Introduction
Biomarkers are biomolecules in the blood or other
body fluids and tissues used to identify a disease state (1). The occurrence or prognosis of a
disorder or disease is reflected by the normal or abnormal
expression of these biomarkers (2).
Current research on biomarkers has focused primarily on the
expression levels of mRNA, microRNA (miRNA) and long non-coding RNA
(lncRNA), and predicting the associated pathological changes and
phenotypes (3–5).
Colorectal cancer (CRC) has become the third most
common malignant cancer among males and females worldwide (6). Epidemiological studies have reported a
5-year survival rate of 54% for CRC (7). A recent study found that the 5-year
survival rate of CRC has increased by 10% and, due to the different
stages of the disease and different characteristics, this disease
remains a serious public health issue (8).
There is currently no efficacious neoadjuvant
treatment, surgery, chemotherapy, radiotherapy, or immunotherapy
for colon cancer. At present, to the best of our knowledge, there
are no accurate tumor markers for colon cancer and identification
of such biomarkers may contribute to early cancer diagnosis and
screening (9,10). Identification of mRNAs and lncRNAs as
potential biomarkers requires complex and expensive deep sequencing
processes for validation (11).
Therefore, the current study aimed to identify genetic biomarkers
and therapeutic targets that can be used to detect CRC and predict
patient survival.
Materials and methods
Data collection
GSE21510 (12) and
GSE32323 (13) gene expression data
were analyzed using the Affymetrix Human Genome U133 Plus 2.0
Array. The GSE21510 dataset includes data from 123 patients with
CRC and 25 healthy controls, whereas the GSE32323 dataset includes
17 samples from patients with CRC and 17 samples from healthy
controls. Both microarray datasets were retrieved from the Gene
Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/).
Identification of differentially
expressed (DE) genes
R (version 3.5.2) (14) and R Studio (version 1.1.383)
(15) were used for data analysis.
Prior to the analysis of DE genes, principal component analysis
which was performed using ggord package (version 1.0.0; zenodo.org/badge/latestdoi/35334615) on
the samples from the two datasets to ensure that the data can be
used. DE genes between patients with colon cancer and normal
subjects were identified using the limma (version 3.25.15;
bioinf.wehi.edu.au/limma) software
package in R with the empirical Bayesian approach for linear models
(16). The empirical Bayesian
approach is equivalent to shrinkage of the estimated sample
variances towards a pooled estimate, resulting in a far more stable
inference when the number of arrays is small (17). Only genes which met the criteria of
P<0.01 and |log2 fold-change| >1.5 were selected as DE genes
in the present study. Venn diagram was used to visualize
intersection of genes from GSE21510 and GSE32323 datasets. Finally,
1,126 genes were selected for further analysis.
Functional and pathway enrichment
analysis
Pathway analysis was based on the Kyoto Encyclopedia
of Genes and Genomes (KEGG) database (www.genome.jp/kegg) with P<0.05. Clusterprofiler
(version 3.13.0) (18) was used to
analyze pathway enrichment and obtain false discovery rates.
Construction of the biological
network
Protein-protein interaction (PPI) network data were
downloaded from the Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING version 11.0) database (19). A PPI was used to visualize how
proteins interact with each other. At the same time, the main
proteins in the network were annotated. A network was constructed
so that the connections between target DE genes could be
visualized.
Gene distribution, tumor stage and
survival analysis
STRING analysis using a spring model was used to
generate confidence scores, two genes which were significantly
differentially expressed, based on the confidence scores, were
identified through analysis of the PPI network. Two large clinical
sample databases, The Cancer Genome Atlas (TCGA; cancergenome.nih.gov) and Genotype-Tissue Expression
(GTEx; gtexportal.org), were used to determine
the survival of patients with CRC expressing CDKN1A and CDKN2B, and
patients without these genes by searching the key words ‘colon
cancer’. The distribution of these two genes was verified in the
TGCA colon cancer database. The database here were based upon data
generated by the TCGA Research Network (https://www.cancer.gov/tcga). The expression levels of
CDKN1A and CDKN2B were also determined at different clinical stages
according to the Tumor-Node-Metastasis staging system (20) in CRC. Expression level analysis was
performed using a one-way ANOVA, using the pathological stage as
variable for calculating differential expression. Gene Expression
Profiling Interactive Analysis (GEPIA; gepia.cancer-pku.cn) was
used to map the survival plots, and the patient's clinical
information was obtained from TCGA (21).
