Introduction
Small-cell lung cancer (SCLC) is a highly aggressive
malignancy with early development of widespread metastases, in
addition to a limited number of effective treatments and
particularly poor prognoses (1–3). SCLC
accounted for ~15% of all diagnosed novel lung cancer cases
worldwide between 1995 and 2011 (4,5).
Recently, SCLC-targeted therapy research has
progressed. Delta-like protein 3 (DLL3), an inhibitory Notch
ligand, is particularly upregulated in SCLC (6–8).
Rovalpituzumab tesirine (Rova-T), a novel DLL3-targeted
antibody-drug conjugate, has demonstrated in vivo efficacy
in eradicating DLL3-expressing tumor-initiating cells in SCLC
patient-derived xenograft tumors (7). A phase I clinical trial demonstrated
single-agent antitumor activity of Rova-T in patients with
recurrent SCLC (6,9). The objective response rate and
disease-control rate were 38 (10/26) and 88% (23/26) in the
DLL3-high patient subgroup compared with 0 (0/8) and 50% (4/8) in
the DLL3-low subgroup (6,9). This early clinical trial provides
evidence that DLL3 is a potential predictive molecular marker for
DLL3-targeted treatment. Although a recent study reported that
DLL3-high expression was not a prognostic factor for Japanese
patients with SCLC (10), the
prognostic value of DLL3 for patients from China remains
unknown.
Biopsies are used often in clinical practice to
determine SCLC diagnoses prior to treatment. According to our
previous studies, in Guangdong Provincial People's Hospital between
January 2006 and June 2015, 93% of SCLC cases were diagnosed based
on biopsy specimens (unpublished data). Accurate diagnosis is the
premise of accurate treatment. However, sampling bias caused by
intratumoral and intertumoral heterogeneity may reduce its value as
a biomarker for targeted therapy (11). Therefore, it is crucial for any
ongoing and future clinical trials to examine the DLL3 expression
pattern. At present, the DLL3 expression pattern in SCLC remains
unknown.
SCLC is positive for thyroid transcription factor-1
(TTF-1) in ≤90-95% of cases due to its neuroendocrine
differentiation (12–14). TTF-1 protein is routinely used for
differential diagnosis in clinical pathology labs (15). A previous study revealed a positive
correlation between the expression of DLL3 and TTF-1 in SCLC,
suggesting the potential application of TTF-1 to predict the DLL3
expression level and response to targeted therapy (16). However, a limited number of studies
have been published on the prognostic significance of TTF-1 in
SCLC.
Taking this into consideration, the aim of the
present study was to examine the expression pattern of DLL3 in SCLC
by immunohistochemical staining of formalin-fixed,
paraffin-embedded (FFPE) tumor tissues. Furthermore, the
association between DLL3 and TTF-1 expression in SCLC was analyzed.
Finally, the prognostic value of protein markers DLL3 and TTF-1 was
examined.
Materials and methods
Human tissues
A retrospective study of patients with de
novo SCLC was performed. Hematoxylin and Eosin (H&E)
staining of the tissue sections included nuclear staining with 1%
hematoxylin for 5–15 min at room temperature and counterstaining
with 1% eosin for 2–3 min at room temperature. Expert lung cancer
pathologists Dr Yan-Hui Liu, Dr Li-Xu Yan and Dr Yu-Fa Li from The
Guangdong Provincial People's Hospital of Guangdong Academy of
Medical Sciences (Guangzhou, China) independently reviewed the
diagnoses of the histological samples according to the 2015 World
Health Organization classification (17). Inconsistent diagnoses were submitted
to the expert panel to reach a consensus diagnosis. A total of 335
cases were identified at Guangdong Provincial People's Hospital
(Guangzhou, China) between January 2006 and June 2015. All cases
had adequate tumor tissues and complete clinical and prognostic
data. Patients with only cytology specimens were excluded. Out of
the 335 patients, 11 had paired biopsy of primary site and
lobectomy specimens. A total of 37 patients had paired specimens of
primary and metastatic site, including mediastinal lymph node,
supraclavicular lymph node and distant metastatic site.
Tumor staging was classified according to the
8th Edition of The Tumor-Node-Metastasis Classification
for Lung Cancer (18). A total of
324 of the 335 patients received cytotoxic therapy (75 or 80
mg/m2 cisplatin on day 1 and 100 or 80 mg/m2
etoposide on days 1, 2 and 3), and 11 patients received surgery
with paired biopsy of primary site and lobectomy specimens. The
Research Ethics Committee of Guangdong Provincial People's Hospital
(Guangzhou, China) approved the present study (approval no.
