Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second
most common primary liver cancer diagnosed worldwide (1–3). In the
past decades, the incidence of ICC has been rising worldwide,
including Europe, North America, Asia, Japan and Australia
(4). In a 30-year period the
incidence of ICC increased 165% in the United States to 0.95 cases
per 100,000 (4). Despite the
continuous development of therapeutic options, including surgery,
chemotherapy and radiotherapy, the prognosis for ICC remains poor
(5). The molecular mechanisms
underlying the invasion and metastasis of ICC remain unclear.
Identifying these mechanisms, and therefore, novel molecular
biomarkers, is crucial for early disease detection, prognostic
evaluation and the development novel treatment strategies for
ICC.
The epithelial-mesenchymal transition (EMT), a
series of events during which epithelial cells lose many of their
epithelial characteristics to gain a mesenchymal phenotype, is a
pivotal mechanism in tumor progression and metastasis (6,7). The
hallmark of EMT comprises the downregulation of epithelial
molecules, such as E-cadherin, Keratin 19 and mucin-1, cell surface
associated, and the upregulation of mesenchymal molecules,
including Vimentin, S100 calcium binding protein A4 (S100A4),
N-cadherin, fibronectin and β-catenin (7–9). A
number of transcription factors, including Snail family
transcriptional repressor 1 (Snail), Snail family transcriptional
repressor 2 (Slug), Twist family BHLH transcription factor (Twist),
Zinc finger E-box binding homeobox 1 (Zeb1) and Zeb2, are also
known to serve a central role in the activation of EMT (8–10). This
process has been associated with tumor invasion, metastasis and a
poor prognosis in various types of gastrointestinal tumor,
including esophageal, gastric, colorectal and hepatic carcinomas
(11–14).
Numerous EMT-associated proteins have been suggested
to be associated with tumor progression in ICC (15–19).
However, to the best of our knowledge, no previous study has been
performed to evaluate the EMT process in ICC through the
measurement of a large number of EMT-associated markers.
In the present study, the association between the
expression of six representative EMT-associated proteins, including
E-cadherin, N-cadherin, Vimentin, Snail, Slug and S100A4, and
survival in ICC was assessed. In addition, the clinicopathological
significance and prognostic value of the expression profile of
EMT-related proteins, based on the number of alterations in the
expression of these proteins, were also investigated to evaluate
the clinical significance of EMT-associated proteins in patients
with ICC.
Materials and methods
Patients
A panel of 109 surgical ICC tissue specimens was
obtained from patients undergoing curative resection at the
Shandong Provincial Hospital Affiliated to Shandong University
(Jinan, China) between January 2010 and December 2015.
Clinicopathological parameters, including age, sex, tumor size,
histological differentiation, surgical margin, lymph node
metastasis and Tumor-Node-Metastasis (TNM) stage (20), were obtained by reviewing clinical
and pathological records (Table I).
The patients included 60 males and 49 females (mean, 57.4 years;
range, 39–75 years); all patients were followed-up. The follow-up
period ranged from 5–73 months (mean, 26.3 months). None of the
patients received neoadjuvant chemotherapy, radiotherapy or
immunotherapy prior to surgery. The tumor stage was diagnosed by
two certified pathologists of Shandong Provincial Hospital
Affiliated to Shandong University, according to the TNM
classification defined by the Union for International Cancer
Control (20). The study was
approved by the Institutional Ethics Committee of Shandong
Provincial Hospital Affiliated to Shandong University, and informed
written consent was obtained from each patient.
 | Table I.Association between EMT status and
clinicopathological features in patients with intrahepatic
cholangiocarcinoma. |
Table I.
Association between EMT status and
clinicopathological features in patients with intrahepatic
cholangiocarcinoma.