Results
Identification of DE genes
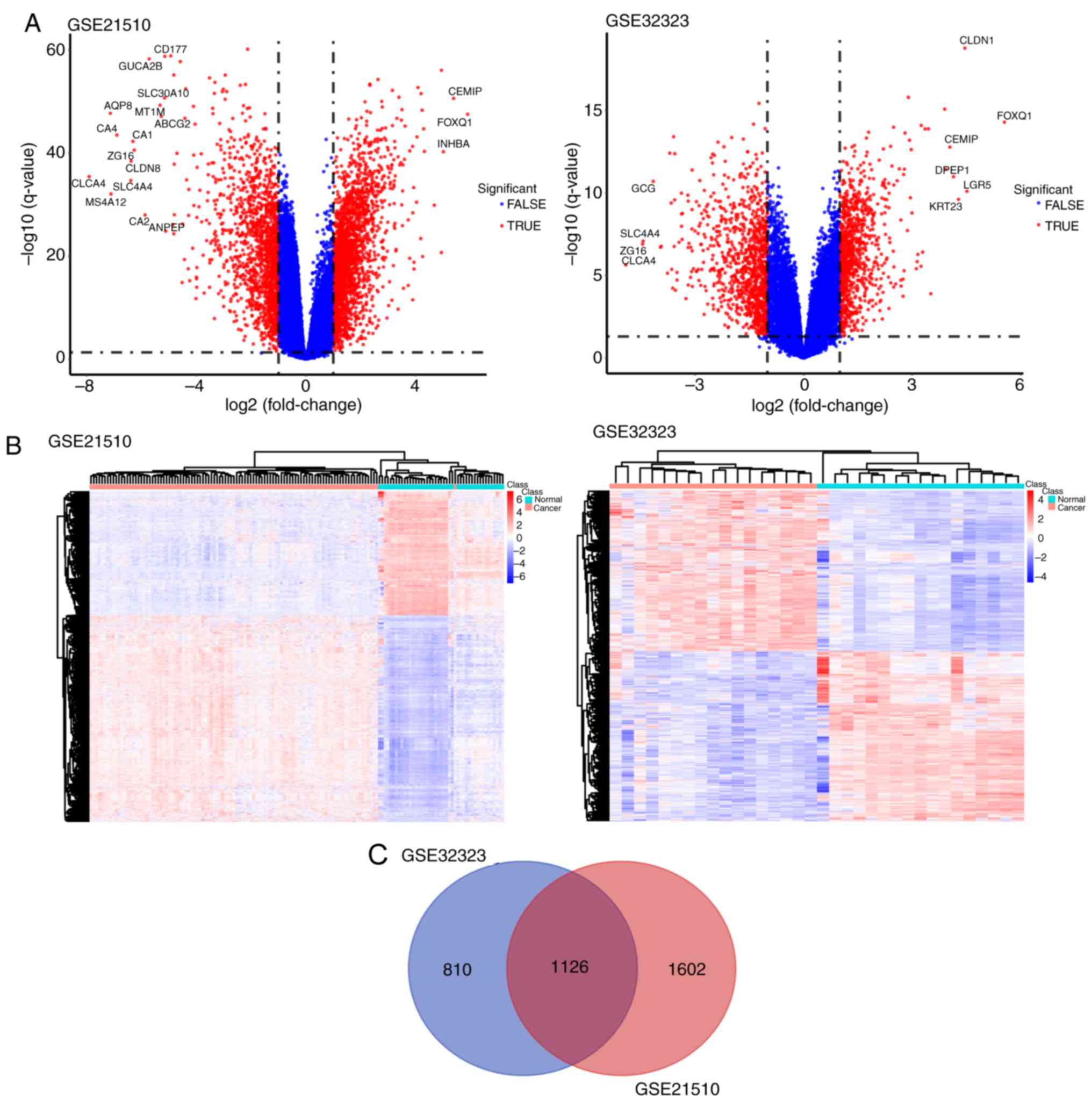
The results of the principal component analysis
revealed that differential tissue analysis can be performed between
normal and tumor tissue samples (Fig.
