Introduction
As the most aggressive skin cancer, melanoma is
characterized by the rapid progression (1). Surgical resection of primary tumors
usually results in satisfactory outcomes for patients at early
stages (2). However, the development
of melanoma is usually accompanied by tumor metastasis to regional
lymph nodes or even distant organs, which lacks radical treatment
(3). At present, the 5-year survival
rate of metastatic melanoma patients remains <20% (4). The unclear pathogenesis of melanoma is
the major challenge for clinical treatment of this disease
(5). Identification of novel
therapeutic targets is always needed to improve the survival of
melanoma patients.
Long non-coding RNAs (lncRNAs) are RNA transcripts
consisting of >200 nucleotides with no protein-coding capacity
(6). Different from messenger RNAs,
lncRNAs participate in cellular processes by regulating gene
expression at post-transcriptional and translational levels, or
even through epigenetic pathways (6,7). There
is mounting evidence that lncRNAs are critical determinants in
human diseases, and dysregulated lncRNA expression is closely
correlated with the occurrence of many cancers (8). Regulation of lncRNA expression has been
proven as potential therapeutic target for cancer treatment
(9). However, function of most
lncRNAs remains unclear. LncRNA LINC-PINT is a recently identified
tumor suppressor in different types of cancer, such as
lymphoblastic leukemia (10,11). In the present study we investigate
the involvement of LINC-PINT in melanoma, and explored its
interactions with BANCR, which promotes melanoma (12).
Materials and methods
Research subjects
A total of 60 patients with melanoma [35 males and
25 females; age range, 28 to 69 years; mean age, 49.7±5.6 (standard
deviation) years] were enrolled in Chongqing Traditional Chinese
Medicine Hospital (Chongqing, China) between January 2015 and
January 2018. All patients were diagnosed pathologically by at
least 3 experienced pathologists. The inclusion criteria for
enrolment in the current study were as follows: i) Patients with
melanoma patients with no history of other malignancies; and ii)
patients willing to participate. The exclusion criteria were as
follows: i) Patients complicated with other skin diseases or other
severe diseases, including other types of cancer; and ii) patients
who had been treated within 3 months prior to admission. According
to the American Joint Committee on Cancer staging (13), there were 23, 18 and 19 cases at
stage I, II and III, respectively. According to the thickness of
primary tumors, there were 15 cases <1 mm, 16 cases between 1–2
mm, 15 cases between 2–4 mm and 14 cases >4 mm. The current
study was approved by the Ethics Committee of Chongqing Traditional
Chinese Medicine Hospital (Chongqing, China). All patients signed
informed consent.
Specimens and cell lines
Tumor tissues and adjacent (within 2 cm around
tumors) healthy tissues were collected through biopsy and were
stored in a liquid nitrogen sink at −196°C prior to use. Tissues
were stored in liquid nitrogen prior to use. The melanoma cell
lines A375-P and A375-MA2 (ATCC; American Type Culture Collection,
Manassas, VA, USA) were used in the current study. Cells were
cultured using ATCC-formulated Dulbecco's Modified Eagle Medium
(American Type Culture Collection) containing 10% fetal bovine
serum (American Type Culture Collection) in an incubator at 37°C
and 5% CO2.
Total RNA extraction and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
RNAzol® reagent (GeneCopoeia, Inc.,
Rockville, MD, USA) was used to extract total RNA from tissue
specimens and in vitro cultured A375-P and A375-MA2 cells.
Tissues were ground in liquid nitrogen prior to the addition of
RNAzol® reagent. A RevertAid RT Reverse Transcription
Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
synthesize cDNA through reverse transcription using the following
conditions: 25°C for 5 min, 55°C for 30 min and 80°C for 15 min. To
detect the expression of LINC-PINT and BANCR, SYBR™-Green Master
mix (Thermo Fisher Scientific, Inc.) was used to prepare all PCR
reaction systems. CFX96 Touch™ Real-Time PCR Detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to perform
all PCR reactions with 18S RNA as endogenous control. Primer
sequences were as follows: LINC-PINT, forward,
5′-CGTGGGAGCCCCTTTAAGTT-3′ and reverse, 5′-GGGAGGTGGCGTAGTTTCTC-3′;
BANCR forward, 5′-ACAGGACTCCATGGCAAACG-3′ and reverse,
5′-ATGAAGAAAGCCTGGTGCAGT-3′; and 18S forward,
5′-GCTTAATTTGACTCAACACGGGA-3′ and reverse,
5′-AGCTATCAATCTGTCAATCCTGTC-3′. The following thermocylcing
conditions were used: 95°C for 30 sec, followed by 40 cycles of
95°C for 10 sec and 58°C for 35 sec and a final extension step at
72°C for 40 sec. Expression of LINC-PINT and BANCR was normalized
to 18S using the 2−ΔΔCq method (14).
