (CK)-7, CK-19, and positive for thyroglobulin (Fig. 2). The diagnosis of massive pleural
fluid due to metastatic papillary thyroid cancer was established.
The patient's poor general condition and refusal prevented us from
further treatment. Thereafter, the patient followed up with
palliative care and died of the disease one month after the
diagnosis of pleural fluid due to thyroid cancer. Diagnosis of
pleural fluid due to papillary thyroid cancer was confirmed at
autopsy. Informed patient consent was obtained from the family of
the patient.
Discussion
Massive pleural fluid caused by thyroid cancer is
very rare. To our best knowledge, there have been reported only 51
cases (3–19). According to a review by
Vassilopoulou-Sellin and Sneige at the MD Anderson Cancer Center,
10 (0.6%) had malignant pleural fluid that developed during the
course of the disease among 1772 differentiated papillary thyroid
cancer patients (4). In a recent
review by Olson et al at the Johns Hopkins Hospital, only 6
patients with pleural fluid resulting from metastasis of thyroid
primaries over the last 26 years, which comprised 0.67% of all
malignant pleural fluids (11). Most
of the 21 patients had papillary thyroid cancer except for a few
cases with both papillary and undifferentiated carcinomas (19), follicular thyroid cancer (16) and Hürthle cell thyroid cancer
(9), which is usually classified
with a certain type of follicular thyroid cancer (9). In our case, we compared the
pathological findings of the specimens obtained from pleural fluids
and those surgically resected specimens, which were 6-year
previously in our hospital. They were consistent and confirm the
diagnosis of papillary thyroid cancer. Vassilopoulou-Sellin and
Sneige reported that pleural fluid appeared 61 to 132 months after
the initial diagnosis of thyroid cancer in 4 patients (4). Together with the result of our case,
these facts imply that pleural fluid may develop in long-term
survivors with this disease.
With regards to distant metastases,
Vassilopoulou-Sellin and Sneige reported that all their 10 patients
had radiologically apparent lung metastases at the time pleural
fluid was found (4). Other reports
also pointed out lung metastases (3,5,7,12–14,17).
Vernon et al reported a patient with a left iliac crest
metastasis and multiple bilateral pulmonary metastases were also
found (5). On the other hand, only
one of the 6 patients had bone metastases, but others had no
pathological evidence of distant metastasis in their institutional
review by Olson et al (11).
Our patient had multiple bilateral pulmonary metastases, which had
been detected a few months prior to the pleural fluid.
The diagnosis of massive pleural fluid in our
patient was not difficult with pleural immunocytochemical findings.
In general, however, it may be difficult because of its rarity of
pleural dissemination from thyroid cancer and the similarity of the
symptoms and signs to those of advanced chronic pulmonary diseases.
Negative cytological results of pleural fluid might be required in
evaluation of differential diagnosis of other causes of pleural
fluid. Our patient had a history of tuberculous pleuritis,
therefore, we had to include it as a differential diagnosis.
As for the therapy, in general, in patients with
pleural fluid due to chemo-sensitive cancers, systemic chemotherapy
is indicative for them (23–25). In those with chemo-insensitive
cancers, on the other hand, chest tube drainage and pleurodesis are
treatment choices for them (23–25).
Vassilopoulou-Sellin and Sneige reported that pleural fluids in
their patients were treated with local radioisotopes or sclerosing
agents, systemic radioiodine or chemotherapy, or both (4). They also reported that appearance of
pleural fluid preceded death by 1 to 20 months (median, 11 months)
and pleural fluid was associated with greatly shortened survival
time in all cases (3,4,7,9–11,14–19).
In our patient, chest tube drainage and pleurodesis were
considered, but only thoracentesis was performed because of his
contra-lateral calcified pleural thickening and poor respiratory
condition and died of thyroid cancer one month after the diagnosis
of pleural fluid.
In summary, pleural metastasis from papillary
thyroid cancer is a potential cause of massive pleural fluid,
although very rare. The current case extended the clinical spectrum
of metastatic potential to the pleura of papillary thyroid cancer.
As for the therapy, long-term palliation can be the treatment
choice for these patients as there has not established any standard
therapy for them.
Acknowledgements
The authors would like to thank Mr Jyunichi
Hakamtsuka (Division of Central Clinical Laboratory, Mito Medical
Center, University of Tsukuba) for his technical assistance with
cytological analysis.
Funding
No funding was received
Availability of data and materials
The datasets during and/or analyzed during the
current study available from the corresponding author on reasonable
request.
Authors' contributions
TT, TS, HS, KKa were responsible for the design of
the study and interpretation of the data. They also have revised
critically the manuscript for important intellectual content. HS,
KKa, NT, NH were responsible for the data acquisition, selection
and analysis and clinical interpretation of the data. TT, HS, KKu,
NT were responsible for the data analysis and interpretation. All
authors contributed to the writing of the manuscript. All authors
read and approved the final version of manuscript.
Ethics approval and consent to
participate
The study was conducted in the Division of
Respiratory Medicine, Mito Medical Center, University of Tsukuba
and approved by the Ethics Committee of Division of Respiratory
Medicine, Mito Medical Center, University of Tsukuba. An informed
consent was obtained from the patient included in this study.