1). GSE32323 and GSE21510 datasets were selected and underwent
DE gene analysis using the limma package in R. A total of 1,936
genes were identified as either upregulated or downregulated in the
GSE32323 dataset and 2,728 DE genes were identified in the GSE21510
dataset (fold change ≥1.5 or ≤-1.5; P<0.01). Among the
identified DE genes, 1,126 were designated and listed as common
significantly DE genes through the Venn diagram analysis. All genes
plotted in red represented DE genes and the remaining genes were
plotted in blue (Fig. 2A). The DE
genes are presented in Fig. 1.
Significantly DE genes were presented in a heatmap and clustering
was observed between cancer and normal samples (Fig. 2B). The corresponding Venn diagram is
presented in Fig. 2C.
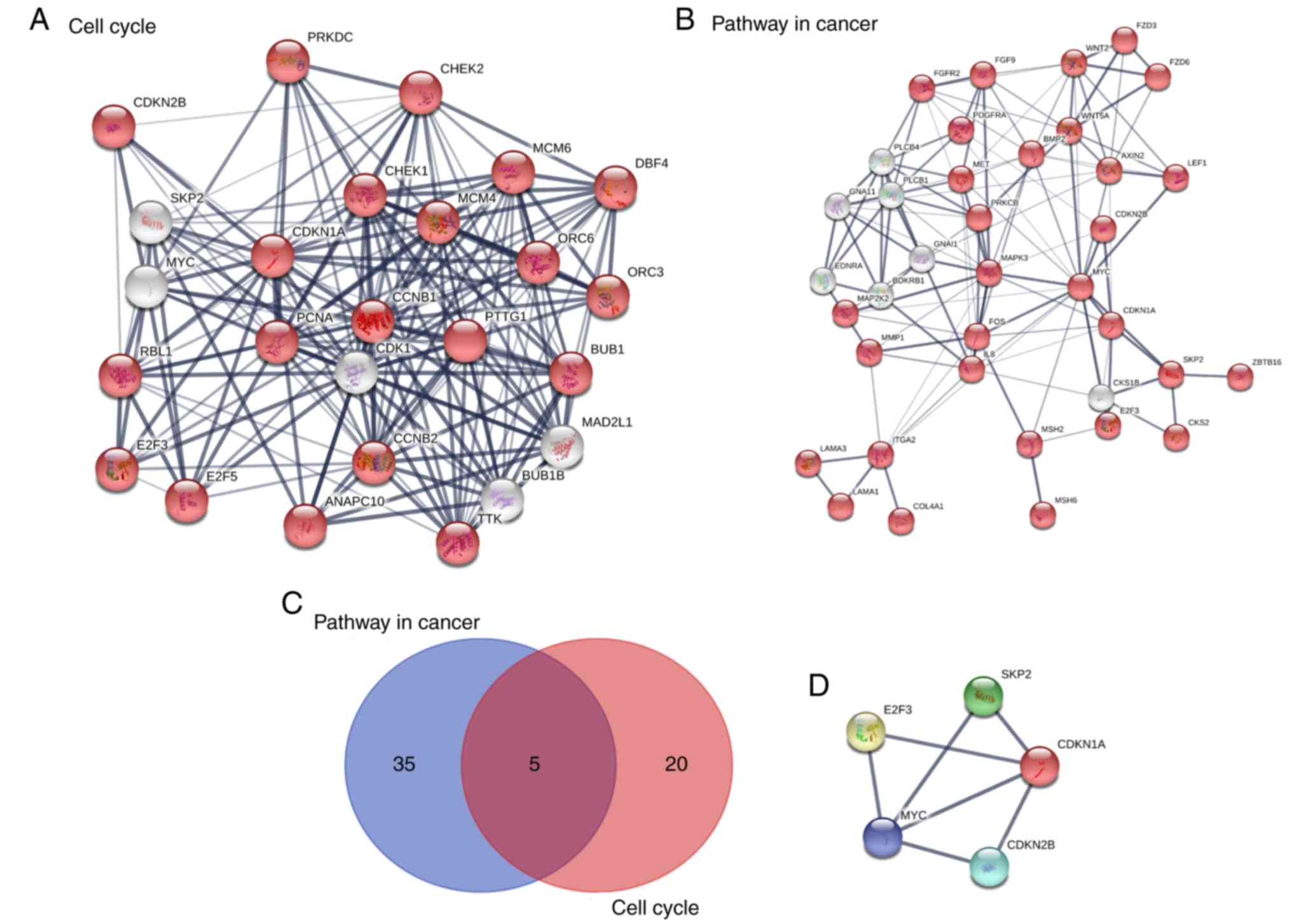
KEGG pathway analysis of DE genes and
construction of the PPI network
A total of 1,126 genes were subjected to KEGG
pathway analysis. All DE gene names were submitted to the STRING
database. The DE genes were significantly enriched in the ‘cell
cycle’, ‘mineral absorption’, ‘pancreatic secretion’, ‘pathways in
cancer’, ‘metabolic pathways’, ‘aldosterone-regulated sodium
reabsorption’ and ‘Wnt signaling pathway’. In terms of the
signaling pathway enrichment, these DE genes were enriched in the
cell cycle and tumor-associated pathways (Table I). An intersection between these two
signaling pathways was determined using Venn diagrams. E2F
transcription factor 2 (E2F2), S-phase kinase associated protein 2
(SKP2), MYC, cyclin-dependent kinase inhibitor 1A (CDKN1A) and
cyclin-dependent kinase inhibitor 2B (CDKN2B) were indicated to be
the common hub genes (Fig. 3).
