Introduction
Pancreatic cancer is a deadly disease, the deadliest
of all the solid tumors; almost all patients diagnosed with
pancreatic cancer will eventually succumb to the disease (1). This disease has a low survival rate at
just 5 years, which has only increased by 3% over the last 20
years, from 5% in 1997 to 8% in 2017 in the United States (2,3).
However, the incidence of pancreatic cancer has increased by 49%
within the USA alone over the past 20 years (2,3). By the
year 2030, pancreatic cancer is predicted to be the second leading
cause of cancer-associated mortality; thus, it is crucial for the
public and for physicians to start investing time in pancreatic
cancer research for the purposes of improving the current situation
and future treatment options (4).
This type of cancer has a possible curative treatment in surgical
resection. However, only 15–20% of patients with this disease will
be able to undergo surgical resection, as most are diagnosed in the
late stages of disease when surgery is no longer feasible (5). Thus, researching new diagnostic
biomarkers to identify pancreatic cancer at an earlier stage is
currently an area of great interest.
Previously, high-throughput sequencing led to the
discovery of novel small non-coding RNAs known as transfer RNA
(tRNA)-derived fragments (tRFs) (6–8). tRFs
cover all the domains of life (archaea, bacteria and eukarya)
(9–11). They were thought to be random
products of tRNA, but research has revealed that they are their own
biological entities (9,10). Humans produce tRFs, consisting of
dicer or angiogenin, including yeast with endonucleases, such as
ribonuclease T2 (1). Transfer RNA
(tRNA)-derived fragments (tRFs) comprise two groups, sized 30 to 35
nucleotides (nt), named tRNA halves (tiRNA), including those that
are stress-induced and those with a full-length tRNA anticodon loop
cleaved by endonucleases, which generates the tiRNAs. Another group
are approximately 20 nt in length, and are referred to as tRFs,
which can be separated from the tRNAs to form a 5′- and a
3′-sequence and stemming out of the 5′- and the 3′-site of a mature
tRNA (7,12,13). The
precursor tRNAs generate the 5′-lead and 3′-tail sequences that are
also classified as tRFs (8,14,15).
There have been reports of tRFs in an acquired
metabolic disorder (16), breast
cancer (17), developmental
disorders (18), lung (19) and colorectal cancer (20). This demonstrates that tRFs may serve
a significant role in tumorigenesis. The mechanisms of tRFs are
diverse. tRFs regulate gene expression post-translation; with the
main factor being displaced for eukaryotic initiation factor 4G
translation, tRFs directly inhibit protein synthesis (21–23). A
3′-tRF from lymphoma B-cells suppresses proliferation and regulates
responses to DNA damage in a similar way to microRNAs (24). tRFs were indicated to compete for
binding sites against the binding protein Y-BOX binding protein 1
(YBX1), which may suppress cell proliferation and invasion through
the stabilization of oncogenic transcripts. Thus, tRFs counteract
the protein YBX1 and suppress tumor progress (17).
In summary, the aforementioned studies demonstrate
that tRFs play important roles in tumorigenesis. The studies also
suggest that tRFs may be novel biomarkers of diseases, yet the
expression profile of tRFs in pancreatic cancer remains unknown.
Thus, in the present study, tRF expression levels in clinical
samples of pancreatic cancer were analyzed using tRF and tiRNA
sequencing. Subsequently, the expression levels of selected tRFs
and tiRNAs were analyzed using quantitative PCR (qPCR), and
bioinformatics predictions were analyzed.
Materials and methods
Sample collection
The three patients with pancreatic cancer involved
in the present study were recruited in June 2017 at the Affiliated
Changzhou No. 2 People's Hospital of Nanjing Medical University
(Jiangsu, China). All patients were female and 51–69 years of age,
with an average age of 61.7 years. Three pairs of pancreatic cancer
and tissues adjacent to the tumor sites at a distance of 2 cm were
selected for tRFs and tiRNAs sequencing. The paired pancreatic
cancer and adjacent normal tissue samples were obtained from the
postoperative specimens; the adjacent normal tissue samples were
used as the control group for the tRFs and tiRNAs sequencing.
