Introduction
Colon cancer is one of the common types of nausea
and digestive tract tumors, which has become a disease that
seriously affects human health (1).
The molecular mechanism of colon cancer development and progression
is still unclear. Long non-coding RNAs (lncRNAs) are a subgroup of
non-coding RNAs composed of more than 200 nucleotides, playing an
important role in many biological processes such as
dose-compensation effect, epigenetic regulation, cell cycle and
cell differentiation regulation (2).
Previously, it was believed that lncRNA genes had no biological
function and were therefore called junk DNA. Recent studies have
found that lncRNAs are abnormally expressed in many tumors such as
lung cancer, glioma, liver cancer and breast cancer (3–6). lncRNA
THOR is a conserved lncRNA identified in testicular tissue in 2017
(7). It was reported that lncRNA
THOR can significantly promote the proliferation of osteosarcoma
cells and renal cancer cells (8,9). In
addition, it was found that lncRNA THOR could enhance the stem cell
characteristics of nasopharyngeal carcinoma cells, thereby
increasing the resistance of nasopharyngeal carcinoma cells to
chemotherapy (10). Early
high-throughput sequencing revealed that the expression of lncRNA
THOR in colon cancer tissues was significantly higher than that in
adjacent normal tissues. However, effect of lncRNA THOR on the
biological behavior of colon cancer cells has not been reported.
This study analyzed the effect of lncRNA THOR on proliferation and
migration of colon cancer cells.
Materials and methods
General information
Thirty female Balb/c nude mice weighing 20 g were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. The mice
(6 per cage) were kept in SPF animal room at 25°C with a 12 h
light/12 h dark cycle and free access to food and water and
humidity was 50%. Colon cancer cell line SW620 was purchased from
ATCC. CCK8 cell proliferation assay kit and BeyoClick™ EdU-488 cell
proliferation assay kit were purchased from Biyuntian Biotechnology
Co., Ltd. Transwell kit was purchased from Corning. MTT assay kit
and RNA extraction kit were purchased from Sigma-Aldrich; Merck
KGaA. RNA extraction kit (RC101), reverse transcription kit
(R323-01) and universal high-sensitivity dye quantitative PCR
detection kit (Q431-02) were purchased from Nanjing Nuoweizan
Biological Co., Ltd. Control shRNA lentivirus, and Lenti-THOR-shRNA
lentivirus were obtained from Shandong Weizhen Biological Co., Ltd.
The study was approved by the Ethics Committee of Jinan Central
Hospital Affiliated to Shandong University (Jinan, China).
lncRNA THOR knockdown cell line
Colon cancer cell line SW620 [SW-620]
(ATCC® CCL-227™) was cultured in DMEM medium (10% FBS)
twice to restore cell status. After digestion with 0.25% trypsin,
the cell concentration was adjusted to 1×105 cells/ml
and inoculated in 12-well plate with 1 ml per well. After 12 h of
cell adherence, the control shRNA lentivirus and Lenti-THOR-shRNA
lentivirus were added respectively, and the cell-to-virus titer
ratio was 1:100. Culture medium was replaced by fresh medium at 24
h after transfection, followed by cell culture for additional 4 h.
Then cells were treated with 1 µg/ml puromycin for 24 h, and viable
cells were observed under the microscope (ZEISS) to observe GFP
fluorescence. When GFP in all cells is expressed, stable cell line
is successfully constructed. Lenti-THOR-shRNA stable expression
cell line was used as the experimental group, and control shRNA
stable expression cell line was used as the control group.
Preparation of a reverse transcription-quantitative
PCR (RT-qPCR) system. The same number of living cells were
collected both from the control and experimental groups. Total RNA
was extracted using an RNA extraction kit, and then the RNA was
reverse-transcribed into cDNA using a reverse transcription kit to
serve as the template for RT-qPCR. The temperature protocol for
reverse transcription was 37°C for 30 min, 85°C for 15 sec and 4°C
for storage. cDNA template was diluted 1:100 before use. PCR
reaction systems included: 2X SYBR-Green premix 10 µl, template 8.8
µl, upstream primers 0.6 µl and downstream primers 0.6 µl. PCR
reaction conditions were: 95°C for 30 sec, followed by 40 cycles of
95°C for 10 sec and 60°C for 30 sec. Sequences of primers used in
PCR reactions are shown in Table I.
β-actin was the reference gene. The method of quantification was
2−ΔΔCq (11).
 | Table I.Sequences of primers used in PCR
reactions. |
Table I.