| Variables | Cases (n=109) | Group 1 (low EMT
expression, n=75) | Group 2 (high EMT
expression, n=34) | P-value |
|---|
| Sex |
| Male | 60 | 41 | 19 |
|
|
Female | 49 | 34 | 15 | 0.906 |
| Age (years) |
| ≤60 | 56 | 38 | 18 |
|
|
>60 | 53 | 37 | 16 | 0.826 |
| Tumor size
(cm) |
| ≤4 | 47 | 33 | 14 |
|
|
>4 | 62 | 42 | 20 | 0.783 |
| Macroscopic
types |
|
Mass-forming type | 74 | 52 | 22 |
|
|
Non-mass-forming type | 35 | 23 | 12 | 0.632 |
| Histological
differentiation |
|
Well/moderate | 69 | 49 | 20 |
|
|
Poor/undifferentiated | 40 | 26 | 14 | 0.514 |
| Surgical
margin |
|
Positive | 28 | 17 | 11 |
|
|
Negative | 81 | 58 | 23 | 0.284 |
| Vascular
invasion |
|
Positive | 38 | 25 | 13 |
|
|
Negative | 71 | 50 | 21 | 0.619 |
| Lymph node
metastasis |
|
Positive | 32 | 17 | 15 |
|
|
Negative | 77 | 58 | 19 | 0.023a |
| T stage |
|
T1+T2 | 66 | 45 | 21 |
|
|
T3+T4 | 43 | 30 | 13 | 0.861 |
| TNM stage (17) |
|
I+II | 61 | 52 | 9 |
|
|
III+IV | 48 | 23 | 25 |
<0.001a |
Immunohistochemistry
Briefly, 4 µm thick sections of the 4%
formalin-fixed, paraffin-embedded surgical specimens were baked at
60°C for at least 2 h, and then were dewaxed in xylene and
rehydrated using a descending alcohol series (100% for 5 min, 85%
for 5 min, 75% for 5 min, distilled water). They were placed in a
glass container filled with 10 mmol/l citrate buffer (pH 6.0) and
heated in a microwave for 15 min for antigen retrieval. Endogenous
peroxidase activity was blocked by incubation in 3%
H2O2 at room temperature for 15 min. Goat
serum (1%) (cat. no., AR1009; Wuhan Boster Biological Technology,
Ltd.) was applied to sections to block nonspecific binding for 5
min at room temperature. Sections were then incubated overnight at
4°C with primary antibodies, including: Rabbit anti-E-cadherin
(cat. no., ab40772; 1:200; Abcam), anti-N-cadherin (cat. no.,
ab76011; 1:200; Abcam), anti-Vimentin (cat. no., ab92547; 1:200;
Abcam), anti-S100A4 (cat. no., ab124805; 1:200; Abcam), anti-Snail
(cat. no., 13099-1-AP; 1:100; ProteinTech Group, Inc.) or anti-Slug
(cat. no., 12129-1-AP; 1:100; ProteinTech Group, Inc.). The
sections were then treated with HRP-labelled universal secondary
antibody (cat. no., K5007; Dako) for 30 min at 37°C. Slides were
washed with PBS in triplicate and 3,3′-diaminobenzidine solution
was added for 2–3 min at room temperature, which was incubated
until the desired staining was achieved. Then the sections were
counterstained with hematoxylin for 3–5 min at room temperature,
and then dehydrated and mounted. The slides were observed using a
light microscope (magnification, ×400).
Evaluation of immunohistochemical
staining
The degree of immunostaining of the sections was
blindly evaluated semi-quantitatively by two pathologists of
Shandong Provincial Hospital Affiliated to Shandong University,
unaware of any clinical information. For each section, five
high-power fields using light microscope (magnification, ×400) were
randomly selected. For the evaluation of E-cadherin expression,
staining within the membrane was considered as positive
immunostaining. For evaluation of N-cadherin, Vimentin, Snail, Slug
and S100A4 expression, staining in the cytoplasm and/or the nucleus
was considered positive immunostaining. The expression of
E-cadherin was considered low if the tumor cells exhibited weaker
staining patterns than the normal epithelium, or when no staining
was observed. The staining of N-cadherin, Vimentin, Snail, Slug and
S100A4 were evaluated on the basis of staining intensity and the
proportion of positive cells. The tissue sections were scored based
on the percentage of immunostained cells as follows: 0, <5; 1,
5–25; 2, 26–50 and 3, >51%. Sections were also scored on the
basis of staining intensity: 0, negative; 1, weak; 2, moderate and
3, strong (21). A final score was
obtained by multiplying the intensity and percentage scores. Tumors
were divided into low (total score of 0–2) and high (a total score
of >2) expression groups.
Statistical analysis
The associations between alterations in the
expression of EMT proteins and clinicopathological variables were
examined by a χ2 test. Survival curves were produced
using the Kaplan-Meier method and compared with the log-rank test.
Univariate and multivariate analyses were performed to identify
independent prognostic factors using the Cox proportional hazards
regression model. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS 17.0 software (SPSS Inc., Chicago, IL,
USA).