The staging of pancreatic cancer is dependent on a
Tumor-Node-Metastasis (TNM) system that was jointly designed by the
American Joint Committee on Cancer and the Union for International
Cancer Control (25). The patients'
clinical stages were T2N1M0, T2N2M0 and T2N0M0 (Table I). The paired pancreatic cancer
samples, along with associated normal tissues, were obtained from
post-operative specimens and immediately preserved in −80°C liquid
nitrogen. Each patient provided a written informed consent form,
and the Ethics Committee of the Affiliated Changzhou No. 2 People's
Hospital of Nanjing Medical University (Changzhou, China) approved
the study protocol.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Number | Age, years | Sex | Tumor size, cm | Tumor stage | Pathological
description |
|---|
| 1 | 65 | Female | 2.5×2.5×3.0 | T2N1M0 | Middle-differentiated
adenocarcinoma invaded nerve, metastasis in 2/7 lymph nodes |
| 2 | 51 | Female | 2.5×2.5×2.5 | T2N2M0 | Middle-differentiated
adenocarcinoma invaded nerve and Vater, metastasis in 5/11 lymph
nodes |
| 3 | 69 | Female | 3.5×3.5×3.0 | T2N0M0 | Middle,
low-differentiated adenocarcinoma invaded duodenal wall, no lymph
node metastasis |
tRFs and tiRNAs sequencing
The pancreatic cancer and adjacent normal tissue
samples were used to extract total RNA. The amount of purified
total RNA was 1–2 µg. Prior to the tRF and tiRNA sequencing, the
quantity of each RNA was measured in a Nanodrop instrument (Thermo
Fisher Scientific, Inc.). The integrity of each RNA was checked
using agarose gel (2%) electrophoresis (2 µg per lane). tRFs and
tiRNAs have heavy RNA modifications that interfere with the small
RNA-sequencing (RNA-seq) library construction (26). Prior to tRF and tiRNA sequencing, the
following pretreatments were performed: 3′-aminoacyl (positively
charged) deacetylation to 3′-OH for 3′adaptor ligation,
2′,3′-cyclic phosphate removal to 3′-OH for 3′adaptor ligation,
5′-OH phosphorylation to 5′-P for 5′adaptor ligation, and m1A and
m3C demethylation for efficient RT. Sequencing libraries were
size-selected (50-bp single-read at 10 Mb reads) for the RNA
biotypes to be sequenced using an automated gel cutter (Thermo
Fisher Scientific, Inc.). These libraries were qualified and
quantified using an Agilent Bioanalyzer 2100 (Agilent Technologies,
Inc.). For standard small RNA sequencing using the Illumina NextSeq
instrument (Illumina, Inc.), the sequencing type was 50-bp
single-read at 10 Mb reads. Comprehensive data and Student's paired
t-test statistical analysis were performed using the Arraystar tRFs
and tiRNA-seq data analysis package (version 4.1; DNASTAR). When
comparing two groups of profile differences (such as disease vs.
control), the normalized tag number of tRNAs annotated in the
Genomic tRNA Database (http://gtrnadb.ucsc.edu) was used, including the tag
count of each sample, the ‘fold change’ (i.e., the ratio of the
group averages) between the groups for each tRF/tiRNA was computed.
The statistical significance of the difference was estimated using
a paired Student's t-test. tRFs/tiRNAs with fold change ≥2 and
P≤0.05 were selected as significantly differentially expressed (DE)
tRFs/tiRNAs. The analysis outputs and the differentially expressed
genes can be filtered and ranked according to fold change, P-value,
etc., using the Data and Sort & Filter functions in Microsoft
Excel (any version; Microsoft Corporation).
Reverse transcription-quantitative
(RT-q)-PCR
In the present study, four tRFs and tiRNAs were
selected for PCR validation. Total RNA was extracted from the
pancreatic cancer samples and the adjacent normal tissue samples
using cetyltrimethylammonium bromide buffer (Invitrogen; Thermo
Fisher Scientific, Inc.). The RNA was reverse transcribed using the
rtStar™ First-Strand cDNA Synthesis kit (3′ and 5′adaptor; catalog
no. AS-FS-003; Arraystar). The temperature conditions for RT were
37°C for 15 min followed by 85°C for 5 sec. tRFs and tiRNAs exhibit
heavy RNA modifications that interfere with PCR (26); therefore, prior to PCR, the following
pretreatments were performed: 3′-aminoacyl (positively charged)
deacetylation to 3′-OH for 3′adaptor ligation, 2′,3′-cyclic
phosphate removal to 3′-OH for 3′adaptor ligation, 5′-OH
phosphorylation to 5′-P for 5′adaptor ligation, and m1A and m3C
demethylation for efficient RT. Following this, a 3′adaptor
ligation was performed, connecting the 5′adaptor and RT ensued. The
primers for qPCR were designed by KangChen Biotech Co., Ltd.
(Table II). Subsequently, qPCR was
performed using the ViiA 7 real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with FAM fluorophore
(Ai Mei Jie Technology Co. Ltd.). The thermocycling conditions were
as follows: 95°C for 5 sec, followed by 30 cycles of 60°C for 30
sec and dissociation at 95°C for 1 min and 55°C 1 min. The
2−ΔΔCq method (27) was
used to analyze the PCR data, and U6 was used as a reference gene.
A Mann-Whitney U test was used to determine the statistical
significance of the qPCR expression values. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using GraphPad Prism (version
6.0; GraphPad Software, Inc.).
 | Table II.tRFs and tiRNAs selected for
validation via quantitative PCR. |
Table II.
tRFs and tiRNAs selected for
validation via quantitative PCR.
| Gene | Primer
sequence | Product length,
bp |
|---|
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ | 89 |
|
| R:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
|
| AS-tDR-000064 | F:
5′-CAGTCCGACGATCATCCCAC-3′ | 45 |
|
| R:
5′-TGCTCTTCCGATCTTGGTGG-3′ |
|
| AS-tDR-000069 | F:
5′-AGTTCTACAGTCCGACGATCTCTC-3′ | 49 |
|
| R:
5′-CTCTTCCGATCTTGGAGGTTC-3′ |
|
| AS-tDR-000102 | F:
5′-CTACAGTCCGACGATCTCCCC-3′ | 45 |
|
| R:
5′-TCTTCCGATCTTGGTGGAGATG-3′ |
|
| AS-tDR-001391 | F:
5′-GGCTCGTTGGTCTAGGGGTATG-3′ | 52 |
|
| R:
5′-ACGTGTGCTCTTCCGATCTGAA-3′ |
|
Predicted targets of various tRFs and
tiRNAs expressions
miRanda (http://www.microrna.org/microrna/home.do) and
TargetScan (http://www.targetscan.org/) were used to predict
targets of various tRFs and tiRNAs expressions. miRanda and
TargetScan assign binding scores of tRFs and target genes; a higher
score indicates stronger binding. The 35 target genes with the
highest binding scores were selected as the candidate target
genes.