Patient consent for publication
Informed consent for publication was obtained from
the family of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Quint LE, Park CH and Iannettoni MD:
Solitary pulmonary nodules in patients with extrapulmonary
neoplasms. Radiology. 217:257–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massin JP, Savoie JC, Gamier H, Guiraudon
C, Leger FA and Bacourt F: Pulmonary metastases in differentiated
thyroid carcinoma. Study of 58 cases with implications for the
primary tumor treatment. Cancer. 53:982–992. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hyman MP: Papillary and undifferentiated
thyroid carcinoma presenting as a metastatic papillary serous
effusion. A case report. Acta Cytol. 23:483–486. 1979.PubMed/NCBI
|
|
4
|
Vassilopoulou-Sellin R and Sneige N:
Pleural effusion in patients with differentiated papillary thyroid
cancer. South Med J. 87:1111–1116. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vernon AN, Sheeler LR, Biscotti CV and
Stoller JK: Pleural effusion resulting from metastatic papillary
carcinoma of the thyroid. Chest. 101:1448–1450. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kovacs CS, Nguyen GK, Mullen JC and
Crockford PM: Cardiac tamponade as the initial presentation of
papillary thyroid carcinoma. Can J Cardiol. 10:279–281.
1994.PubMed/NCBI
|
|
7
|
Nomori H, Horio H, Mimura T and Morinaga
S: Massive hemoptysis from an endobronchial metastasis of thyroid
papillary carcinoma. Thorac Cardiovasc Surg. 45:205–207. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siddaraju N, Viswanathan VK, Saka VK, Basu
D and Shanmugham C: Fine needle aspiration of follicular variant of
papillary thyroid carcinoma presenting with pleural effusion: A
case report. Acta Cytol. 51:911–915. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu KF, Hsieh CB, Duh QY, Chien CF, Li HS
and Shih ML: Hürthle cell carcinoma of the thyroid with
contralateral malignant pleural effusion. Onkologie. 32:47–49.
2009.PubMed/NCBI
|
|
10
|
Jeon MJ, Yim JH, Kim EY, Kim WG, Kim TY,
Kim WB and Shong YK: Four cases of malignant pleural effusion in
patients with papillary thyroid carcinoma. Endocrinol Metab.
26:330–334. 2011. View Article : Google Scholar
|
|
11
|
Olson MT, Nuransoy A and Ali SZ: Malignant
pleural effusion resulting from metastasis of thyroid primaries: A
cytomorphological analysis. Acta Cytol. 57:177–183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenstengel A, Lim EM, Millward M and Lee
YG: A distinctive colour associated with high iodine content in
malignant pleural effusion from metastatic papillary thyroid
cancer: A case report. J Med Case Rep. 7:1472013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abe T, Suzuki M, Shimizu K, Shinagawa N,
Oizumi S, Matsuno Y, Miyazaki M, Tanino M, Tanaka S and Nishimura
M: Anaplastic transformation of papillary thyroid carcinoma in
multiple lung metastases presenting with a malignant pleural
effusion: A case report. J Med Case Rep. 8:4602014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakamoto RI, Sumida LC, Lum CA and
Tauchi-Nishi PS: Recurrent papillary thyroid carcinoma with pleural
metastasis diagnosed by effusion cytology: A report of cases with
clinicopathologic correlation. Hawaii J Med Public Health.
74:51–56. 2015.PubMed/NCBI
|
|
15
|
Vyas M and Harigopal M: Metastatic thyroid
carcinoma presenting as malignant pleural effusion: A cytologic
review of 5 cases. Diagn Cytopathol. 44:1085–1089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomoda C, Ogimi Y, Saito F, Masaki C,
Akaishi J, Matsuzu K, Suzuki A, Uruno T, Ohkuwa K, Shibuya H, et
al: Outcome and characteristics of patients with malignant pleural
effusion from differentiated thyroid carcinoma. Endocr J.
63:257–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim H, Park YW, Oh YH, Sim J, Ro JY and
Pyo JY: Anaplastic transformation of papillary thyroid carcinoma
only seen in pleural metastasis: A case report with review of the
literature. Head Neck Pathol. 11:162–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kosmas K, Tsonou A, Mitropoulou G, Salemi
E, Kazi D and Theofanopoulou A: Malignant pleural effusion from
papillary thyroid carcinoma diagnosed by pleural effusion cytology:
A case report. Diagn Cytopathol. 46:204–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uchida T, Yamaguchi H, Nagamine K,
Yonekawa T, Nakamura E, Shibata N, Kawano F, Asada Y and Nakazato
M: Rapid pleural effusion after discontinuation of lenvatinib in a
patient with pleural metastasis from thyroid cancer. Endocrinol
Diabetes Metab Case Rep. 2019:EDM1801582019.PubMed/NCBI
|
|
20
|
Heffner JE: Diagnosis and management of
malignant pleural effusions. Respirology. 13:5–20. 2008.PubMed/NCBI
|
|
21
|
Casal RF, Eapen GA, Morice RC and Jimenez
CA: Medical thoracoscopy. Curr Opin Pulm Med. 15:313–320. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kastelik JA: Management of malignant
pleural effusion. Lung. 191:165–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parwani AV, Chan TY and Ali SZ:
Significance of psammoma bodies in serous cavity fluid: A
cytopathologic analysis. Cancer. 102:87–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zahid I, Routledge T, Billè A and Scarci
M: What is the best treatment for malignant pleural effusions?
Interact Cardiovasc Thorac Surg. 12:818–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodriguez-Panadero F and Romero-Romero B:
Management of malignant pleural effusions. Curr Opin Pulm Med.
17:269–273. 2011. View Article : Google Scholar : PubMed/NCBI
|