Vectors and cell transfection
pcDNA3.1 vectors expressing LINC-PINT and BANCR were
designed and constructed by Sangon Biotech Co., Ltd. (Shanghai,
China). A375-P and A375-MA2 cells were cultured overnight to reach
70–80% confluence, followed by cell transfection performed using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol, with 10 nM LINC-PINT and
BANCR vectors or empty vectors (negative control, NC).
Untransfected cells were were used as control (C) cells. Cells were
harvested 24 h following transfection and used for subsequent
experimentation. Transfection efficiency was determined using
RT-qPCR.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) (Beyotime Institute
of Biotechnology, Haimen, China) was used to measure the cell
proliferation rate 24 h following transfection. Cells were
collected and single cell suspensions were prepared. Cell density
was adjusted to 5×104 cells/ml. Each well of a 96-well
plate was filled with 100 µl cell suspension. The plate was
incubated at 37°C in a 5% CO2 incubator, followed by the
addition of 10 µl CCK-8 solution 24, 28, 72 and 96 h later. The
cells were subsequently incubated for an additional 4 h at 37°C.
After adding 10 µl DMSO, optical density values at a wavelength of
450 nm were measured to assess cell proliferation.
Statistical analysis
Three biological replicates were performed for each
experiment. GraphPad Prism software (version 6; GraphPad Software,
Inc., La Jolla, CA, USA) was used to process the data and perform
statistical analysis. Data are expressed as the mean ± standard
deviation. Comparisons of expression levels of LINC-PINT and BANCR
between melanoma and healthy adjacent tissues were performed using
a paired t-test. Comparisons of LINC-PINT and BANCR among the tumor
thickness groups, as well as comparisons of expression levels of
LINC-PINT and BANCR and cell proliferation data among cell groups
were performed by one-way ANOVA followed by a Tukey post hoc test.
Associations between LINC-PINT and BANCR were analyzed by linear
regression. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of LINC-PINT and BANCR is