Functional DE-tRF and tiRNA enrichment
analysis
The Database for Annotation, Visualization and
Integrated Discovery (DAVID version 6.8; http://david.ncifcrf.gov/) is a powerful tool that
assists in exploiting the functions of the genes of interest. tRFs
and tiRNAs with fold change ≥2 and P≤0.05 were selected as
significantly DE. As tRFs and tiRNAs can act on target genes to
downregulate their expression, to identify the possible biological
pathways or functions that could be regulated by the DE-tRFs and
tiRNAs, the target genes were analyzed using DAVID. Target genes
were screened for significantly enriched Kyoto Encyclopedia of
Genes and Genomes (KEGG; http://www.kegg.jp/) pathways and Gene Ontology (GO;
http://geneontology.org), assessing the
biological processes, molecular functions and cellular components.
The GO project offers a controlled vocabulary for describing genes
and the attributes of gene products within all organisms. There are
three domains covered by GO: Cellular components, biological
processes and molecular functions. The Bioconductor's (https://www.bioconductor.org) top GO function was used
for the Fisher's exact test when locating whether additional
overlap existed between the GO annotation list and the DE list,
compared with the expected amount. The top GO indicated a P-value
that demonstrated the importance of the GO enrichment term for DE
genes. Therefore, the lower the P-value, the greater the
significance of the GO term (P≤0.05 was suggested). The pathway
analysis model was used for the functional gene mapping analysis of
the KEGG pathway. The P-value (Fisher's P-value, EASE-score or
hypergeometric P-value) indicated the importance of the pathway
that was associated with specific conditions; the lower the
P-value, the more important the pathway. The KEGG and GO selection
criteria had P-values of <0.05.
Results
tRF and tiRNA sequencing
A total of 48 DE-tRFs and tiRNAs were screened from
the pancreatic cancer samples and adjacent normal tissue samples,
including 46 upregulated tRFs and tiRNAs and 2 downregulated tRFs
and tiRNAs (Table III). A heat map
of tRFs and tiRNAs is presented in Fig.
1.
 | Figure 1.Heat map indicating the expression
levels of various tRFs and tiRNAs. Each square represents a gene,
and its color represents the amount of expression of the gene. The
higher the amount of expression, the darker the color (red is
upregulated, green is downregulated). On the heatmap, dark green,
dark orange, light orange, purple, green, blue, olive, magenta,
pink and dark red represent low, intermediate to high tRFs and
tiRNAs expression, respectively. The colors in the hatmap
represent relative tRFs and tiRNAs expression as indicated in the
color key. tRFs, transfer RNA-derived fragments; tiRNAs,
tRNA halves; T, tumor tissue; N, normal tissue. |
 | Table III.The 48 various tRF and tiRNA
expressed within the pancreatic cancer samples. |
Table III.
The 48 various tRF and tiRNA
expressed within the pancreatic cancer samples.
| A, Upregulated
tDRs |
|---|
|
|---|
| tDRs_ID | tRNA | tDR type | Fold change,
T/N | P-value |
|---|
| AS-tDR-000546 | Glu-CTC-1-1 | tRF-5 | 3.515352854 | 0.028505313 |
| AS-tDR-011144 | Glu-TTC-2-1 | tiRNA-5 | 3.316925679 | 0.024712693 |
| AS-tDR-007301 | Glu-TTC-2-1 | tiRNA-5 | 2.218473266 | 0.030904399 |
| AS-tDR-001267 | Glu-TTC-2-1 | tiRNA-5 | 2.919735986 | 0.011554406 |
| AS-tDR-000882 | Lys-CTT-1-1 | tRF-5 | 3.900728682 | 0.027078399 |
| AS-tDR-001130 | Val-CAC-1-1 | tRF-5 | 10.18152502 | 0.023608439 |
| AS-tDR-005700 | Val-CAC-1-1 | tRF-3 | 7.86901301 | 0.008481533 |
| AS-tDR-000076 | Val-CAC-1-1 | tRF-3 | 8.846767284 | 0.025715623 |