Results
Expression of EMT-associated proteins
in primary ICC
Fig. 1 shows
representative images of immunohistochemical staining for the
EMT-associated proteins in the tumor tissue samples. Low E-cadherin
expression was observed in 63 (57.8%) of the samples. Regarding the
mesenchymal markers and transcription factors, 39 (35.8%) of the
samples exhibited high Vimentin, 42 (38.5%) exhibited high S100A4
expression; 35 (32.1%), 38 (34.9%) and 27 (24.8%) samples revealed
upregulated N-cadherin, Snail and Slug protein expression,
respectively.
 | Figure 1.Representative immunohistochemical
staining for the EMT-associated proteins in intrahepatic
cholangiocarcinoma. The low expression of (A) E-cadherin, and high
expression of (B) N-cadherin, (C) Snail, (D) Slug, (E) Vimentin and
(F) S100A4 were observed in tumor tissues (the black arrows,
magnification, ×400). Snail, Snail family transcriptional repressor
1; Slug, Snail family transcriptional repressor 2; S100A4, S100
calcium binding protein A4; EMT, epithelial-mesenchymal
transition. |
Association between EMT-associated
protein expression and patient survival time
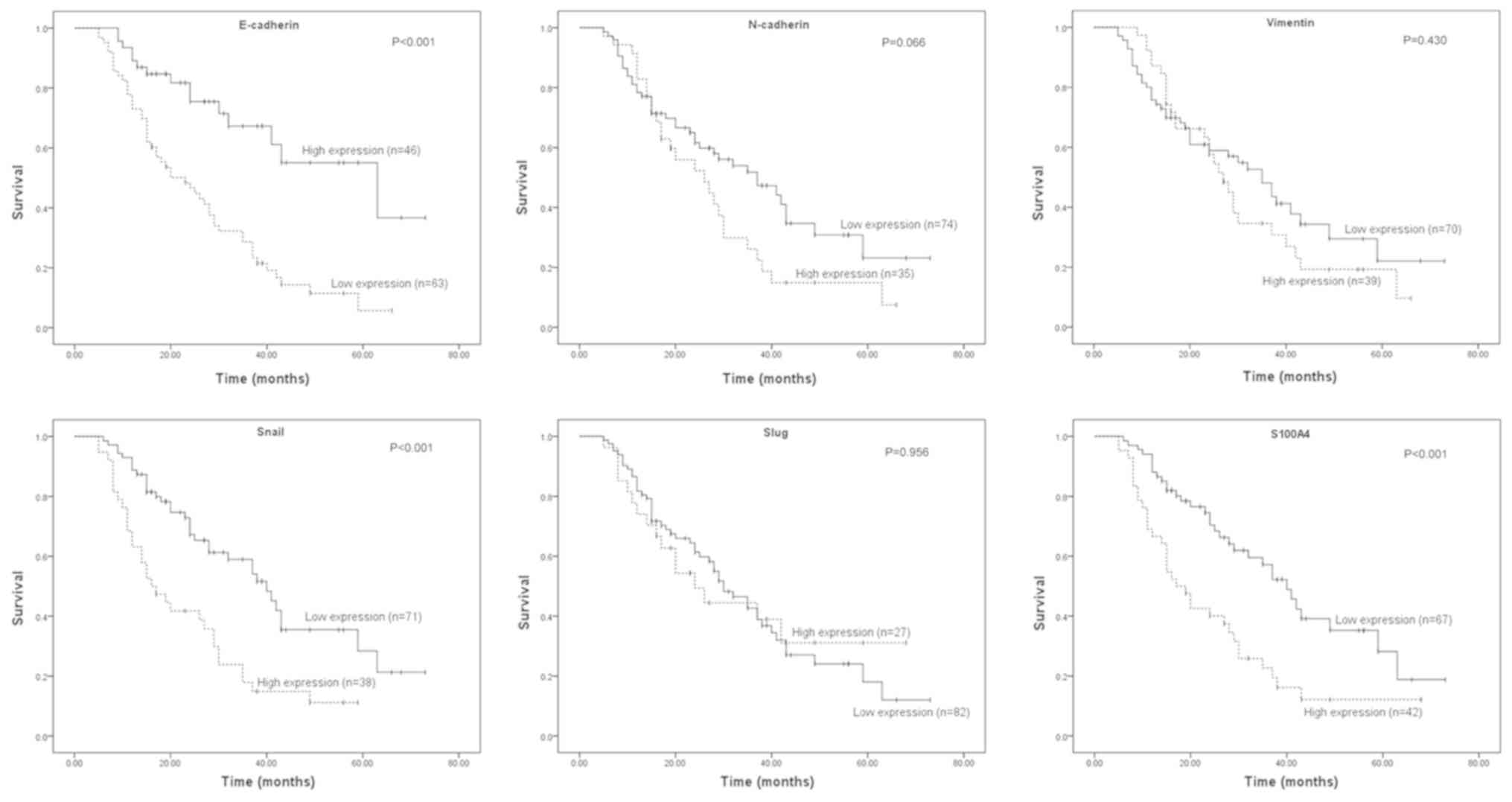
The log-rank test was used to identify the
differences in patient survival time with respect to the expression
of the six EMT-associated proteins (Fig.