altered in melanoma tissues
The expression levels of LINC-PINT and BANCR in 60
patients with melanoma were detected by RT-qPCR. Compared with
healthy adjacent tissues, LINC-PINT was significantly downregulated
in tumor tissues (Fig. 1A;
P<0.05). By contrast, BANCR was significantly upregulated in
tumor tissues compared with healthy adjacent tissues (Fig. 1B; P<0.05).
Expression of LINC-PINT and BANCR is
affected by tumor thickness
Primary tumors were classified based on thickness.
There were 15 cases <1 mm, 16 cases between 1–2 mm, 15 cases
between 2–4 mm and 14 cases >4 mm. The expression levels of
LINC-PINT significantly decreased (Fig.
2A; P<0.05), while the expression levels of BANCR
significantly increased (Fig. 2B;
P<0.05) with increasing tumor thickness.
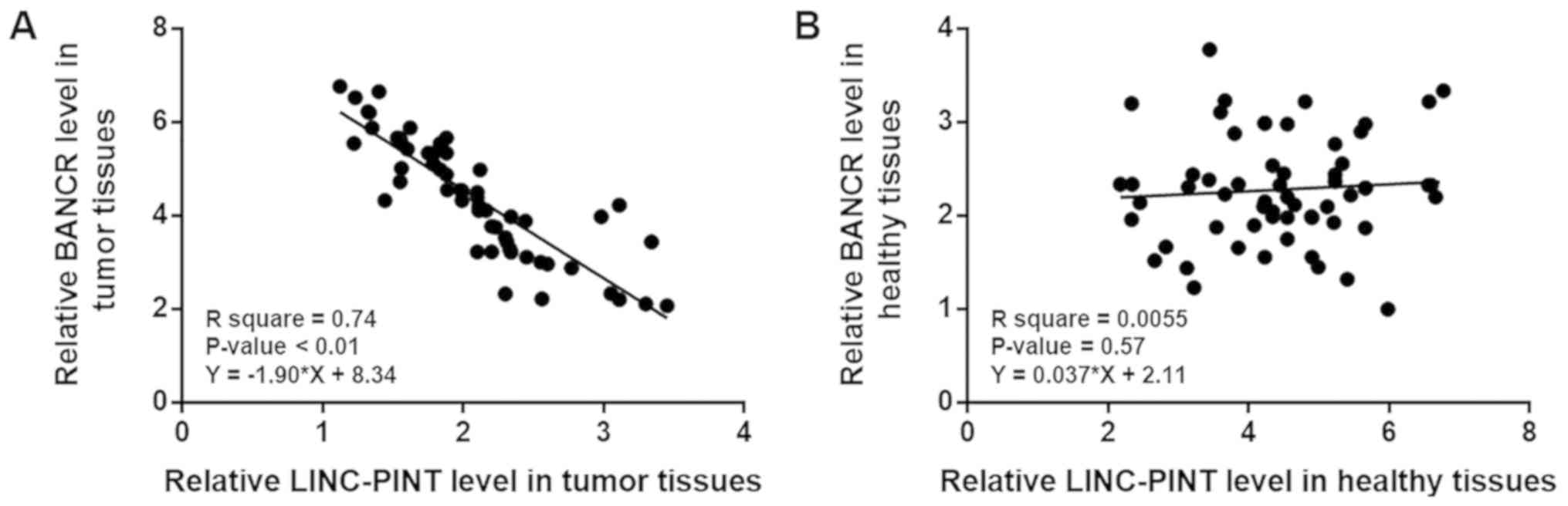
LINC-PINT and BANCR are inversely
associated
Associations between LINC-PINT and BANCR were
analyzed by linear regression. The expression levels of LINC-PINT
and BANCR were significantly and inversely associated in melanoma
tissues (Fig. 3A; P<0.01).
However, LINC-PINT and BANCR expression levels were not
significantly associated in healthy adjacent tissues (Fig. 3B; P=0.57).
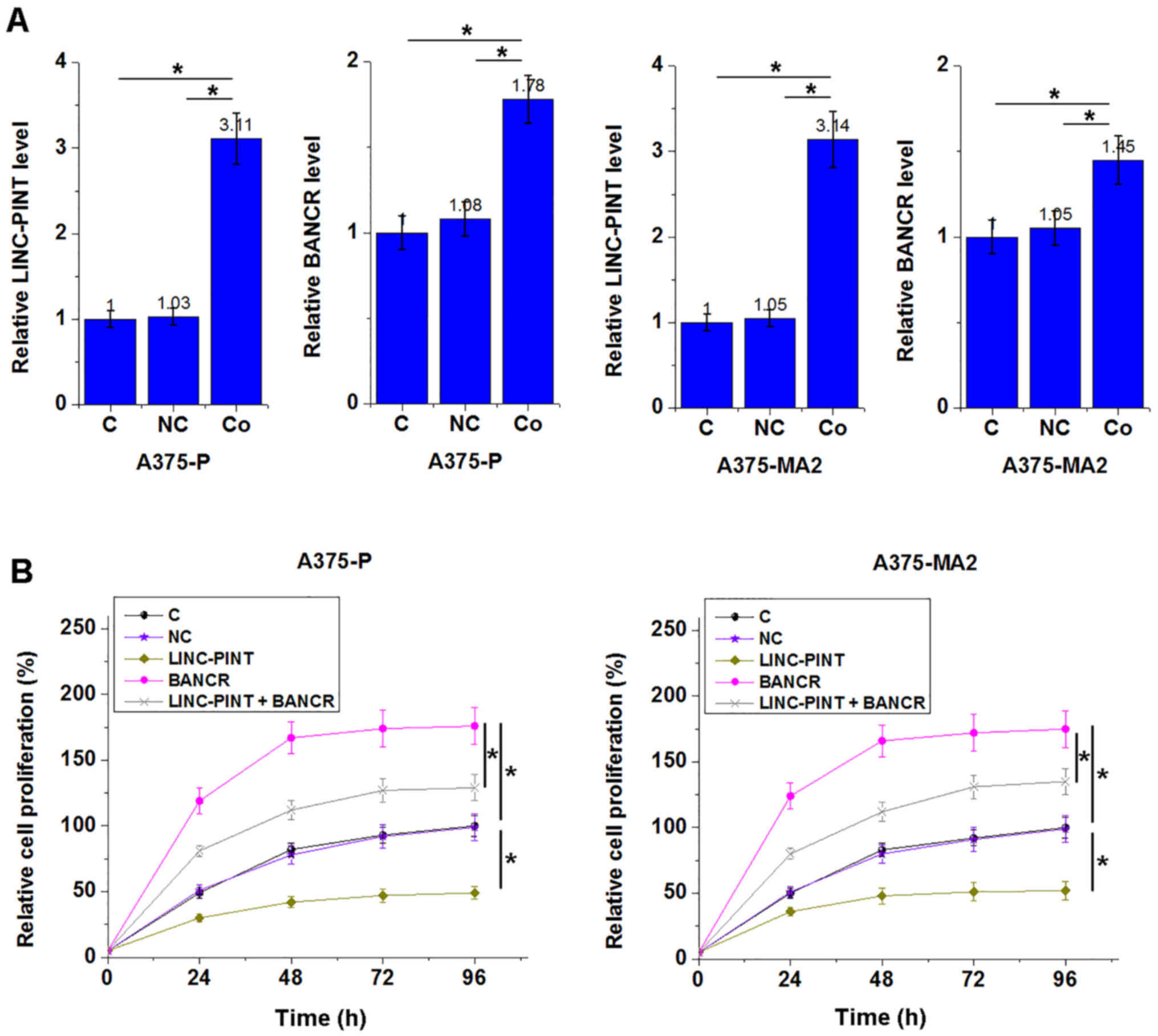
LINC-PINT is a likely upstream
inhibitor of BANCR in melanoma cells
The significantly inverse association between
LINC-PINT and BANCR in tumor tissues indicated the possible
interactions between LINC-PINT and BANCR. To further investigate
the interaction between LINC-PINT and BANCR, vectors expressing
LINC-PINT and BANCR were transfected into A375-P and A375-MA2
melanoma cell lines. Overexpression in A375-P and A375-MA2 cells
was achieved 24 h following transfection and compared with
untransfected cells (C group) and cells transfected with empty
vectors (NC group; Fig. 4A;
P<0.05). Compared with the C and NC groups, cells overexpressing
LINC-PINT revealed significantly downregulated BANCR levels
(Fig. 4B; P<0.05), while cells
with BANCR overexpression revealed no significant changes in the
LINC-PINT expression level compared with the C and NC groups
(Fig. 4C; P>0.05).