 | Table I.Functional and pathway enrichment
analysis of the differentially expressed genes in colorectal
cancer. |
Table I.
Functional and pathway enrichment
analysis of the differentially expressed genes in colorectal
cancer.
| Term | Genes | False discovery
rate | P-value |
|---|
| hsa04110: Cell
cycle | CDK1, E2F2, E2F5,
DBF4, RBL1, SKP2, PRKDC, TTK, CHEK1, ANAPC10, CHEK2, PTTG1, MCM4,
MCM6, CCNB1, CDKN1A, CCNB2, MAD2L1, CDKN2B, PCNA, BUB1, BUB1B,
ORC6, MYC, ORC3 |
6.71×10−4 |
5.10×10−7 |
| hsa04978: Mineral
absorption | SLC11A2, SLC26A3,
CLCN2, MT1M, HMOX1, MT2A, MT1E, MT1H, MT1X, MT1G, MT1F | 0.57 |
4.36×10−4 |
| hsa04972:
Pancreatic secretion | KCNMA1, CLCA1,
CLCA4, SLC12A2, PRKCB, CEL, SLC26A3, PLCB4, ATP2A3, PLA2G2A, CPA3,
CA2, SLC4A4, PLCB1, SLC9A1 | 2.29 |
1.76×10−3 |
| hsa05200: Pathways
in cancer | FGFR2, WNT5A,
CKS1B, E2F2, PPARD, GNAI1, FGF9, GNA11, CXCL8, BDKRB1, ZBTB16,
MMP1, EDNRA, WNT2, FOS, PLCB4, CDKN2B, AXIN2, PLCB1, TRAF5, MYC,
MSH6, BMP2, COL4A1, EPAS1, MAP2K2, MSH2, MET, SKP2, LEF1, ITGA2,
FZD3, PRKCB, FZD6, LAMA1, CDKN1A, LAMA3, MAPK3, CKS2, PDGFRA | 3.20 |
2.47×10−3 |
| hsa01100: Metabolic
pathways | B3GALT5, B3GALT4,
ADH1C, ADH1B, GPAT3, PRIM1, ASPA, PTGIS, ST3GAL4, CPOX, NANP,
LPCAT2, GLCE, PLCE1, NME1, AKR1B10, PLA2G2A, ACAA1, PRPS1, XDH,
GCNT3, AHCY, GCNT2, GNE, CTPS2, PPAT, B3GNT6, CDA, GCSH, DNMT3B,
MAOA, AK1, MAOB, HGD, GART, TST, CEL, POLD4, GGT6, RPE, HMGCS2,
MTR, AHCYL2, PC, ATP5D, CYP2C18, ANPEP, PSPH, CKB, ST6GALNAC6,
TDO2, PLCB4, HPSE, P4HA1, MGLL, TWISTNB, PLCB1, ATP6V0D1, HYAL1,
POLR1D, ACADS, DHRS9, POLR1C, POLR1B, ST6GALNAC1, ACADVL, ATP6V1C2,
ADO, PTGDS, ADK, TGDS, AOC1, UGP2, ALPI, SORD, FUT8, HSD17B2, UGDH,
UPP1, PIPOX, GLS2, DGKA, ALDH1A1, CKMT2, FUT3, FUT1, PLCD1, UGT2A3,
ACSL4, PAPSS2, PLA2G16, NAT2, SI, PCK1, GBA3, GBA2, MBOAT1, SMPD1,
PSAT1, PAICS | 3.87 |
2.99×10−3 |
| hsa04310: Wnt
signaling pathway | WNT5A, PPARD, MMP7,
LEF1, FZD3, PRKCB, FZD6, WNT2, GPC4, PLCB4, SFRP1, SFRP2, WIF1,
RUVBL1, AXIN2, PLCB1, MYC | 14.59 |
1.19×10−2 |
Common hub gene expression and cancer
stage analysis
Differential expression analysis of genes was
performed through the TCGA and Genotype-Tissue Expression (GTEx)
databases, in order to verify that the five aforementioned common
hub genes serve important roles in the development of colon cancer.