GDREC2016373H).
Immunohistochemistry (IHC)
The specimens were fixed in 10% neutral buffered
formalin for 24 to 48 h at room temperature. Pretreated FFPE tumor
specimens were used for testing. Each tumor tissue block was
sectioned at 4 µm. Slides were stained with a DLL3-specific
antibody (dilution, 1:100; cat. no. ab103102; Abcam, Cambridge, UK)
at 37°C for 32 min. The OptiView DAB IHC Detection Kit (cat. no.
760–700; Ventana Medical Systems, Inc.), including blocking reagent
and secondary antibody, was used according to the manufacturer's
protocol. IHC staining was performed using an automated
immunostaining instrument (Ventana BenchMark XT; Ventana Medical
Systems, Inc., Tucson, AZ, USA) according to the manufacturer's
protocol. For the detection of TTF-1, IHC was performed with
anti-TTF-1 antibody (undiluted; cat. no. 790-4398; Ventana Medical
Systems, Inc.) combined with the same detection kit and procedures
as DLL3. DLL3-positive SCLC tissue was used as a positive control,
and DLL3-negative lung adenocarcinoma tissue was used as a negative
control; positive and negative control tumor slides were included
in each assay. An Olympus BX51 light microscope (magnification,
×20-400), equipped with a DP72 camera and DP2-BSW software
(Olympus, Tokyo, Japan), was used. Positive TTF-1 staining was
defined as >5% of tumor cells stained for the marker for TTF-1-
targeted treatment (13).
Scoring for DLL3
The results of immunohistochemical staining for DLL3
were semi-quantitatively evaluated using an immunohistochemical
H-score (HS) method (7,11,19).
Staining intensity of DLL3 was categorized into the following four
groups: i) 0, no membrane or cytoplasmic staining; ii) 1+, weak
membranous with or without cytoplasmic staining; iii) 2+, moderate
membranous with or without cytoplasmic staining; and iv) 3+, strong
membranous (observable with ×10 objective) with or without
cytoplasmic staining. HS (range, 0–300) was calculated using the
formula: HS=Σ(i+1) × Pi, in which i=staining intensity and
Pi=percentage of stained cells (7,11,19).
Statistical analysis
Statistical analyses were conducted using SPSS
version 20.0 (IBM Corp., Armonk, NY, USA). Wilcoxon matched-pairs
test was used to analyze DLL3 expression in paired samples. The
receiver operating characteristic (ROC) curve was used to define
the optimal cut-off value for DLL3 in predicting 5-year overall
survival rate (36.1%) of SCLCs. Pearson's χ2 test was
used to analyze the potential association between DLL3 level and
TTF-1 and the clinicopathological features. The concordance
analyses between paired specimens were estimated using Kappa test.
Kaplan-Meier curve with log-rank test was used to analyze the
impact of DLL3 expression levels on survival of patients.
Univariate and multivariate analysis was performed using a Cox
proportional hazard regression model. Multivariable models were
constructed using a forward selection (likelihood ratio, LR) test,
starting with variables with P<0.05 in univariable analyses.
Two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The clinical characteristics of the 335 patients
with SCLC are summarized in Table I.
The median age was 63 years (range, 34–87), and the majority of the
patients, 91.3%, were male (306/335), while 72.2% were smokers
(242/335). Distant metastasis at diagnosis was observed in
approximately one-half (50.7%) of the patients (170/335).
 | Table I.Association analysis between DLL3 and
TTF-1 expression and clinicopathological characteristics of
patients with small-cell lung cancer. |
Table I.
Association analysis between DLL3 and
TTF-1 expression and clinicopathological characteristics of
patients with small-cell lung cancer.