LINC-PINT overexpression inhibits
melanoma cell proliferation through BANCR
LINC-PINT and BANCR expression levels were
significant increased following co-transfection with LINC-PINT and
BANCR expression vectors compared with the C and NC groups
(Fig. 5A; P<0.05). Compared with
untransfected cells (C group) and cells transfected with empty
vectors (NC group), cell proliferation was decreased in cells
overexpressing LINC-PINT at 96 h (Fig.
5B; P<0.05). BANCR overexpression increased proliferation
compared with the C and NC groups (Fig.
5B; P<0.05). Co-transfection with LINC-PINT and BANCR
expression vectors attenuated the effects of LINC-PINT
overexpression (Fig. 5B;
P<0.05).
Discussion
LINC-PINT is a recently identified tumor suppressor
in different types of cancer, including retinoblastoma and gastric
cancer (10,11); however, its role in melanoma remains
unknown. The current study, to the best of our knowledge, was the
first to show the downregulated expression pattern of LINC-PINT in
melanoma, and suggested that LINC-PINT may be a tumor suppressor in
this disease. Furthermore, the current study demonstrated that the
actions of LINC-PINT in melanoma are likely achieved through the
interaction with BANCR.
BANCR is a well-characterized oncogenic lncRNA in
different types of cancer, including retinoblastoma and gastric
cancer (12,15,16).
Upregulation of BANCR promoted tumor growth and metastasis, and
indicated poor survival of patients with retinoblastoma and gastric
cancer (15,16). Li et al (12) demonstrated that BANCR promoted cancer
cell proliferation in malignant melanoma. Consistent with the
aforementioned result, the current study revealed upregulated
expression of BANCR in melanoma tissues compared with healthy
adjacent tissues. Furthermore, overexpression of BANCR promoted
proliferation of melanoma cells in vitro. The results
obtained in the current study further demonstrated the oncogenic
roles of BANCR in melanoma.
The oncogenic or tumor suppression roles of lncRNAs
are achieved through the interactions with downstream tumor
suppression or oncogenic pathways (17,18).