Sequences of primers used in PCR
reactions.
| Genes | Forward primers
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| lncRNA THOR |
ACAATCGAGCAAGGCAGTGA |
TGGCCAAGACCTGCTGTTAG |
| β-actin |
GGCCCAGAATGCAGTTCGCCTT |
AATGGCACCCTGCTCACGCA |
| c-myc |
CCCTCCACTCGGAAGGACTA |
GCTGGTGCATTTTCGGTTGT |
| SOX9 |
GCTGCGAAGTGGAAACCATC |
CCTCCTTCTGCACACATTTGAA |
| IGF2BP1 |
CAGGAGATGGTGCAGGTGTTTATC C |
GTTTGCCATAGATTCTTCCCTGAGC |
Cell proliferation analysis (CCK8 and
EDU methods)
Control and experimental cells were trypsinized at a
cell density of 5×105 cells/ml, and seeded in 96-well
plates with 100 µl per well. Cells were cultured for 6 h at 37°C in
a 5% CO2 incubator. After that, 10 µl of CCK solution
was added 12, 24, 36, 48 and 72 h later. After that, cells were
cultivated for additional 3 h. Finally, OD values at 450 nm were
measured using a microplate reader (BioTek) to reflect cell
proliferation.
Cells of the control and experimental groups were
digested and inoculated into a 12-well plate. After the cells were
completely adhered, the EdU was diluted to 50 µM with a cell
culture medium, and 100 µl was added to each well. Cells were
incubated for 2 h in a 37°C and 5% CO2 incubator. After
that, cells were washed three times with PBS, and then fixed with
4% paraformaldehyde at room temperature for 20 min. After washing
three times with PBS, nuclei were stained with DAPI. After washing
three times with PBS, cells were observed under a microscope.
Cell migration analysis (Transwell
method)
The control and experimental cells were trypsinized
to prepare a single cell suspension, and the cell density was
adjusted to 5×105 cells/ml, and inoculated into the
Transwell upper chamber, 100 µl per well. The lower chamber was
filled with fresh cell culture medium. Cells were incubated in a
37°C and 5% CO2 incubator for 24 h, and were fixed with
paraformaldehyde. Then the upper chamber membrane was fixed with 1%
crystal violet (MXB Biotechnologies) at room temperature for 5 min.
Migrating cells were observed under an optical microscope, and 20
visual fields were selected for both the experimental and control
groups to calculate the average number of migrating cells.
Xenograft tumor
After cells of the control and experimental groups
were trypsinized, cell density was adjusted to 1×108/ml.
Thirty Balb/c nude mice were randomly divided into the control and
experimental groups. Tumor cells (100 µl) were injected into the
fat pad of each mouse, and mice were raised in SPF-level animal
house. Tumor formation and growth in the mice were observed. Tumor
length (L) and width (W) were measured. Tumor volume was calculated
by the following formula: V = 1/2 × L × W2.
Statistical analysis
All data were analyzed by SPSS 17.0 statistical
software (SPSS, Inc.). Measurement data were expressed as mean ±
standard deviation and compared by t-test. P<0.05 indicated a
difference with statistical significance.
Results
Comparison of lncRNA THOR levels in
the control and experimental groups
The lncRNA THOR knockdown stable cell line was
established by lentiviral-mediated THOR-shRNA. The expression level
of lncRNA THOR in the control group (1.21±0.21) was significantly
higher than that in the experimental group (0.28±0.10), and the
difference was statistically significant (P<0.05) (Fig. 1).