| Variables | Number of patients,
N=335 (%) | DLL3-Low, N=126
(%) | DLL3-High, N=209
(%) | P-value | TTF-1 negative, N=65
(%) | TTF-1 positive, N=270
(%) | P-value |
|---|
| Age (years) |
| ≤60 | 143 (42.7) | 51 (40.5) | 92 (44.0) | 0.525 | 25 (38.5) | 118 (43.7) | 0.443 |
|
>60 | 192 (57.3) | 75 (59.5) | 117 (56.0) |
| 40 (61.5) | 152 (56.3) |
|
| Sex |
|
Female | 29 (8.7) | 16 (12.7) | 13 (6.2) | 0.041a | 8 (12.3) | 21 (7.8) | 0.244 |
| Male | 306 (91.3) | 110 (87.3) | 196 (93.8) |
| 57 (87.7) | 249 (92.2) |
|
| Smoking history |
|
Non-smokersc | 93 (27.8) | 44 (34.9) | 49 (23.4) | 0.023a | 17 (26.2) | 76 (28.1) | 0.747 |
|
Smokers | 242 (72.2) | 82 (65.1) | 160 (76.6) |
| 48 (73.8) | 194 (71.9) |
|
| Distant
metastasis |
|
Negative | 165 (49.3) | 69 (54.8) | 96 (45.9) | 0.117 | 38 (58.5) | 127 (47.0) | 0.098 |
|
Positive | 170 (50.7) | 57 (45.2) | 113 (54.1) |
| 27 (41.5) | 143 (53.0) |
|
| TNM stage (18) |
| I | 12 (3.6) | 5 (4.0) | 7 (3.3) | 0.661b | 4 (6.2) | 8 (3.0) | 0.244 |
| II | 17 (5.1) | 7 (5.6) | 10 (4.8) |
| 4 (6.2) | 13 (4.8) |
|
|
III | 136 (40.6) | 57 (45.2) | 79 (37.8) |
| 30 (46.2) | 106 (39.3) |
|
| IV | 170 (50.7) | 57 (45.2) | 113 (54.1) |
| 27 (41.5) | 143 (53.0) |
|
| TTF-1 |
|
Negative | 65 (19.4) | 34 (27.0) | 31 (14.8) | 0.006a | – | – | – |
|
Positive | 270 (80.6) | 92 (73.0) | 178 (85.2) |
| – | – |
|
Cut-off value for DLL3
The optimal cut-off value for DLL3 in predicting
overall survival of patients with SCLC was determined by ROC curves
(data not shown). DLL3-low was defined as an H-score ≤150 and
DLL3-high was defined as an H-score >150.
Expression pattern of DLL3 protein in
SCLCs
Tumor cells labeled by DLL3 exhibited a membranous
staining with or without a cytoplasmic staining pattern as
previously reported (6).
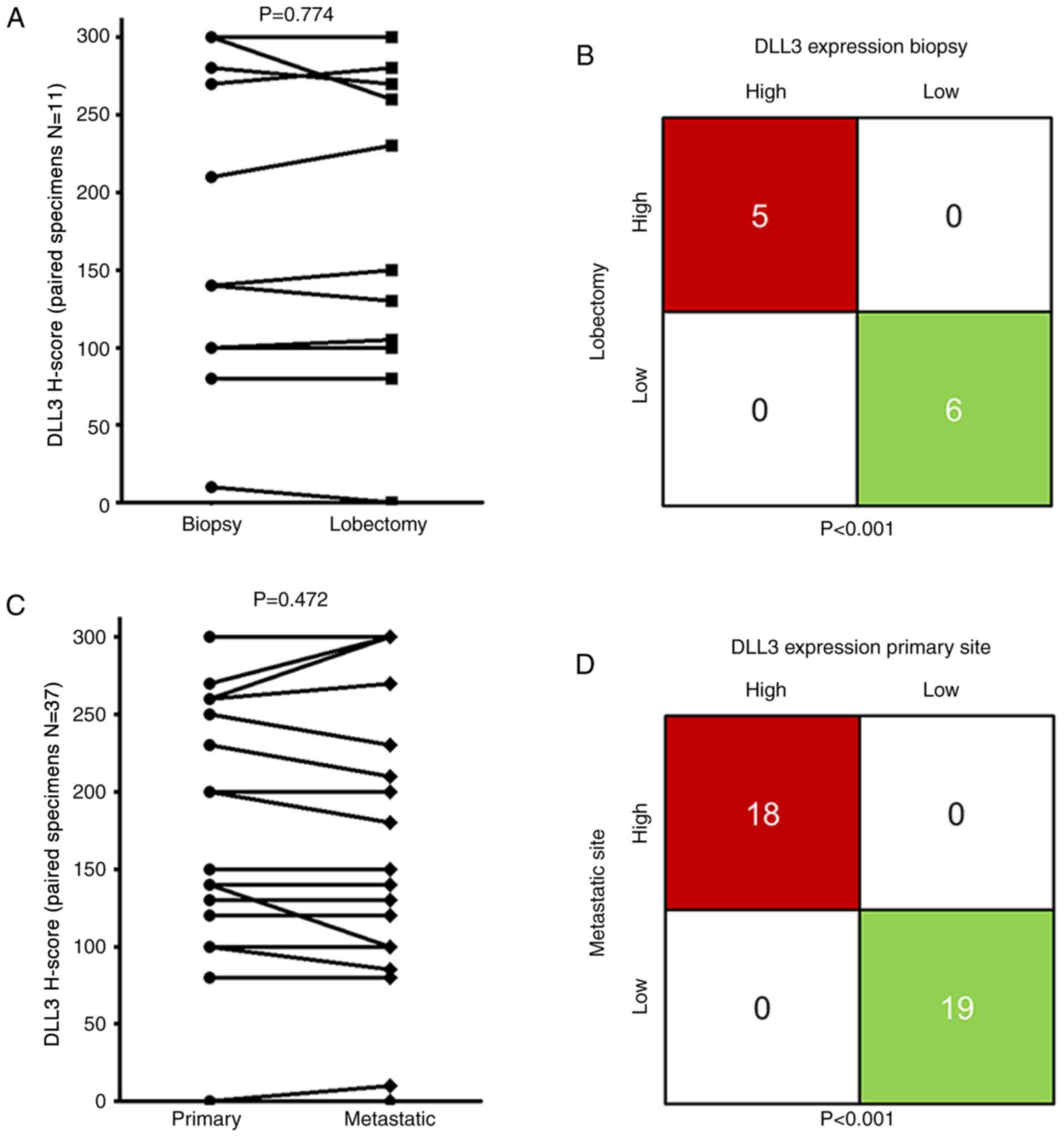
Representative images of DLL3 protein are indicated in Fig. 1. Intratumoral distribution of DLL3