| AS-tDR-000602 | Glu-TTC-1-1 | tRF-5 | 4.750337157 | 0.047733824 |
| AS-tDR-011075 | Glu-TTC-1-1 | tiRNA-5 | 6.476649011 | 0.033682034 |
| AS-tDR-001271 | Glu-TTC-1-1 | tiRNA-5 | 4.07538365 | 0.028804625 |
| AS-tDR-000017 | Gly-CCC-2-1 | tRF-5 | 172.3909573 | 0.037686709 |
| AS-tDR-005996 | Leu-AAG-1-1 | tRF-3 | 8.00593945 | 0.008751401 |
| AS-tDR-007240 | Leu-AAG-1-1 | tRF-3 | 8.343736727 | 0.017853307 |
| AS-tDR-000064 | Leu-AAG-1-1 | tRF-3 | 7.269608569 | 0.012542769 |
| AS-tDR-012559 | Leu-AAG-1-1 | tRF-3 | 9.5247251 | 0.017505317 |
| AS-tDR-006872 | Leu-AAG-1-1 | tRF-3 | 7.046389294 | 0.030169694 |
| AS-tDR-005835 | Val-AAC-2-1 | tRF-3 | 6.171351562 | 0.034156521 |
| AS-tDR-000619 | Glu-TTC-4-1 | tRF-5 | 184.2695023 | 0.02810437 |
| AS-tDR-007303 | Gly-GCC-4-1 | tRF-5 | 6.198056479 | 0.014263475 |
| AS-tDR-006895 | Ala-CGC-1-1 | tRF-3 | 168.4545482 | 0.009518626 |
| AS-tDR-000102 | Ala-CGC-1-1 | tRF-3 | 8.371135493 | 0.00433924 |
| AS-tDR-007001 | Gln-CTG-1-1 | tRF-3 | 156.4192978 | 0.008799826 |
| AS-tDR-000069 | Gln-CTG-1-1 | tRF-3 | 8.166729416 | 0.008389211 |
| AS-tDR-006440 | Gln-CTG-1-1 | tRF-3 | 237.5981942 | 0.003454101 |
| AS-tDR-005699 |
Val-AAC-chr6-84 | tRF-3 | 266.3540372 | 0.001098637 |
| AS-tDR-009340 |
Val-AAC-chr6-84 | tRF-3 | 231.4538129 | 0.027702861 |
| AS-tDR-000100 | Ala-AGC-2-1 | tRF-3 | 12.7381447 | 0.004182236 |
| AS-tDR-006235 | Ala-AGC-2-1 | tRF-3 | 216.309212 | 0.001144849 |
| AS-tDR-006247 | Ala-AGC-2-1 | tRF-3 | 217.0466322 | 0.013086257 |
| AS-tDR-006032 | Cys-GCA-11-1 | tRF-3 | 369.7327691 | 0.009656002 |
| AS-tDR-006924 | Cys-GCA-11-1 | tRF-3 | 168.3240454 | 0.018648999 |
| AS-tDR-000067 | Cys-GCA-11-1 | tRF-3 | 10.73455381 | 0.022127251 |
| AS-tDR-006255 | Val-CAC-3-1 | tRF-3 | 171.1292469 | 0.043234111 |
| AS-tDR-006288 | Val-CAC-3-1 | tRF-3 | 200.0867071 | 0.005066061 |
| AS-tDR-006001 | Leu-CAG-1-1 | tRF-3 | 144.0176019 | 0.00182195 |
| AS-tDR-000082 | Leu-CAG-1-1 | tRF-3 | 8.426255616 | 0.001442425 |
| AS-tDR-006875 | Leu-CAG-1-1 | tRF-3 | 129.3419781 | 0.002049951 |
| AS-tDR-006198 | Leu-CAG-1-1 | tRF-3 | 170.0118027 | 0.011517777 |
| AS-tDR-006051 | Gln-TTG-1-1 | tRF-3 | 255.8832937 | 0.008529051 |
| AS-tDR-000071 | Gln-TTG-1-1 | tRF-3 | 13.1479288 | 0.002794513 |
| AS-tDR-006580 | Asp-GTC-2-1 | i-tRF | 165.7441719 | 0.007588423 |
| AS-tDR-009829 |
pre-Leu-AAG-1-2 | tRF-1 | 226.3986073 | 0.002930597 |
| AS-tDR-006548 | Val-TAC-1-1 | tRF-3 | 208.6565351 | 0.003334287 |
| AS-tDR-000098 | Ala-AGC-1-1 | tRF-3 | 207.9900163 | 0.023503917 |
| AS-tDR-000080 | Leu-TAG-2-1 | tRF-3 | 158.4247545 | 0.00803969 |
|
| B, Downregulated
tDRs |
|
| tDRs_ID | tRNA | tDR type | Fold change,
T/N | P-value |
|
| AS-tDR-007337 |
pre-Pro-TGG-3-2 | tRF-1 | −196.1316037 | 0.028326535 |
| AS-tDR-001391 | Pro-CGG-1-1 | tiRNA-5 | −146.1942975 | 0.012735921 |
Reverse transcription-quantitative
(RT-q)-PCR
In the present study, four DE-tRFs and tiRNAs
(AS-tDR-000064, AS-tDR-000069, AS-tDR-000102 and AS-tDR-001391),
which exhibited abundant expression and were significantly
different between the pancreatic cancer samples and adjacent normal
tissue samples were selected for qPCR validation; the results
demonstrated that AS-tDR-000064, AS-tDR-000069 and AS-tDR-000102
were insignificantly upregulated to in the pancreatic cancer
samples compared with adjacent normal tissue samples, and
AS-tDR-001391 was insignificantly downregulated (Table IV), which was comparable with the
sequencing results presented in Table
III. However, the P-values did not indicate significant
differences due to the small sample size.
 | Table IV.Quantitative PCR results of tRFs and
tiRNAs selected for validation. |
Table IV.