A total of 275 cancer samples and 349 normal samples were selected
from the TCGA and GTEx databases. E2F2, SKP2, MYC and CDKN1A showed
significantly increased expression in tumor samples compared with
normal tissue, while CDKN2B had significantly reduced expression in
tumor samples compared with normal tissue in the TCGA database
(Fig. 4A-E). Using patient
information included in the TCGA and GTEx databases, the present
study revealed that these five genes were expressed in different
stages of colon cancer (Fig. 4F-J);
however, there was no statistically significant difference between
the stages (F-value >0.05).
 | Figure 4.Expression of (A) CDKN1A, (B) CDKN2B,
(C) MYC, (D) E2F2 and (E) SKP2 in clinical colon cancer (red,
tumor; gray, normal). The expression level changes in different
stages of (F) CDKN1A, (G) CDKN2B, (H) MYC (I) E2F2 and (J) SKP2.
Differential gene expression analysis was analyzed using a one-way
ANOVA, with the pathological stage as the variable used for
calculating differential expression. Samples were obtained from The
Cancer Genome Atlas and Genotype-Tissue Expression datasets. |Log
fold-change| cut-off=1; *P<0.05. CDKN1A, cyclin-dependent kinase
inhibitor 1A; COAD, colon adenocarcinoma; T, tumor; N, normal;
CDKN2B, cyclin-dependent kinase inhibitor 2B; E2F2, E2F
transcription factor 2; SKP2, S-phase kinase associated protein
2. |
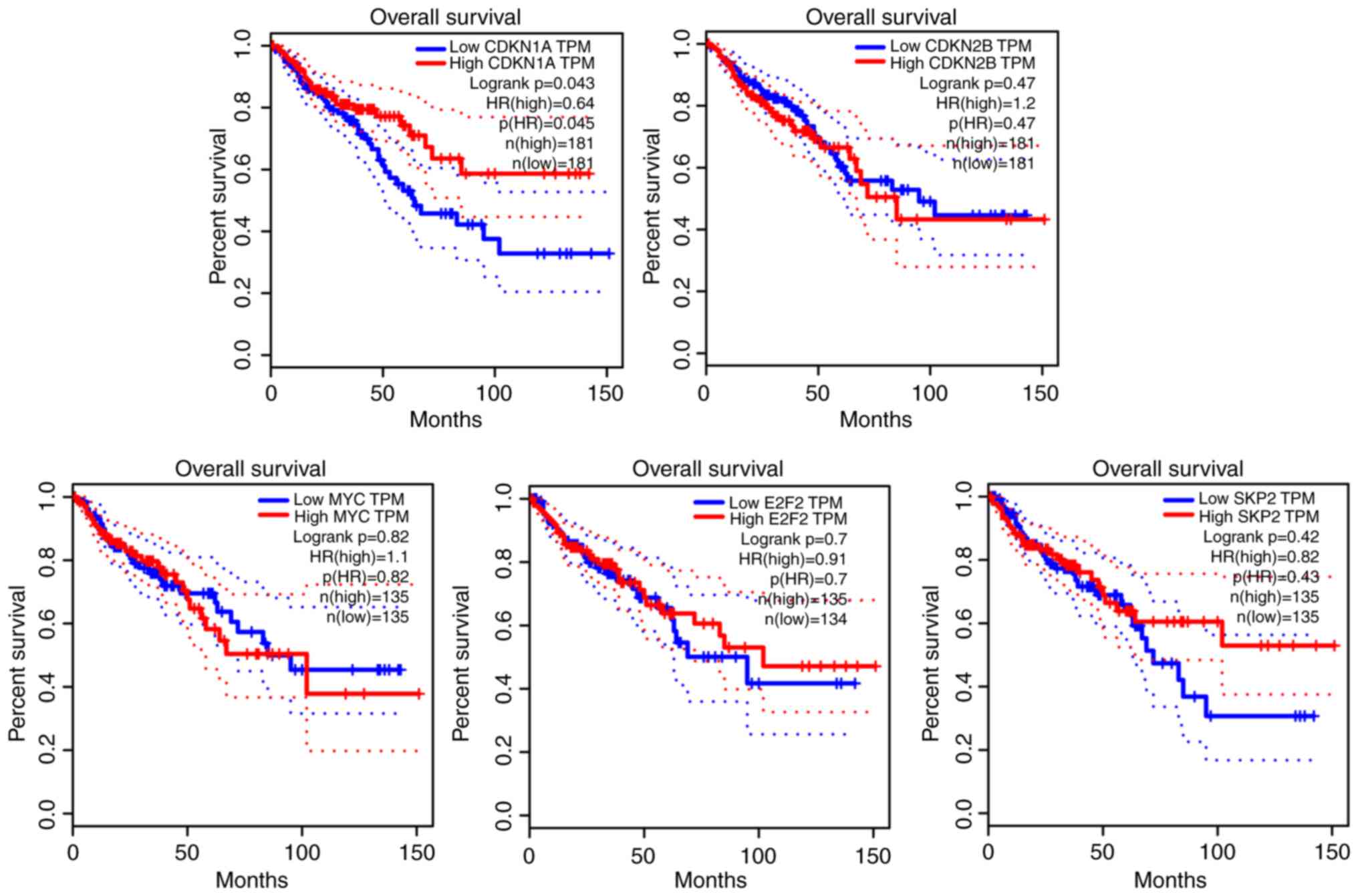
Survival analysis
Clinical information regarding 362 cases of colon
cancer was retrieved from the TGCA database. Data analysis revealed