indicated homogeneity in 11 lobectomy specimens (Fig. 2). To compare intratumoral expression
of DLL3, paired biopsy of primary site and lobectomy specimens were
used (Fig. 3A). No difference in
DLL3 H-scores was observed in paired biopsy [median HS, 140;
interquartile range (IQR), 100–280] and lobectomy specimens (median
HS, 150; IQR 100–270; Wilcoxon test; P=0.774; H-score). Concordant
staining, either high or low for DLL3 in paired biopsy and
lobectomy specimens, was observed in 11 out of the 11 (100%) SCLCs.
Kappa test indicated significant concordance between the paired
specimens (P<0.001; Fig. 3B).
To compare intertumoral expression of DLL3, paired
biopsies of primary and metastatic sites were used; no difference
of DLL3 H-scores was observed in paired primary sites (median HS,
150; IQR, 100–260) and metastatic sites (median HS, 150; IQR,
100–285; P=0.472; Fig. 3C).
Concordant staining, either high or low for DLL3 in paired
specimens of primary and metastatic sites, was also observed in 37
of the 37 (100%) SCLCs (P<0.001; Fig.
3D).
Association of DLL3 and TTF-1 with
clinicopathological characteristics
SCLC with high DLL3 expression levels were more
frequent in males (P=0.041), smokers (P=0.023) and TTF-1 expression
(P=0.006) compared with SCLCs with low DLL3 expression levels
(Table I). There was no significant
association of DLL3 expression level with age, distant metastasis
status or TNM stage. No significant association of TTF-1 expression
with clinical characteristics was observed.
DLL3 combined with TTF-1 predicts
survival of SCLC
The median follow-up time for the patients with
SCLCs was 11.3 months (range, 0.1–75.1). The extent to which
analysis of DLL3 and TTF-1 levels delineate prognosis in SCLC was
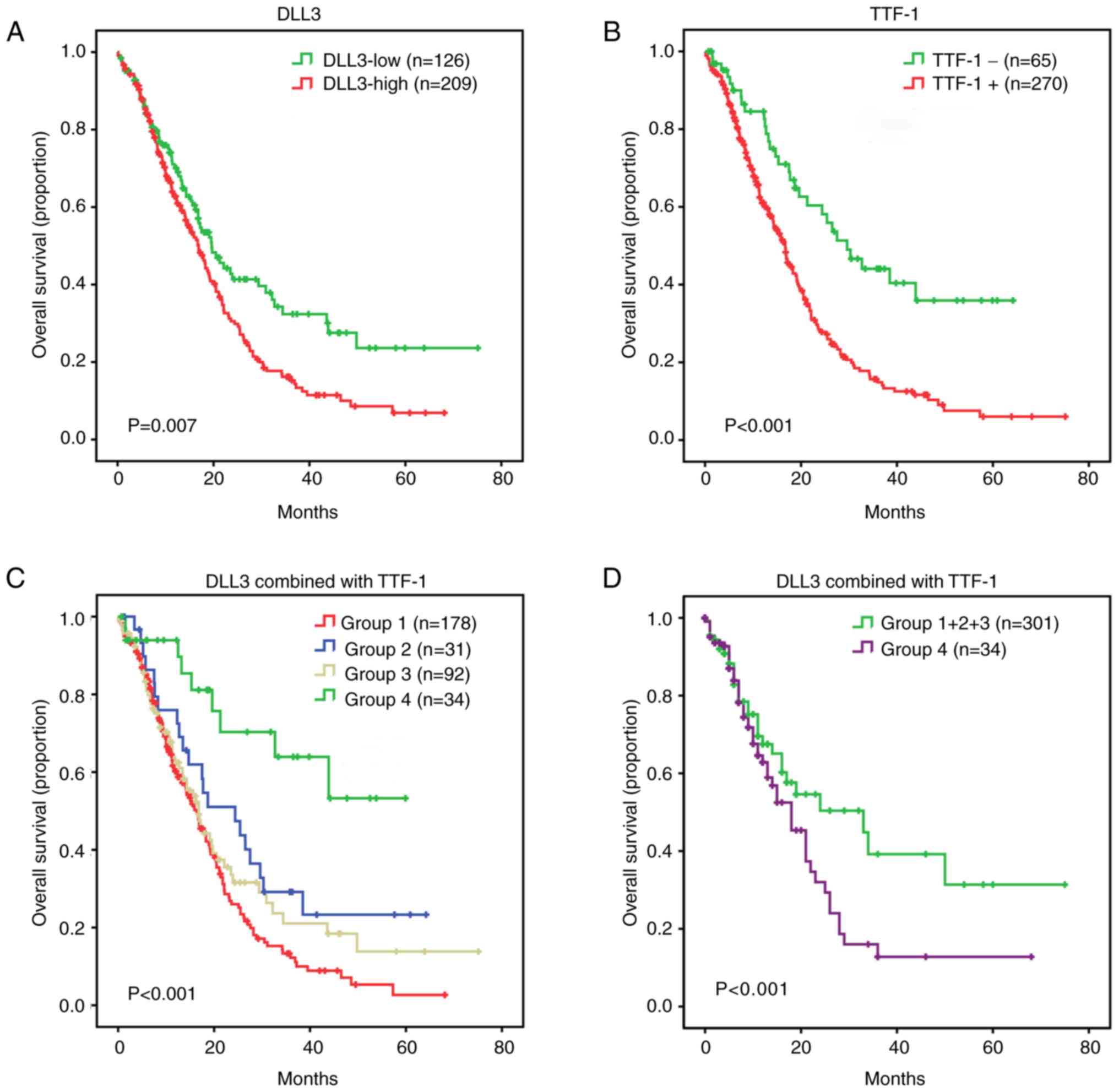
investigated. A Kaplan-Meier survival curve indicated that patients
with SCLC with a high DLL3 expression level exhibited a lower