Quantitative PCR results of tRFs and
tiRNAs selected for validation.
| tDRs_ID | tRNA | tDR type | Fold change,
T/N | P-value |
|---|
| AS-tDR-000064 | Leu-AAG-1-1 | tRF-3 | 1.45 | 0.54775 |
| AS-tDR-000069 | Gln-CTG-1-1 | tRF-3 | 1.20 | 0.80352 |
| AS-tDR-000102 | Ala-CGC-1-1 | tRF-3 | 1.36 | 0.51009 |
| AS-tDR-001391 | Pro-CGG-1-1 | tiRNA-5 | 0.24 | 0.07624 |
Predicted target genes of various tRFs
and tiRNAs and functional DE-tRF and tiRNA enrichment analysis
DE-AS-tDR-000064 was predicted to have 2,450 target
genes by miRanda and TargetScan; DE-AS-tDR-000069 was predicted 445
target genes; DE-AS-tDR-000102 was predicted 746 target genes; and
DE-AS-tDR-001391 was predicted 216 target genes. GO analyses
revealed that the target genes of AS-tDR-000064 were mostly
enriched in ‘Regulation of cellular process’ (Biological Process),
‘Synapse’ (Cellular Component) and ‘Enzyme binding’ (Molecular
Function) (Fig. 2). The target genes
of AS-tDR-000069 were mostly enriched in ‘Signaling’ (Biological
Process), ‘Plasma membrane part’ (Cellular Component) and
‘Phosphatidylinositol 3-kinase binding’ (Molecular Function)
(Fig. 3). The target genes of
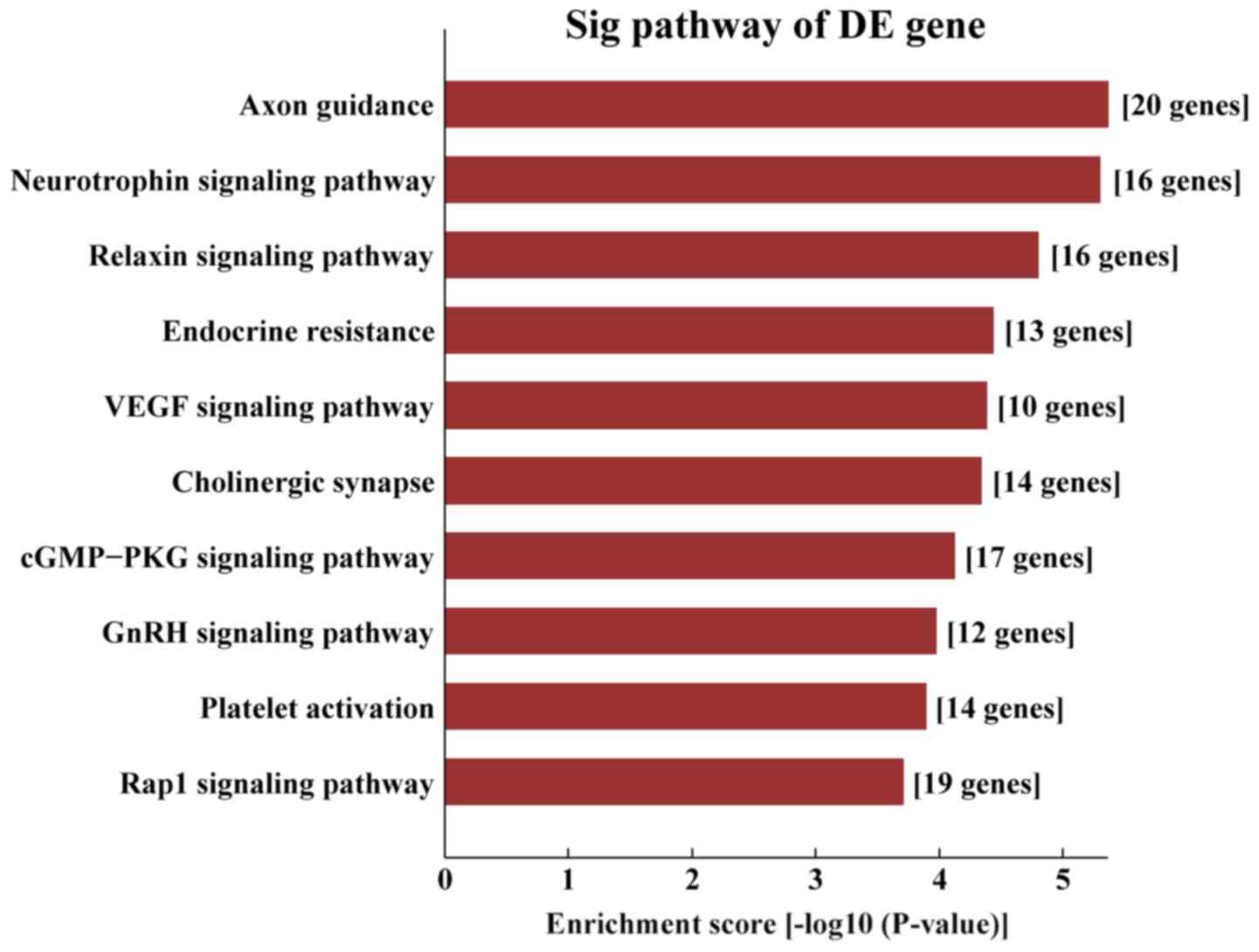
AS-tDR-000102 were mostly enriched in ‘Axon development’
(Biological Process), ‘Synapse part’ (Cellular Component) and
‘Sequence-specific DNA binding’ (Molecular Function) (Fig. 4). The target genes of AS-tDR-001391
were mostly enriched in ‘Neuromuscular process’ (Biological
Process), ‘Neuron part’ (Cellular Component) and ‘PDZ domain
binding’ (Molecular Function) (Fig.