2). During the follow-up period, a total of 67 (61.5%) of the
patients succumbed to ICC. In terms of epithelial markers, the
survival time for patients with tumors with low expression of
E-cadherin was significantly lower than the rate for patients with
high expression of E-cadherin (P<0.001). In terms of mesenchymal
markers and transcription factors, increased expression of S100A4
(P<0.001) and Snail (P<0.001) was associated with a lower
survival rate compared with the low expression group. However,
there were no significant differences in survival time between the
high and low expression groups with respect to N-cadherin
(P=0.066), Slug (P=0.956) and Vimentin (P=0.430) expression.
Association between EMT status and the
survival of patients
According to the number of downregulated epithelial
proteins and upregulated mesenchymal and transcription proteins in
each patient, the EMT status of patients was categorized into 2
groups: i) Group 1: (low EMT expression, score, 0–3; n=75), with
the alteration number of ≤3; ii) Group 2: (high EMT expression,
score, ≥4; n=34), with the alteration number of ≥4).
The characteristics of the patients from each group
are outlined in Table I.
χ2 analysis showed that patients with high EMT
expression exhibited a significantly greater likelihood of lymph
node metastasis (P=0.023) and a higher TNM stage (P<0.001).
The cumulative survival rates at 1, 2 and 5 years
were 61.8, 25.2 and 3.2%, respectively, in the high EMT expression
group, whereas they were 88.0, 74.1 and 31.5% in the low EMT
expression group. The survival rate for patients with high EMT
expression was significantly lower than that in patients with low
EMT expression (P<0.001; Fig.
3).
Univariate and multivariate analysis were performed
to identify independent prognostic factors by using the Cox
proportional hazards regression model. Univariate analysis
demonstrated that the significant prognostic factors included EMT
status (P<0.001), lymph node metastasis (P=0.025), vascular
invasion (P=0.027), surgical margin (P<0.001) and TNM stage
(P<0.001). The above five significant factors were analyzed by
multivariate analysis and it was indicated that EMT status
(P=0.004), surgical margin (P=0.013) and TNM stage (P<0.001)
were independent prognostic factors for overall survival rate
(Table II).
 | Table II.Univariate and multivariate analysis
of prognostic factors for overall survival rate. |
Table II.
Univariate and multivariate analysis
of prognostic factors for overall survival rate.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 1.079 | 0.666–1.749 | 0.757 | – | – | – |
| Age (≤60 vs.
>60) | 0.940 | 0.582–1.519 | 0.800 | – | – | – |
| Tumor size (≤4 vs.
>4 cm) | 1.221 | 0.748–1.995 | 0.425 | – | – | – |
| Macroscopic types
(mass-forming vs. non-mass-forming) | 1.543 | 0.935–2.546 | 0.090 | – | – | – |
| Histological
differentiation (well/moderate vs. poor/undifferentiated) | 0.867 | 0.519–1.448 | 0.586 | – | – | – |
| Surgical margin
(positive vs. negative) | 3.876 | 2.196–6.841 |
<0.001a | 2.218 | 1.185–4.152 | 0.013a |
| Vascular invasion
(positive vs. negative) | 1.741 | 1.065–2.844 | 0.027a | 1.296 | 0.739–2.275 | 0.365 |
| Lymph node
metastasis (positive vs. negative) | 1.813 | 1.077–3.052 | 0.025a | 1.398 | 0.806–2.426 | 0.233 |
| T stage (T1+T2 vs.