overall survival compared with patients with DLL3-low expression
(log-rank test; P=0.007; Fig. 4A).
TTF-1+ SCLCs experienced a decrease in overall survival compared
with TTF-1- SCLCs (P<0.001; Fig.
4B). Based on the DLL3 and TTF-1 features, the SCLC cohort was
divided into the following four subgroups: Group 1 consisting of
DLL3-high/TTF-1+; Group 2 consisting of DLL3-high/TTF-1-; Group 3
consisting of DLL3-low/TTF-1+; and Group 4 consisting of
DLL3-low/TTF-1-. A Kaplan-Meier survival curve of the four
different subgroups indicated significant differences (P<0.001;
Fig. 4C). Group 4, representing
DLL3-low/TTF-1- had improved overall survival compared with Group
1+2+3 (P<0.001; Fig. 4D). Group 1
had inferior survival compared with Group 2 (P=0.024) and Group 4
(P<0.001; data not shown). Group 1 and 3 (P=0.192), and Group 2
and 3 (P=0.298) exhibited a similar survival pattern (data not
shown). A univariate model was fitted for each patient
characteristic, including age, sex, smoking history, distant
metastasis, clinical stage, DLL3, TTF-1, and combination of DLL3
and TTF-1. Univariate analysis indicated that non-distant
metastasis (P<0.001), early clinical stage (P<0.001),
DLL3-low (P=0.008), TTF-1- (P<0.001) and DLL3-low/TTF-1-
(P<0.001) were prognostic factors for improved overall survival
of patients with SCLC. All eight characteristics were included in
the multivariate analysis using a forward selection (LR) test in
SPSS software, with P<0.05 as the entry criterion. Therefore,
only significant characteristics were included in the final
multivariate cox model. Multivariate analysis indicated that early
clinical stage (P<0.001) and DLL3-low/TTF-1- (P=0.001) were
independent prognostic factors for improved overall survival of
patients with SCLC (Table II).
 | Table II.Cox regression analyses of overall
survival of patients with small-cell lung cancer. |
Table II.