5).
KEGG pathway analysis demonstrated that the target
genes of AS-tDR-000064 were mostly enriched in the ‘Ras signaling
pathway’ (Fig. 6), the target genes
of AS-tDR-000069 were mostly enriched in ‘the cancer pathways’
(Fig. 7), the target genes of
AS-tDR-000102 were mostly enriched in ‘axon guidance’ (Fig. 8) and the target genes of
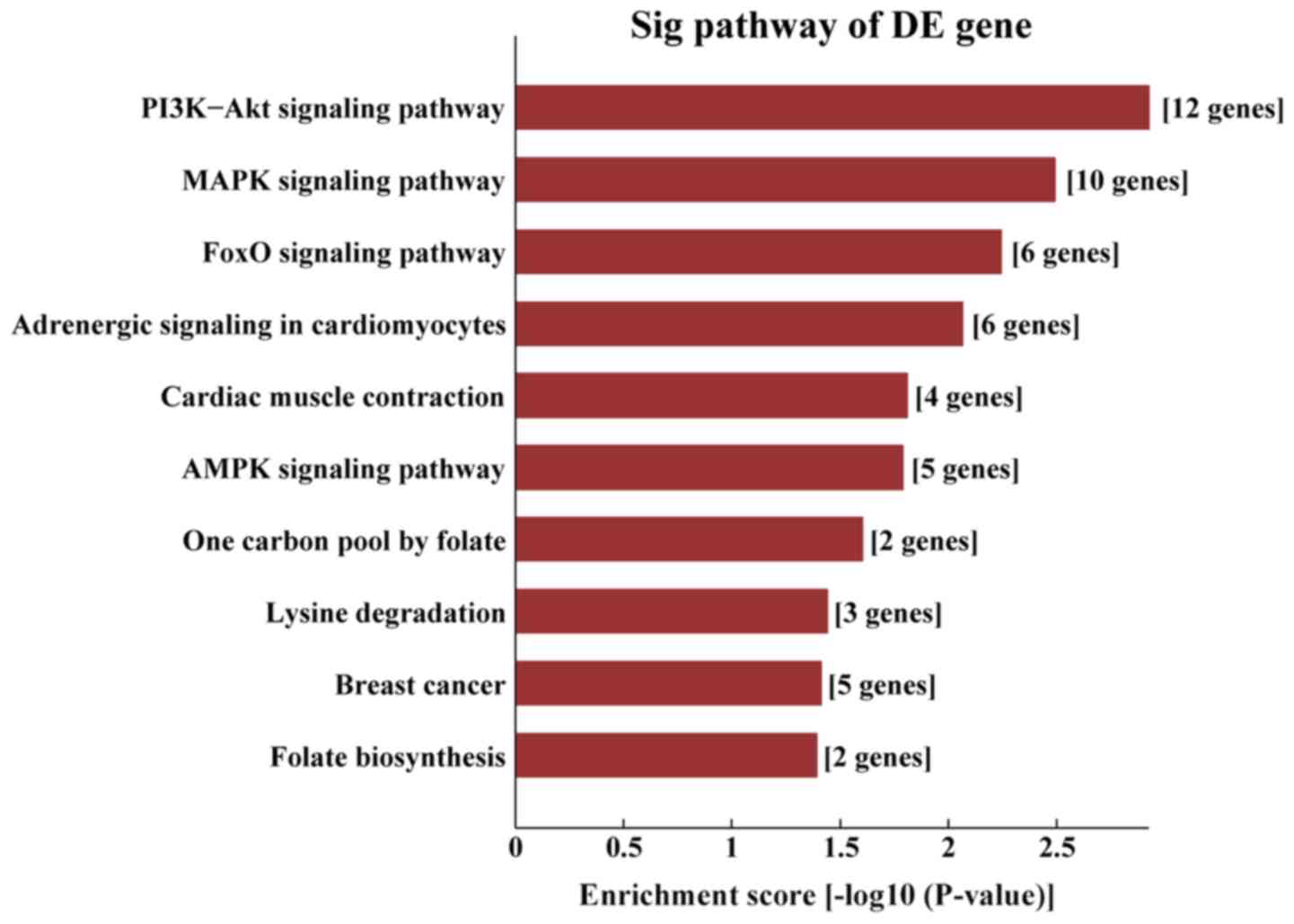
AS-tDR-001391 were mostly enriched in ‘the PI3K/protein kinase-B
(Akt) signaling pathway’ (Fig.