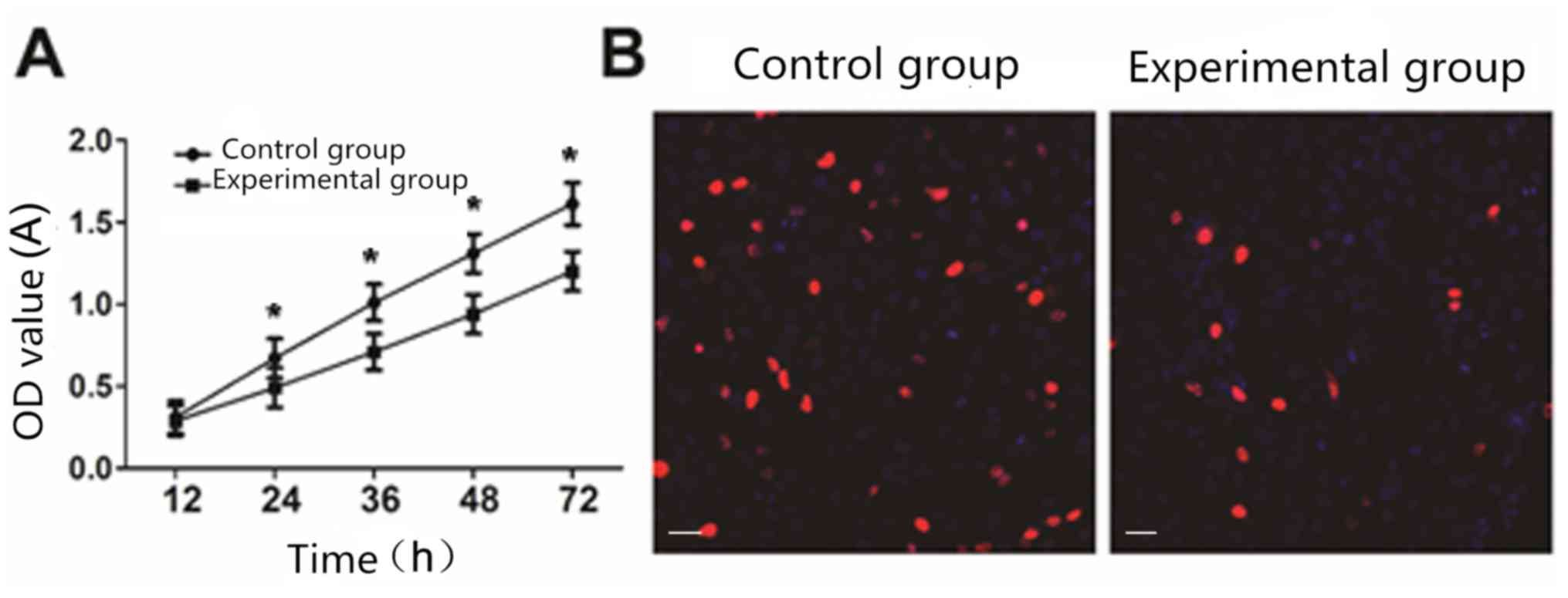
Effect of lncRNA THOR on cell
proliferation
CCK8 analysis showed that compared with the control
cells, cell proliferation ability of the experimental group was
significantly reduced (P<0.05) (Fig.
2A). EdU staining analysis showed that the cell proliferation
ability of the experimental group was significantly decreased
compared with that of the control group (P<0.05) (Fig. 2B).
Effect of lncRNA THOR on cell
migration
Compared with the number of migrating cells in the
control group (91.23±11.44), the number of migrating cells in the
experimental group (49.32±8.14) decreased significantly (P<0.05)
(Fig. 3).
Effect of lncRNA THOR on tumor
growth
Control and experimental group cells were inoculated
into nude mice, and cell proliferation rate in vivo was
analyzed. Compared with the control cells, growth rate of tumors in
the experimental group was significantly reduced (P<0.05)
(Fig. 4).
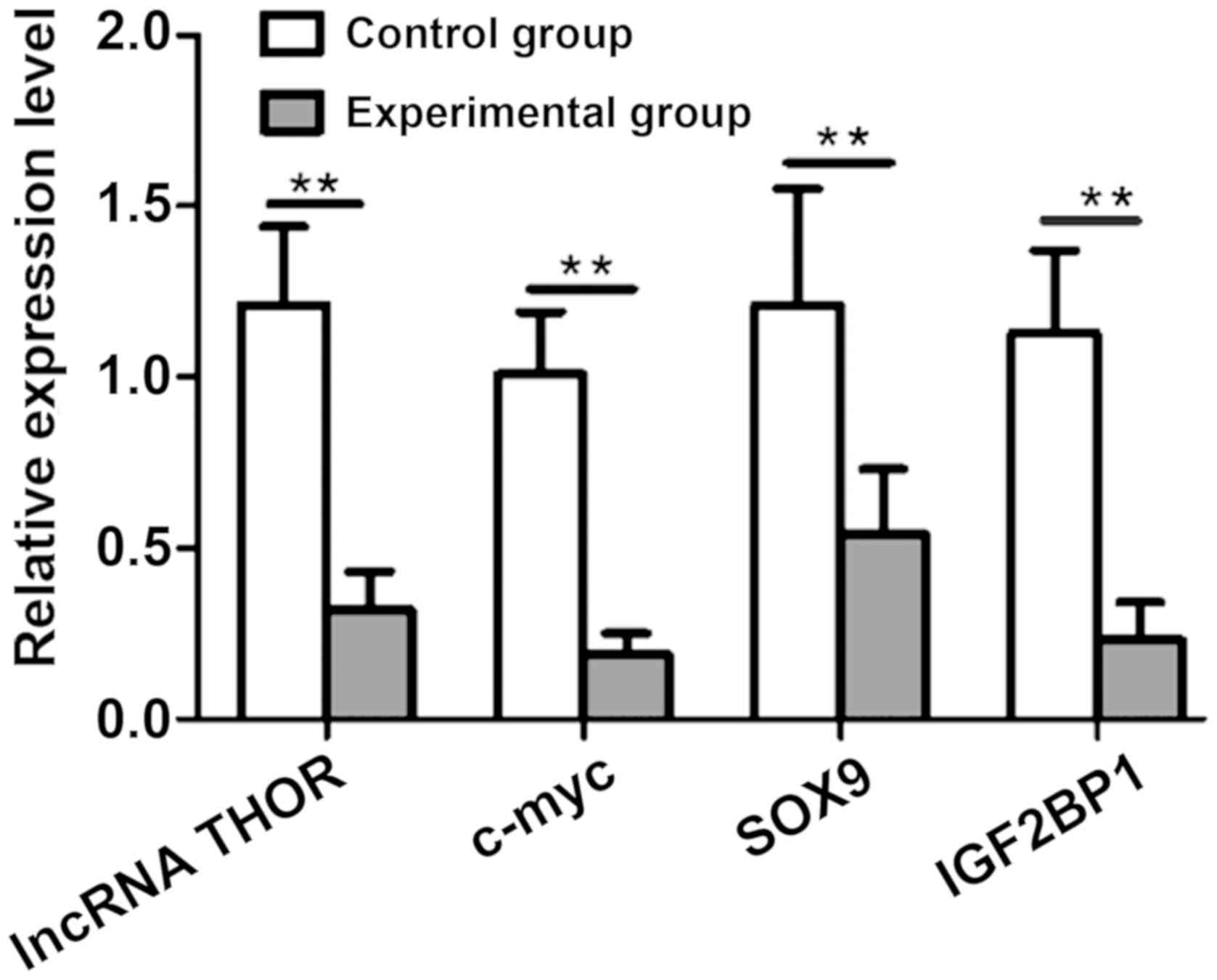
Effect of lncRNA THOR knockdown on its
target genes
Effect of lncRNA THOR knockdown on its target genes
at mRNA level was further analyzed by RT-qPCR. Compared with the
control group, the mRNA levels of IGF2BP1, SOX9 and c-myc in the
experimental group were significantly reduced (P<0.05) (Fig. 5).
Discussion
Colon cancer, as the third most common type of
malignant tumor in the world, seriously affects human health
(12). Despite the efforts made in
cancer diagnosis, many cancer patients are still diagnosed at
advanced stages. Therefore, novel early diagnosis biomarkers are
needed (13). Studies have shown
that lncRNAs participate in many biological processes by regulating
gene expression (14). It has been