that 181 patients with high CDKN1A expression had significantly
improved survival (P<0.05). However, the differential expression
of CDKN2B, MYC, E2F2 and SKP2 was not significantly associated with
the survival of patients from the TGCA database (P>0.05). The
results of the survival analysis are presented in Fig. 5.
Discussion
The present study analyzed data from GSE21510 and
GSE32323 gene expression datasets. Comparison of data from patients
with CRC and healthy patients resulted in identification of 1,126
DE genes with P<0.01 and fold-change ≥1.5 or ≤-1.5. All DE genes
between CRC and non-CRC samples were used for PPI network
construction, and for KEGG pathway enrichment and survival
analyses.
The interrelated pathways were subsequently
analyzed. The identified genes interacted directly or indirectly
with others. The analyzed DE genes were associated with a number of
pathways. ‘Cell cycle’, ‘mineral absorption’, ‘pancreatic
secretion’, ‘pathways in cancer’ and ‘Wnt signaling pathway’ were
the top pathways in the enrichment analysis. ‘Cell cycle’ and
‘pathways in cancer’ associated with the MAPK and p53 signaling
pathways are the most important components of cancer research
(22,23). In addition, the Wnt signaling pathway
plays an important role in numerous biological processes, including
embryonic development, cell cycle regulation, inflammation and
cancer (24). These alterations
converge into an increased tumorigenicity, sustained proliferation
and enhanced metastatic potential.