T3+T4) | 1.003 | 0.611–1.647 | 0.991 | – | – | – |
| TNM stage (17)
(I+II vs. III+IV) | 7.714 | 4.293–13.859 |
<0.001a | 4.919 | 2.585–9.359 |
<0.001a |
| EMT status (high
vs. low) | 4.180 | 2.529–6.908 |
<0.001a | 2.305 | 1.311–4.050 | 0.004a |
Discussion
EMT serves a crucial role in cancer invasion,
metastasis and progression (6,7).
Although numerous EMT-associated markers have been reported to be
effective prognostic factors for patients who have undergone
curative resection in many types of digestive tumours, including
esophageal, gastric, colorectal and hepatic carcinomas (11–14), few
studies have focused on the expression and prognostic value of EMT
markers in ICC (15–19). Therefore, further investigation into
the prognostic value and clinical significance of EMT in ICC is
required. In the present study, the expression of six
EMT-associated proteins was analyzed in a relatively large cohort
of patients with ICC, in addition to their association with overall
survival rate. Furthermore, the association of EMT status, based on
the number of expression changes to EMT markers, with
clinicopathological factors and prognosis was also investigated to
determine the clinical significance of EMT in ICC. To the best of
our knowledge, this is the first study to evaluate the clinical
role of EMT, taking into consideration the expression of several
EMT proteins in ICC.
E-cadherin is expressed in the membranes of
epithelial cells and serves a vital role in cell adhesion and
movement (22). The loss of
E-cadherin expression promotes the migration and invasion of tumor
cells, and is a critical step of EMT in the development of
malignant carcinomas (23). A number
of transcription factors, including Snail, Slug, Twist, Zeb1 and
Zeb2, induce EMT by downregulating E-cadherin, and upregulating
mesenchymal factors, such as N-cadherin, Vimentin and S100A4,
through a number of different signalling cascades, such as the
signal transducer and activator of transcription 3,
mitogen-activated protein kinase and Wnt pathways (8–10,24). In
the present study, the downregulation of an epithelial marker
(E-cadherin) and the upregulation of mesenchymal markers (Vimentin,
N-cadherin and S100A4) were detected. The EMT transcription factors
Snail and Slug were also highly expressed in ICC tissue samples.
These representative characteristic changes confirmed the
occurrence of EMT in ICC tissue. Furthermore, survival analysis
showed that reductions in E-cadherin expression, and increased
expression of S100A4 and Snail was associated with significantly
shorter overall survival time.
S100A4 is a typical fibroblast marker of EMT;
furthermore, it is involved in the regulation of various biological
processes, including cell proliferation, extracellular matrix
remodelling, cell motility, cell detachment and angiogenesis
(25). S100A4 expression has been
reported to be significantly associated with cancer aggressiveness
and a worse prognosis for patients with several types of cancer,
such as pancreatic, bladder, gallbladder, breast, ovarian,
colorectal and gastric cancer, and non-small cell lung carcinoma,
and may be a useful marker of metastatic potential with prognostic
significance (26). S100A4
expression has also been reported to increase the invasiveness and
metastasis of cholangiocarcinoma in vitro and in vivo
(27). In our previous study, the
high expression of S100A4 was identified as an independent
predictor for reduced overall survival time in ICC (21). The outcomes in the present larger
scale study were consistent with the aforementioned studies.
Among the EMT transcription factors, the Snail
family, including Snail and Slug, are the most extensively studied
(28–31). Slug and Snail have been identified to
serve key roles in the development of several types of carcinoma,
including renal, breast, prostate and ovarian carcinomas (28–31). The
Snail family facilitates the metastatic potential of tumors by
promoting cell migration, inhibiting cell-cell adhesion and
enhancing tumor invasiveness (32).
The overexpression of Snail potently inhibits the expression of
E-cadherin and induces EMT (33,34). The
inhibition of Slug expression by RNA interference is associated
with upregulated E-cadherin expression and decreased cell invasion
in vitro (35). In patients
with ICC or hilar cholangiocarcinoma, high expression of Snail was
reported to be associated with aggressive tumor characteristics and
poor prognosis (16,32). In ICC, the expression of Slug has
been associated with lymph node invasion, lymphovascular invasion
and distant metastasis, as well as acting as an independent
indicator of poor prognosis (36).