9).
Discussion
The technological advances in sequence technology,
along with the increase in sncRNA research, have resulted in the
discovery of a number of new small RNAs, including tRFs and tiRNAs.
Initially, tRFs and tiRNAs were described as a large group of
species, and information regarding their functions within cells has
been increasing. While studies have described tRFs and tiRNAs
expression in human cell lines, the actual retention within human
tissue remains unknown (8,17,28,29).
In the present study, an Illumina NextSeq instrument
was used to analyze standard small RNA sequences. Using the
criteria of log2FC≥2 and P-value<0.05 with the Arraystar tRFs
and tiRNA-seq data analysis package (version 4.1; DNASTAR), a total
of 48 DE-tRFs and tiRNAs were screened from the pancreatic cancer
samples and the adjacent normal tissue samples, including 46
upregulated tRFs and tiRNAs and two downregulated tRF and tiRNA. Of
these, four tRFs and tiRNAs were selected for further validation by
qPCR, and the results demonstrated that AS-tDR-000064,
AS-tDR-000069 and AS-tDR-000102 were upregulated in the pancreatic
cancer samples, though not significantly, compared with adjacent
normal tissue samples, and AS-tDR-001391 was downregulated, though
not significantly, which was consistent with the sequencing data,
with AS-tDR-001391 expression levels being the most different
between the pancreatic cancer samples and the adjacent normal
tissue samples. MiRanda and TargetScan were used to predict targets
of differentially expressed tRFs and tiRNAs. The target genes were
analyzed using DAVID to screen for significantly enriched KEGG
pathways and Gene Ontology biological processes, molecular
functions and cellular components.
It should be noted that the present study had
limitations. First, although the regulatory roles of AS-tDR-000064,
AS-tDR-000069, AS-tDR-000102 and AS-tDR-001391, which are
associated with tumors, have been demonstrated by sequencing, their
expression levels were not validated further, and thus, the
expression and role of systemic tumor-associated pathways in
functional experiments were not demonstrated. Secondly, as only
three patients were included in the present study, additional
consideration was not given to the different stages of the patients
with pancreatic cancer recruited for tRFs and tiRNAs sequencing.
Finally, the results from the present study are based only on tRFs
and tiRNAs sequencing analysis and bioinformatics predictions, and
further verification is required. These results form the foundation
of a primary study and can be used to an extent in future research,
particularly regarding selecting the direction of future studies on
pancreatic cancer.
In the present study, after the sequencing results
were obtained, further qPCR verification was performed in the
samples. tRFs and tiRNAs (AS-tDR-000064, AS-tDR-000069,
AS-tDR-000102 and AS-tDR-001391) with high expression and a large
statistical difference between the cancer samples and adjacent
normal tissue samples based on sequencing were selected for
verification. Whether the upregulation or downregulation of these 4
tRFs and tiRNAs are associated with the generation and/or
progression of human pancreatic cancer requires further
investigation in a large clinical cohort. In addition, the results
of the present study are based bioinformatics analysis alone.
Previous studies have revealed tRFs and tiRNAs to be present in
acquired metabolic disorders (16),
breast cancer (17), lung cancer
(19), colorectal cancer (20), B-cell lymphoma (24), stress injuries (29), neurodegenerative diseases (30) and prostate cancer (31), among others, but, to the best of our
knowledge, tRFs and tiRNAs in pancreatic cancer have not been
reported.
The importance of the assessed tRFs and tiRNAs in
the enrichment of cancer-associated pathways was demonstrated in
the present study, including ‘the Ras signaling pathway’, ‘cancer
pathways’, ‘axon guidance’ and ‘the PI3K/Akt signaling pathway’,
which suggests potential uses of AS-tDR-000064, AS-tDR-000069,
AS-tDR-000102 and AS-tDR-001391 as diagnostic and therapeutic
biomarkers for pancreatic cancer. However, to confirm these
results, further experiments are required. The expression levels of
tRF-1391 in clinical samples of pancreatic cancer and pancreatic
cancer cell lines will be validated as the next step in its
potential as a tumor marker.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Foundation for Changzhou's High-level Health Talent Cultivation
(grant no. 2016CZBJ007), the Social Development Foundation of
Science and Technology of Jiangsu (grant no. BE2016658), the
Changzhou Sci&Tech Program (grant no. CE20165020) and Project
of Changzhou Medical Innovation Team (grant no. CCX201807).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XQ and CZ designed the study. The experiment was
performed by LJ. XQ and CZ provided some of the samples and
experiment methods. LJ wrote the paper. CZ reviewed and edited the
manuscript. All the authors reviewed and approved the revised
manuscript.
Ethics approval and consent to
participate
The Affiliated Changzhou No. 2 People's Hospital of
Nanjing Medical University (Changzhou, China) approved the current
study. All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers.
2:160222016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parker SL, Tong T, Bolden S and Wingo PA:
Cancer statistics, 1997. CA Cancer J Clin. 47:5–27. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawaji H, Nakamura M, Takahashi Y,
Sandelin A, Katayama S, Fukuda S, Daub CO, Kai C, Kawai J, Yasuda
J, et al: Hidden layers of human small RNAs. BMC Genomics.