reported that lncRNA CCTA1, ATB and HOTAIR participate in the
regulation of cell behavior of colon cancer by mediating the
downstream signaling pathways (15,16).
Because of the important role of lncRNA in tumorigenesis, lncRNAs
have been used as a novel tumor marker and tumor therapeutic
target. This study analyzed the role of lncRNA THOR in the
pathogenesis of colon cancer.
At cellular level, lncRNA THOR knockdown led to
significantly inhibited proliferation and migration of colon cancer
SW620 cells. At animal level, lncRNA THOR knockdown mediated the
significantly inhibited growth of tumors in mice. It indicated that
lncRNA THOR plays an important role in the development of colon
cancer.
Sox9 plays an important role in early embryonic
development, cell fate determination and differentiation of tissues
and organs, sex determination, occurrence and development of
nervous system and cartilage (17).
The high expression of Sox9 is related to the size of the tumors,
TNM stage, lymph node metastasis and differentiation of colorectal
cancer patients (18). lncRNA THOR
maintains the stability and activity of IGF2BP1 through
conservative interaction with IGF2BP1 mRNA (7). The results showed that knockdown of
lncRNA THOR resulted in significant downregulation of the target
genes SOX9 and IGF2BP1 mRNA, suggesting that lncRNA THOR has
important significance in stabilizing SOX9 and IGF2BP1 mRNA. C-myc,
as an oncogene, plays an important role in tumorigenesis (19). This study also found that knockdown
of lncRNA THOR in colon cancer cells resulted in a significant
decrease in c-myc mRNA level, which further demonstrated that
lncRNA THOR, was involved in the occurrence and progress of colon
cancer genes.