Cox regression analyses of overall
survival of patients with small-cell lung cancer.
|
| Univariate Cox
model | Multivariate Cox
modelb |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≤60 years vs.
>60 years) | 1.05 | 0.80–1.37 | 0.753 |
| NS |
|
| Sex (male vs.
female) | 1.08 | 0.68–1.72 | 0.743 |
| NS |
|
| Smoking history (no
vs. yes) | 1.01 | 0.74–1.36 | 0.968 |
| NS |
|
| Distant metastasis
(negative vs. positive) | 2.01 | 1.52–2.64 |
<0.001a |
| NS |
|
| TNM stage (18) (I
vs. II vs. III vs. IV) | 1.82 | 1.49–2.22 |
<0.001a | 1.73 | 1.43–2.10 |
<0.001a |
| DLL3 (low vs.
high) | 1.49 | 1.11–1.99 | 0.008a |
| NS |
|
| TTF-1 (negative vs.
positive) | 2.27 | 1.53–3.34 |
<0.001a |
| NS |
|
| Combination of DLL3
and TTF-1 |
| (Group 4 vs. Group
1–3) | 3.71 | 1.90–7.25 |
<0.001a | 3.26 | 1.67–6.39 | 0.001a |
Discussion
The aim of the present study was to examine the
expression pattern of DLL3 protein in pretreated tumor tissues of
patients with SCLC. The main finding was that the intratumoral and
intertumoral distribution of DLL3 protein in SCLC is homogeneous,
supporting the conclusion that biopsy specimens are a reliable
source for DLL3 evaluation for targeted therapy. In addition, the
clinical and prognostic significance of DLL3 and TTF-1 for SCLC
were examined, given the fact that high DLL3 in SCLCs was
associated with the smoking history of the patient, TTF-1
expression and poor survival. One of the most notable findings of
the present study was that DLL3-low/TTF-1- was an independent
prognostic marker and defined a distinct subpopulation of patients
with SCLC with improved overall survival.
Rova-T, a novel DLL3-targeted conjugate, has been
reported to exhibit single-agent antitumor activity in preclinical
and early clinical studies with a strong correlation between DLL3
expression level and antitumor activity (6,7). These
results indicate that DLL3 is a potential predictive biomarker for
therapy with Rova-T. Multiple clinical trials of Rova-T are ongoing
(20). Therefore, DLL3 expression
pattern is essential from a clinical point of view. The present
study indicated high consistency of DLL3 expression levels in
paired specimens, adding novel information on DLL3 expression and
further demonstrating its predictive value for DLL3-targeted
agents.
However, these clinical trials support the role of
DLL3 as a predictive marker for the therapeutic utility of Rova-T
therapy. However, the prognostic value of DLL3 in SCLC remains
unclear. One of the main purposes of the present study was to
investigate the prognostic value of DLL3. The present study
indicated that DLL3-high was an inferior survival marker for
SCLC.
Previous preclinical studies suggested that DLL3 may
lead to high-grade neuroendocrine tumorigenesis, by inhibiting the
Notch receptor activation (7,21). SCLC
is positive for TTF-1 in ≤90-95% of cases, due to its
neuroendocrine differentiation (12–14).
Therefore, the association between DLL3 and TTF-1 protein
expression was analyzed. It was indicated that high expression of
DLL3 in SCLCs are associated with the expression of TTF-1,
suggesting that DLL3 may be associated with the neuroendocrine
phenotype. The findings of the present study are in agreement with
a recent study with a small sample size, reporting that TTF-1 and
DLL3 were highly associated in SCLC (16).
The prognostic value of TTF-1 for patients with SCLC
is supported by a limited number of previous studies (13,22,23).
Patients with SCLC with TTF-1 expression had worse disease-free
survival and overall survival (22).
The results of the present study demonstrated that TTF-1 predicts
inferior survival, which reinforces the prognostic value of TTF-1
in SCLC. In addition, the prognostic value of the combination of
DLL3 and TTF-1 was examined. It was indicated that the combination
of DLL3 and TTF-1 was an independent prognostic marker, and had a
higher prognostic value compared with a single marker. The cohort
was further divided into four subgroups based on TTF-1 and DLL3
protein expression levels. It was indicated that DLL3-low/TTF-1-
was an independent marker and defined a distinct molecular subgroup
of patients with SCLC exhibiting the optimal prognosis. The
combination of the two markers has potential clinical value to
stratify patients with SCLC into subgroups of different
prognosis.