Previous studies have revealed that lncRNAs may interact with other
non-coding RNAs, including microRNAs, to participate in cancer
biology (17–19). However, studies on the interactions
between different lncRNAs are rare. The present study revealed that
LINC-PINT is downregulated in melanoma tissues compared with
healthy adjacent tissues and may serve a role as tumor suppressor
in this disease. Furthermore, the present study suggested that
LINC-PINT may exert its effects in melanoma by serving as an
upstream inhibitor of BANCR. BANCR has previously been revealed to
activate the mitogen-activated protein kinase (MAPK) signaling
pathway to promote the development of melanoma (12). A previous study demonstrated that
LINC-PINT interacts with MAPK in acute myocardial infarction
(20). Additionally, BANCR has been
reported to interact with MAPK (13). Therefore, LINC-PINT may interact the
BANCR/MAPK signaling pathway to inhibit melanoma cell
proliferation, and MAPK may mediate the interaction between
LINC-PINT and BANCR. However, the current study did not investigate
the role of MAPK. Future studies are required to elucidate the role
of MPAK in the interaction between LINC-PINT and BANCR. The results
obtained in the current study enriched the understanding of the
molecular mechanisms in melanoma.
Notably, LINC-PINT overexpression failed to
significantly affect the migration and invasion of melanoma cells
(data not shown). Therefore, LINC-PINT may specifically inhibit the
proliferation, but not other behaviors, of melanoma cells.
In conclusion, LINC-PINT is downregulated in
melanoma, and LINC-PINT overexpression may inhibit melanoma cell
proliferation by downregulating BANCR. The current study suggested
that LINC-PINT may serve as a potential therapeutic target for
melanoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML designed the study. QH, QD and DZ performed all
the experiments, analyzed the data and were major contributors in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of Chongqing Traditional Chinese Medicine Hospital. All
the patients provided written informed consent for participation in
this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
MacKie RM, Hauschild A and Eggermont AM:
Epidemiology of invasive cutaneous melanoma. Ann Oncol. 20 (Suppl
6):vi1–vi7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lund VJ, Chisholm EJ, Howard DJ and Wei
WI: Sinonasal malignant melanoma: An analysis of 115 cases
assessing outcomes of surgery, postoperative radiotherapy and
endoscopic resection. Rhinology. 50:203–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schadendorf D, Hodi FS, Robert C, Weber
JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM and Wolchok JD:
Pooled analysis of long-term survival data from phase II and phase
III trials of ipilimumab in unresectable or metastatic melanoma. J
Clin Oncol. 33:1889–1894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takata M, Murata H and Saida T: Molecular
pathogenesis of malignant melanoma: A different perspective from
the studies of melanocytic nevus and acral melanoma. Pigment Cell
Melanoma Res. 23:64–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Xuan Z and Liu C: Long non-coding
RNAs and complex human diseases. Int J Mol Sci. 14:18790–18808.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi P and Du X: The long non-coding RNAs, a
new cancer diagnostic and therapeutic gold mine. Mod Pathol.
26:155–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marín-Béjar O, Mas AM, González J,
Martinez D, Athie A, Morales X, Galduroz M, Raimondi I, Grossi E,
Guo S, et al: The human lncRNA LINC-PINT inhibits tumor cell
invasion through a highly conserved sequence element. Genome Biol.
18:2022017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garitano-Trojaola A, José-Enériz ES,
Ezponda T, Unfried JP, Carrasco-León A, Razquin N, Barriocanal M,
Vilas-Zornoza A, Sangro B, Segura V, et al: Deregulation of
linc-PINT in acute lymphoblastic leukemia is implicated in abnormal
proliferation of leukemic cells. Oncotarget. 9:12842–12852. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L
and Sha N: Long non-coding RNA BANCR promotes proliferation in
malignant melanoma by regulating MAPK pathway activation. PLoS One.
9:e1008932014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gershenwald JE and Scolyer RA: Melanoma
staging: American joint committee on cancer (AJCC) 8th edition and
beyond. Ann Surg Oncol. 25:2105–2110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su S, Gao J, Wang T, Wang J, Li H and Wang
Z: Long non-coding RNA BANCR regulates growth and metastasis and is
associated with poor prognosis in retinoblastoma. Tumour Biol.
36:7205–7211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Zhang L, Zhang Y and Zhou F:
Increased expression of LncRNA BANCR is associated with clinical
progression and poor prognosis in gastric cancer. Biomed
Pharmacother. 72:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spizzo R, Almeida MI, Colombatti A and
Calin GA: Long non-coding RNAs and cancer: A new frontier of
translational research? Oncogene. 31:4577–4587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu J, Gu H, Lv X, Yuan C, Ni P and Liu F:
LINC-PINT activates the mitogen-activated protein kinase pathway to
promote acute myocardial infarction by regulating miR-208a-3p. Circ
J. 82:2783–2792. 2018. View Article : Google Scholar : PubMed/NCBI
|