Collectively, lncRNA THOR, as an oncogene, promotes
the proliferation and migration of colon cancer cells, which can be
used as a therapeutic target for colon cancer treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and XY drafted the paper and performed PCR. XY
and LW were responsible for CCK8 assay and Transwell method. All
the authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jinan Central Hospital Affiliated to Shandong University (Jinan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Yang W, Chen Z, Chen J, Meng Y,
Feng B, Sun L, Dou L, Li J, Cui Q, et al: Long noncoding RNA
lncSHGL recruits hnRNPA1 to suppress hepatic gluconeogenesis and
lipogenesis. Diabetes. 67:581–593. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shan Y, Ma J, Pan Y, Hu J, Liu B and Jia
L: lncRNA SNHG7 sponges miR-216b to promote proliferation and liver
metastasis of colorectal cancer through upregulating GALNT1. Cell
Death Dis. 9:7222018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mondal T, Juvvuna PK, Kirkeby A, Mitra S,
Kosalai ST, Traxler L, Hertwig F, Wernig-Zorc S, Miranda C, Deland
L, et al: Sense-antisense lncRNA pair encoded by locus 6p22.3
determines neuroblastoma susceptibility via the USP36-CHD7-SOX9
regulatory axis. Cancer Cell. 33:417–434.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao Y, Cheng S, Xiang J and Luo C: lncRNA
CCHE1 increased proliferation, metastasis and invasion of non-small
lung cancer cells and predicted poor survival in non-small lung
cancer patients. Eur Rev Med Pharmacol Sci. 22:1686–1692.
2018.PubMed/NCBI
|
|
6
|
Niknafs YS, Han S, Ma T, Speers C, Zhang
C, Wilder-Romans K, Iyer MK, Pitchiaya S, Malik R, Hosono Y, et al:
The lncRNA landscape of breast cancer reveals a role for DSCAM-AS1
in breast cancer progression. Nat Commun. 7:127912016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hosono Y, Niknafs YS, Prensner JR, Iyer
MK, Dhanasekaran SM, Mehra R, Pitchiaya S, Tien J, Escara-Wilke J,
Poliakov A, et al: Oncogenic role of THOR, a conserved
cancer/testis long non-coding RNA. Cell. 171:1559–1572.e1520. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Chen M, Xu Y, Chen X, Zhou P, Zhao
X, Pang F and Liang W: Long non-coding RNA THOR promotes human
osteosarcoma cell growth in vitro and in vivo. Biochem Biophys Res
Commun. 499:913–919. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye XT, Huang H, Huang WP and Hu WL: lncRNA
THOR promotes human renal cell carcinoma cell growth. Biochem
Biophys Res Commun. 501:661–667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao L, Cheng XL and Cao H: lncRNA THOR
attenuates cisplatin sensitivity of nasopharyngeal carcinoma cells
via enhancing cells stemness. Biochimie. 152:63–72. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shahbazian H, Nasuri Y, Hosseini SM,
Arvandi S and Razzaghi S: A report of the frequency of colorectal
carcinoma and involved lymph nodes in South-West Iran. Indian J Med
Paediatr Oncol. 37:38–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Xie H, Gao Q, Zhan H, Xiao H, Zou
Y, Zhang F, Liu Y and Li J: Colon cancer associated transcripts in
human cancers. Biomed Pharmacother. 94:531–540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim C, Kang D, Lee EK and Lee JS: Long
noncoding RNAs and RNA-binding proteins in oxidative stress,
cellular senescence, and age-related diseases. Oxid Med Cell
Longev. 2017:20623842017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng H, Wang JM, Li M, Tang R, Tang K, Su
Y, Hou Y and Zhang J: Long non-coding RNAs: New biomarkers for
prognosis and diagnosis of colon cancer. Tumour Biol.
39:10104283177063322017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bowles J, Schepers G and Koopman P:
Phylogeny of the SOX family of developmental transcription factors
based on sequence and structural indicators. Dev Biol. 227:239–255.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong C, Wilhelm D and Koopman P: Sox genes
and cancer. Cytogenet Genome Res. 105:442–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weidensdorfer D, Stöhr N, Baude A, Lederer
M, Köhn M, Schierhorn A, Buchmeier S, Wahle E and Hüttelmaier S:
Control of c-myc mRNA stability by IGF2BP1-associated cytoplasmic
RNPs. RNA. 15:104–115. 2009. View Article : Google Scholar : PubMed/NCBI
|