Analysis of the enriched signaling pathways of DE
genes from different clinical samples lead to the identification of
five hub genes, E2F2, SKP2, MYC, CDKN1A and CDKN2B. TGCA and GTEx
datasets were used to further verify that these hub genes were DE
genes and served important roles in colon cancer. The results
revealed that five hub genes were differentially expressed between
tumors and normal tissues. MYC plays a role in cell cycle
progression, apoptosis and cellular transformation (25). Furthermore, MYC enhances protein
synthesis by regulating genes involved in ribosome biogenesis and
protein translation (26). A number
of lncRNAs and transcription factors could affect tumor growth by
acting on MYC. E2F2 was reported to interact with retinoblastoma
protein to regulate the expression of genes involved in the cell
cycle (27). Altered copy number and
activity of this gene have been observed in liver and lung cancer
(28). However, to the best of our
knowledge, the function of E2F2 in CRC has not been verified. SKP2
is a member of the F-box protein family and SKP2-mediated
degradation of cytoglobin has been identified as the key mechanism
for controlling its oscillating levels during the cell cycle
(29). CDKN1A and CDKN2B are potent
cyclin-dependent kinase inhibitors and serve roles in the
regulation of cell cycle progression at the G1 stage
(30,31). CDKN1A and CDKN2B have been reported
in relation to breast (32), liver
(33) and prostate cancer (34). Although an association between CDKN1A
and CDKN2B and colon and rectal cancer has been reported, the
majority of studies reported activities specific to potent
cyclin-dependent kinase inhibitors (32,35,36).
Furthermore, the majority of these studies were based on the roles
of miRNA and lncRNA. Li et al (37) discussed the effect of lncSNHG6 on p21
and CRC. Zhang et al (38)
revealed that upregulation of miRNA-1258 regulated the cell cycle
and inhibited cell proliferation in CRC. Chen et al
(39) reported that baicalein
downregulated ezrin and inhibited the proliferation of human CRC
cell line HCT116. Therefore, the expression status of CDKN1A and
CDKN2B is important in CRC.
There are numerous studies on tumor biomarkers. Del
et al (40) reported that
KRAS and NRAS proto-oncogene GTPase genes were associated with poor
response to anti-epidermal growth factor receptor therapies in
patients with metastatic CRC. Furthermore, Xie et al
(41) reported that phospholipase A
and acyltransferase 3 may increase the risk of CRC in the Chinese
population, while Yu et al (42) reported that Bcl-2 was associated with
favorable prognosis. These reports mainly discussed the association
between biomarkers and colon cancer, taking into consideration two
primary aspects. First, some biomarkers may account for the
phenotypic characteristics of tumors and second, some biomarkers
may predict the prognosis of patients with cancer (43). However, the present study did not
focus on indicators that can reflect both the tumor phenotype and
the clinical prognosis of patients with cancer.
E2F2, SKP2, MYC, CDKN1A and CDKN2B, the hub genes
identified in GSE21510 and GSE32323, were ubiquitously expressed in
colon cancer at different stages of tumorigenesis. The mRNA
expression levels of these five hub genes were further validated in
genomic datasets. Furthermore, the hub DE genes exhibited the
strongest association network among the TCGA datasets. However,
based on the PPI network and the results in (31), it can be hypothesized that E2F2,
SKP2, MYC, CDKN1A and CDKN2B may not be independent prognostic
factors for CRC. Integrative survival analysis indicated that
CDKN1A was associated with favorable prognosis (hazard ratio, 0.64;
95% confidence interval; log-rank, P=0.043). Therefore, CDKN1A is
an indicator of tumor characteristics and may be used to predict
patient prognosis. A previous study also indicated that
differential expression of CDKN1A affected prognosis in the
survival analysis of patients with gastric cancer (44). In this study, the GSE21510 and
GSE32323 datasets were analyzed. However, the limited size of
clinical samples may not be sufficient to reflect all different
types of CRC. Therefore, further examination will be conducted, in
order to verify the results of the present study.
At present, a limited number of tumor biomarkers can
simultaneously predict tumor phenotype and patient prognosis. Based
on the current results, CDKN1A may be a suitable tumor biomarker.
CDKN1A may serve a predictive role for colon cancer phenotype and
prognosis and the marker may be detected without deep sequencing
using a kit or a chip, although this requires additional study.
Future studies will aim to identify more tumor biomarkers and
targets for the diagnosis and treatment of CRC.
Acknowledgements
The authors would like to acknowledge the technical
guidance of Dr Chris Lou and Professor You Zai. The authors would
also like to thank pathologist Dr Si Haipeng for her guidance on
this experiment.