It should be noted that the optimal cut-off value
for DLL3 in the present study was used for predicting overall
survival. Further clinical trials to determine the optimal cut-off
value for DLL3 as a predictive biomarker for DLL3-targeted agents
are required. The main limitation of the present study is the small
sample size of paired specimens, due to a limited number of
patients with SCLC receiving lobectomy (patients with T1-2N0),
dissection or sampling of metastatic site. A total of 1,145
consecutive cases of high-grade pulmonary neuroendocrine carcinomas
were reviewed in Guangdong Provincial People's Hospital between
January 2006 and June 2015, of which 335 SCLC cases had adequate
tissues for IHC detection. In order to reduce selection bias, all
eligible cases were recruited. A second limitation is the
relatively high proportion of early-censored patients, the majority
of whom received treatments at other hospitals following diagnosis
or first-line treatment. The original follow-up data was updated.
The updated one-year and five-year censored rate of the total
cohort were 16.1 (54/335) and 34.6% (116/335), respectively. There
was no bias of early-censored cases between Group 1–3 (46/301,
censored/total) and Group 4 (8/34; P=0.215; data not shown).
Therefore, it is suggested that the survival analyses of the
present study are reliable.
In conclusion, this is the first study, to the best
of our knowledge, to examine DLL3 expression in Chinese patients
with SCLC. Novel information on the homogeneous expression pattern
of DLL3 was indicated in the present study, and evidence supporting
the reliability of biopsy specimens for evaluating DLL3 expression
level in SCLC for targeted therapy has been provided. Additionally,
it was indicated that high DLL3 was associated with smoking
history, TTF-1 expression and poor survival of patients with SCLC.
A total of two subgroups of SCLC with distinct prognoses were
further identified, defined by the combination of TTF-1 and DLL3.
The combination of the two protein markers has potential clinical
value in risk stratification for patients with SCLC.
Acknowledgements
The authors would like to thank Mr. Xin-Chuang Cai
from Guangdong Provincial People's Hospital, Guangdong Academy of
Medical Sciences (Guangzhou, China) for support with the archive
collection.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81202111 and
81673031); The Guangdong Medical Science and Technology Research
Foundation (grant no. A2017566); and The National Clinical Key
Subject Construction Project Fund of China.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LXY, YHL and ZL participated in the conception and
design of the study. YHL, LXY and YFL reviewed all the slides and
collected pathologic data. JTZ, DLL, JHY, JH and CL contributed to
the acquisition and interpretation of the clinical data. LXY and JH
performed immunohistochemical staining. LXY, DLL, JHY, JTZ, XHL and
CL performed the statistical analysis. All authors interpreted the
data. LXY drafted the manuscript, and YHL, ZL and JH edited it. All
authors gave final approval of the version to be published.
Ethics approval and consent to
participate
The Research Ethics Committee of Guangdong
Provincial People's Hospital (Guangzhou, China) approved the
present study (approval no. GDREC2016373H). Due to the
retrospective design of the current study and patient
anonymization, the review board determined that informed consent
was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baize N, Monnet I, Greillier L, Quere G,
Kerjouan M, Janicot H, Vergnenegre A, Auliac JB and Chouaid C:
Second-line treatments of small-cell lung cancers. Expert Rev
Anticancer Ther. 17:1033–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asamura H, Kameya T, Matsuno Y, Noguchi M,
Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, et al:
Neuroendocrine neoplasms of the lung: A prognostic spectrum. J Clin
Oncol. 24:70–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Metro G, Ricciuti B, Chiari R, Baretti M,
Falcinelli L, Giannarelli D, Sidoni A, Mountzios G, Crinò L,
Bellezza G, et al: Survival outcomes and incidence of brain
recurrence in high-grade neuroendocrine carcinomas of the lung:
Implications for clinical practice. Lung Cancer. 95:82–87. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD: Advances in neuroendocrine lung
tumors. Ann Oncol. 21 (Suppl 7):vii65–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rudin CM, Pietanza MC, Bauer TM, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA III,
Robert F, et al: Rovalpituzumab tesirine, a DLL3-targeted
antibody-drug conjugate, in recurrent small-cell lung cancer: A
first-in-human, first-in-class, open-label, phase 1 study. Lancet
Oncol. 18:42–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saunders LR, Bankovich AJ, Anderson WC,
Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang
A, et al: A DLL3-targeted antibody-drug conjugate eradicates
high-grade pulmonary neuroendocrine tumor-initiating cells in vivo.