9:1572008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cole C, Sobala A, Lu C, Thatcher SR,
Bowman A, Brown JW, Green PJ, Barton GJ and Hutvagner G: Filtering
of deep sequencing data reveals the existence of abundant
Dicer-dependent small RNAs derived from tRNAs. RNA. 15:2147–2160.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YS, Shibata Y, Malhotra A and Dutta A:
A novel class of small RNAs: tRNA-derived RNA fragments (tRFs).
Genes Dev. 23:2639–2649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson DM and Parker AR: Stressing out
over tRNA cleavage. Cell. 138:215–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martens-Uzunova ES, Olvedy M and Jenster
G: Beyond microRNA-novel RNAs derived from small non-coding RNA and
their implication in cancer. Cancer Lett. 340:2012013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar P, Anaya J, Mudunuri SB and Dutta A:
Meta-analysis of tRNA derived RNA fragments reveals that they are
evolutionarily conserved and associate with AGO proteins to
recognize specific RNA targets. BMC Biol. 12:782014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thompson DM and Parker R: The RNase Rny1p
cleaves tRNAs and promotes cell death during oxidative stress in
Saccharomyces cerevisiae. J Cell Biol. 185:43–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamasaki S, Ivanov P, Hu GF and Anderson
P: Angiogenin cleaves tRNA and promotes stress-induced
translational repression. J Cell Biol. 185:35–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haussecker D, Huang YA, Parameswaran P,
Fire AZ and Kay MA: Human tRNA-derived small RNAs in the global
regulation of RNA silencing. RNA. 16:673–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar P, Mudunuri SB, Anaya J and Dutta A:
tRFdb: A database for transfer RNA fragments. Nucleic Acids Res.
43:(Database Issue). D141–D145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi
J, Feng GH, Peng H, Zhang X, Zhang Y, et al: Sperm tsRNAs
contribute to intergenerational inheritance of an acquired
metabolic disorder. Science. 351:397–400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goodarzi H, Liu X, Nguyen HC, Zhang S,
Fish L and Tavazoie SF: Endogenous tRNA-derived fragments suppress
breast cancer progression via YBX1 displacement. Cell. 161:790–802.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blanco S, Dietmann S, Flores JV, Hussain
S, Kutter C, Humphreys P, Lukk M, Lombard P, Treps L, Popis M, et
al: Aberrant methylation of tRNAs links cellular stress to
neuro-developmental disorders. EMBO J. 33:2020–2039. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao Y, Sun Q, Liu X, Wang P, Wu R and Ma
Z: tRF-Leu-CAG promotes cell proliferation and cell cycle in
non-small cell lung cancer. Chem Biol Drug Des. 90:730–738. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang B, Yang H, Cheng X, Wang D, Fu S,
Shen W, Zhang Q, Zhang L, Xue Z, Li Y, et al: tRF/miR-1280
suppresses stem cell-like cells and metastasis in colorectal
cancer. Cancer Res. 77:3194–3206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gebetsberger J, Zywicki M, Künzi A and
Polacek N: tRNA-derived fragments target the ribosome and function
as regulatory non-coding RNA in Haloferax volcanii. Archaea.
2012:2609092012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ivanov P, Emara MM, Villen J, Gygi SP and
Anderson P: Angiogenin-induced tRNA fragments inhibit translation
initiation. Mol Cell. 43:613–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang S, Sun L and Kragler F: The
phloem-delivered RNA pool contains small noncoding RNAs and
interferes with translation. Plant Physiol. 150:378–387. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maute RL, Schneider C, Sumazin P, Holmes
A, Califano A, Basso K and Dalla-Favera R: tRNA-derived microRNA
modulates proliferation and the DNA damage response and is
downregulated in B cell lymphoma. Proc Natl Acad Sci USA.
110:1404–1409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edge SB and Compton CC: The American Joint
Committee on Cancer the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Durdevic Z and Schaefer M: tRNA
modifications: Necessary for correct tRNA-derived fragments during
the recovery from stress? Bioessays. 35:323–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martens-Uzunova ES, Jalava SE, Dits NF,
van Leenders GJ, Møller S, Trapman J, Bangma CH, Litman T,
Visakorpi T and Jenster G: Diagnostic and prognostic signatures
from the small non-coding RNA transcriptome in prostate cancer.
Oncogene. 31:978–991. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mishima E, Inoue C, Saigusa D, Inoue R,
Ito K, Suzuki Y, Jinno D, Tsukui Y, Akamatsu Y, Araki M, et al:
Conformational change in transfer RNA is an early indicator of
acute cellular damage. J Am Soc Nephrol. 25:2316–2326. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greenway MJ, Andersen PM, Russ C, Ennis S,
Cashman S, Donaghy C, Patterson V, Swingler R, Kieran D, Prehn J,
et al: ANG mutations segregate with familial and ‘sporadic’
amyotrophic lateral sclerosis. Nat Genet. 38:411–413. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olvedy M, Scaravilli M, Hoogstrate Y,
Visakorpi T, Jenster G and Martens-Uzunova ES: A comprehensive
repertoire of tRNA-derived fragments in prostate cancer.
Oncotarget. 7:24766–24777. 2016. View Article : Google Scholar : PubMed/NCBI
|