In the present study, the increased expression of Snail was
associated with reduced overall survival for patients with ICC
following surgical resection. However, we reported that upregulated
Slug did not predict the unfavorable survival outcomes for patients
with ICC.
Other EMT-associated proteins, such as β-catenin,
N-cadherin and Slug, have also previously been identified as
reliable prognostic indicators and indicators for the likelihood of
tumor invasion in many types of cancer, including ICC (15). However, only the E-cadherin, S100A4
and Snail expression levels were observed to be associated with
poor survival in the present study. The differences in sample size,
antibodies used, patient characteristics, follow-up periods and
immunohistochemistry cut-off values could account for the differing
results (17). The combined
detection of EMT proteins could decrease the likelihood of bias. In
addition, EMT proteins may interact with each other during cancer
progression (19). The detection of
the co-expression of EMT proteins would therefore be expected to
have greater prognostic value than any single EMT protein.
Therefore, in the present study, the association between the
expression of a combination of EMT-related proteins with the
clinicopathological features and prognosis of patients with ICC was
determined. The frequency of EMT proteins dysregulation was used to
reflect the EMT status in each tumor sample. Patients with a higher
number of EMT protein alterations were more likely to exhibit lymph
node metastasis and a higher TNM stage, as well as poorer overall
prognosis. The results collectively demonstrated that EMT is a key
step in the progression of ICC.
In conclusion, the reduced expression of E-cadherin,
and the increased expression of Snail and S100A4 were significantly
associated with reduced overall survival time for patients with ICC
after curative resection. The EMT status, based on the number of
alterations in the expression of EMT-related proteins, was
associated with ICC progression, and may serve as an independent
prognostic indicator for ICC. Further investigations regarding the
upstream or downstream factors of EMT in different mechanisms could
provide the basis for the identification of diagnostic markers and
potential therapeutic targets for ICC.
Acknowledgements
The authors would like to thank Dr Edward C. Mignot
(Shandong University) for the linguistic advice.
Funding
This study was supported by Shandong Provincial Key
Research and Development Program (grant no. 2017GSF218026) and
Shandong Provincial Natural Science Foundation (grant no.
ZR2017PH001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
XT and CZ were responsible for study concept and
design. XT, ZC, QD and ZL performed the experiments. QD and ZL
analysed the data. XT and CZ wrote the present manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Ethics
Committee (Shandong Provincial Hospital Affiliated to Shandong
University), and informed written consent was obtained from each
patient.
Patient consent for publication
Written informed consent was obtained from each
patient to publish the present study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ICC
|
intrahepatic cholangiocarcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bridgewater J, Galle PR, Khan SA, Llovet
JM, Park JW, Patel T, Pawlik TM and Gores GJ: Guidelines for the
diagnosis and management of intrahepatic cholangiocarcinoma. J
Hepatol. 60:1268–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta A and Dixon E: Epidemiology and risk
factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr.
6:101–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Shen F, Han J, Shen YN, Xie GQ,
Wu MC and Yang T: Epidemiology and surgical management of
intrahepatic cholangiocarcinoma. Hepat Oncol. 3:83–91. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaib YH, Davila JA, McGlynn K and
El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in
the United States: A true increase? J Hepatol. 40:472–477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial- mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wen J, Luo KJ, Liu QW, Wang G, Zhang MF,
Xie XY, Yang H, Fu JH and Hu Y: The epithelial-mesenchymal
transition phenotype of metastatic lymph nodes impacts the
prognosis of esophageal squamous cell carcinoma patients.
Oncotarget. 7:37581–37588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MA, Lee HS, Lee HE, Kim JH, Yang HK
and Kim WH: Prognostic importance of epithelial-mesenchymal
transition- related protein expression in gastric carcinoma.
Histopathology. 54:442–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giannelli G, Koudelkova P, Dituri F and
Mikulits W: Role of epithelial to mesenchymal transition in
hepatocellular carcinoma. J Hepatol. 65:798–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaquero J, Guedj N, Clapéron A, Nguyen
Ho-Bouldoires TH, Paradis V and Fouassier L: Epithelial-mesenchymal
transition in cholangiocarcinoma: From clinical evidence to
regulatory networks. J Hepatol. 66:424–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang XY, Zhang C, Cai JB, Shi GM, Ke AW,
Dong ZR, Zhang PF, Fan J, Peng BG and Zhou J: Comprehensive
multiple molecular profile of epithelial mesenchymal transition in
intrahepatic cholangiocarcinoma patients. PLoS One. 9:e968602014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nitta T, Mitsuhashi T, Hatanaka Y,
Miyamoto M, Oba K, Tsuchikawa T, Suzuki Y, Hatanaka KC, Hirano S
and Matsuno Y: Prognostic significance of epithelial-mesenchymal
transition-related markers in extrahepatic cholangiocarcinoma:
Comprehensive immunohistochemical study using a tissue microarray.