Sci Transl Med. 7:302ra1362015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gazdar AF, Bunn PA and Minna JD:
Small-cell lung cancer: What we know, what we need to know and the
path forward. Nat Rev Cancer. 17:725–737. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bauer TM, Spigel D, Ready N, Morgensztern
D, Glisson BS, Byers LA, Burris H, Robert F, Strickland DK,
Pietanza MC, Govindan R Dylla SJ, Peng S and Rudin C: ORAL02.01:
Safety and efficacy of single-agent rovalpituzumab tesirine, a
DLL3-targeted ADC, in recurrent or refractory SCLC: Topic: Medical
oncology. J Thorac Oncol. 11:S252–S253. 2016. View Article : Google Scholar
|
|
10
|
Tanaka K, Isse K, Fujihira T, Takenoyama
M, Saunders L, Bheddah S, Nakanishi Y and Okamoto I: Prevalence of
delta-like protein 3 expression in patients with small cell lung
cancer. Lung Cancer. 115:116–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma SK, Pourat J, Abdel-Atti D, Carlin
SD, Piersigilli A, Bankovich AJ, Gardner EE, Hamdy O, Isse K,
Bheddah S, et al: Noninvasive interrogation of DLL3 expression in
metastatic small cell lung cancer. Cancer Res. 77:3931–3941. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakaeda M, Sato H, Ishii J, Miyata C,
Kamma H, Shishido-Hara Y, Shimoyamada H, Fujiwara M, Endo T, Tanaka
R, et al: Neural lineage-specific homeoprotein BRN2 is directly
involved in TTF1 expression in small-cell lung cancer. Lab Invest.
93:408–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Misch D, Blum T, Boch C, Weiss T, Crolow
C, Griff S, Mairinger T, Bauer TT and Kollmeier J: Value of thyroid
transcription factor (TTF)-1 for diagnosis and prognosis of
patients with locally advanced or metastatic small cell lung
cancer. Diagn Pathol. 10:212015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitamura H, Yazawa T, Sato H, Okudela K
and Shimoyamada H: Small cell lung cancer: Significance of RB
alterations and TTF-1 expression in its carcinogenesis, phenotype,
and biology. Endocr Pathol. 20:101–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukhopadhyay S and Katzenstein AL:
Subclassification of non-small cell lung carcinomas lacking
morphologic differentiation on biopsy specimens: Utility of an
immunohistochemical panel containing TTF-1, napsin a, p63, and
CK5/6. Am J Surg Pathol. 35:15–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardnell RJ, Li L, Sen T, Bara R, Tong P,
Fujimoto J, Ireland AS, Guthrie MR, Bheddah S, Banerjee U, et al:
Protein expression of TTF1 and cMYC define distinct molecular
subgroups of small cell lung cancer with unique vulnerabilities to
aurora kinase inhibition, DLL3 targeting, and other targeted
therapies. Oncotarget. 8:73419–73432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brambilla E, Beasley MB, Austin JHM,
Capelozzi VL, Chirieac LR, Devesa SS, Frank GA, Gazdar A, Ishikawa
Y, Jen J, et al: Small cell carcinoma. WHO classification of tumors
of the lung, pleura, thymus and heart. Travis WD, Brambilla E,
Burke AP, Marx A and Nicholson AG: IARC; Lyon: pp. 62–68. 2015
|
|
18
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Welti J, Rodrigues DN, Sharp A, Sun S,
Lorente D, Riisnaes R, Figueiredo I, Zafeiriou Z, Rescigno P, de
Bono JS and Plymate SR: Analytical validation and clinical
qualification of a new immunohistochemical assay for androgen
receptor splice variant-7 protein expression in metastatic
castration-resistant prostate cancer. Eur Urol. 70:599–608. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sabari JK, Lok BH, Laird JH, Poirier JT
and Rudin CM: Unravelling the biology of SCLC: Implications for
therapy. Nat Rev Clin Oncol. 14:549–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chapman G, Sparrow DB, Kremmer E and
Dunwoodie SL: Notch inhibition by the ligand DELTA-LIKE 3 defines
the mechanism of abnormal vertebral segmentation in spondylocostal
dysostosis. Hum Mol Genet. 20:905–916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hiroshima K, Iyoda A, Shida T, Shibuya K,
Iizasa T, Kishi H, Tanizawa T, Fujisawa T and Nakatani Y:
Distinction of pulmonary large cell neuroendocrine carcinoma from
small cell lung carcinoma: A morphological, immunohistochemical,
and molecular analysis. Mod Pathol. 19:1358–1368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Myong NH: Thyroid transcription factor-1
(TTF-1) expression in human lung carcinomas: Its prognostic
implication and relationship with expressions of p53 and Ki-67
proteins. J Korean Med Sci. 18:494–500. 2003. View Article : Google Scholar : PubMed/NCBI
|