Br J Cancer. 111:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao X, Wang X, Wang Z, Dai L, Zhang G, Yan
Q and Zhou W: Clinicopathological and prognostic significance of
epithelial mesenchymal transition-related protein expression in
intrahepatic cholangiocarcinoma. Onco Targets Ther. 5:255–261.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryu HS, Chung JH, Lee K, Shin E, Jing J,
Choe G, Kim H, Xu X, Lee HE, Kim DG, et al: Overexpression of
epithelial-mesenchymal transition-related markers according to cell
dedifferentiation: Clinical implications as an independent
predictor of poor prognosis in cholangiocarcinoma. Hum Pathol.
43:2360–2370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM Classification of Malignant Tumours. 7th edition.
Wiley-Blackwell; Oxford: pp. 122–128. 2009
|
|
21
|
Tian XG, Wang QZ, Li Y, Hu JH, Wu L, Ding
Q and Zhang C: The expression of S100A4 protein in human
intrahepatic cholangiocarcinoma: Clinicopathologic significance and
prognostic value. Pathol Oncol Res. 21:195–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiozaki H, Tahara H, Oka H, Miyata M,
Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M, et
al: Expression of immunoreactive E-cadherin adhesion molecules in
human cancers. Am J Pathol. 139:17–23. 1991.PubMed/NCBI
|
|
23
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazzucchelli L: Protein S100A4: Too long
overlooked by pathologists? Am J Pathol. 160:7–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boye K and Maelandsmo GM: S100A4 and
metastasis: A small actor playing many roles. Am J Pathol.
176:528–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fabris L, Cadamuro M, Moserle L, Dziura J,
Cong X, Sambado L, Nardo G, Sonzogni A, Colledan M, Furlanetto A,
et al: Nuclear expression of S100A4 calcium-binding protein
increases cholangiocarcinoma invasiveness and metastasization.
Hepatology. 54:890–899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mikami S, Katsube K, Oya M, Ishida M,
Kosaka T, Mizuno R, Mukai M and Okada Y: Expression of Snail and
Slug in renal cell carcinoma: E-cadherin repressor Snail is
associated with cancer invasion and prognosis. Lab Invest.
91:1443–1458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurrey NK, K A and Bapat SA: Snail and
Slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Emadi Baygi M, Soheili ZS, Schmitz I,
Sameie S and Schulz WA: Snail regulates cell survival and inhibits
cellular senescence in human metastatic prostate cancer cell lines.
Cell Biol Toxicol. 26:553–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blechschmidt K, Sassen S, Schmalfeldt B,
Schuster T, Höfler H and Becker KF: The E-cadherin repressor Snail
is associated with lower overall survival of ovarian cancer
patients. Br J Cancer. 98:489–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kong D, Liang J, Li R, Liu S, Wang J,
Zhang K and Chen D: Prognostic significance of snail expression in
hilar cholangiocarcinoma. Braz J Med Biol Res. 45:617–624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Poser I, Dominguez D, de Herreros AG,
Varnai A, Buettner R and Bosserhoff AK: Loss of E-cadherin
expression in melanoma cells involves up-regulation of the
transcriptional repressor Snail. J Biol Chem. 276:24661–24666.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cano A, Perez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang KJ, Wang DS, Zhang SY, Jiao XL, Li
CW, Wang XS, Yu QC and Cui HN: The E-cadherin repressor slug and
progression of human extrahepatic hilar cholangiocarcinoma. J Exp
Clin Cancer Res. 29:882010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang KJ, Zhang BY, Zhang KP, Tang LM, Liu
SS, Zhu DM and Zhang DL: Clinicopathologic significance of slug
expression in human intrahepatic cholangiocarcinoma. World J
Gastroenterol. 16:2554–2557. 2010. View Article : Google Scholar : PubMed/NCBI
|