Funding
This study was supported by National Nature Science
Foundation of China (grant nos. 81774266 and 81804058).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ conceived and designed the experiments. HZ and YJ
performed the experiments. HZ and WL analyzed the data. WL and MW
were significant contributors in the manuscript. MW participated in
the design of this experiment and participated in the review of
proofreading and manuscripts of experimental data. MW agreed to be
responsible for all aspects of the work to ensure proper
investigation and resolution of issues related to the accuracy or
completeness of any part of the work. All authors have read and
approved the final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hodson L, Skeaff CM and Fielding BA: Fatty
acid composition of adipose tissue and blood in humans and its use
as a biomarker of dietary intake. Prog Lipid Res. 47:348–380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang C, Zhang J, Ding M, Xu K, Li L, Mao L
and Zheng J: Ki67 targeted strategies for cancer therapy. Clin
Transl Oncol. 20:570–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Zhang Z, Chen F, Hu T, Peng W, Gu Q
and Sun Y: The diverse oncogenic and tumor suppressor roles of
microRNA-105 in cancer. Front Oncol. 9:5182019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong P, Xiong Y, Yue J, Hanley SJB,
Kobayashi N, Todo Y and Watari H: Long Non-coding RNA NEAT1: A
novel target for diagnosis and therapy in human tumors. Front
Genet. 9:4712018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panoutsopoulou K, Avgeris M and Scorilas
A: miRNA and long non-coding RNA: Molecular function and clinical
value in breast and ovarian cancers. Expert Rev Mol Diagn.
18:963–979. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song N, Kim K, Shin A, Park JW, Chang HJ,
Shi J, Cai Q, Kim DY, Zheng W and Oh JH: Colorectal cancer
susceptibility loci and influence on survival. Genes Chromosomes
Cancer. 57:630–637. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lech G, Słotwiński R, Słodkowski M and
Krasnodębski IW: Colorectal cancer tumour markers and biomarkers:
Recent therapeutic advances. World J Gastroenterol. 22:1745–1755.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puccini A, Berger MD, Zhang W and Lenz HJ:
What we know about stage II and III colon cancer: It's still not
enough. Target Oncol. 12:265–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lan J, Sun L, Xu F, Liu L, Hu F, Song D,
Hou Z, Wu W, Luo X, Wang J, et al: M2 macrophage-derived exosomes
promote cell migration and invasion in colon cancer. Cancer Res.
79:146–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsukamoto S, Ishikawa T, Iida S, Ishiguro
M, Mogushi K, Mizushima H, Uetake H, Tanaka H and Sugihara K:
Clinical significance of osteoprotegerin expression in human
colorectal cancer. Clin Cancer Res. 17:2444–2450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khamas A, Ishikawa T, Shimokawa K, Mogushi
K, Iida S, Ishiguro M, Mizushima H, Tanaka H, Uetake H and Sugihara
K: Screening for epigenetically masked genes in colorectal cancer
Using 5-Aza-2′-deoxycytidine, microarray and gene expression
profile. Cancer Genomics Proteomics. 9:67–75. 2012.PubMed/NCBI
|
|
14
|
R Core Team. R, . A language and
environment for statistical computing. R Foundation for Statistical
Computing. (Vienna, Austria). 2012.
|
|
15
|
RStudio Team. RStudio, . Integrated
development for R. RStudio, Inc. (Boston, MA). 2015.
|
|
16
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krüger T: Editorial change at statistical
applications in genetics and molecular biology. Stat Appl Genet
Mol. 17(pii)2018.doi: 10.1515/sagmb-2018-0046.
|
|
18
|
Yu G, Wang L, Han Y and He Q:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43((Database Issue)): D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henson DE, Hueman MT, Chen D, Patel JA,
Wang H and Schwartz AM: The anatomy of the TNM for colon cancer. J
Gastrointest Oncol. 8:12–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao X, Ponomaryov T, Ornell KJ, Zhou P,
Dabral SK, Pak E, Li W, Atwood SX, Whitson RJ, Chang AL, et al:
RAS/MAPK activation drives resistance to Smo inhibition,
metastasis, and tumor evolution in Shh pathway-dependent tumors.
Cancer Res. 75:3623–3635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma D, Chen X, Zhang PY, Zhang H, Wei LJ,
Hu S, Tang JZ, Zhou MT, Xie C, Ou R, et al: Upregulation of the
ALDOA/DNA-PK/p53 pathway by dietary restriction suppresses tumor
growth. Oncogene. 37:1041–1048. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duchartre Y, Kim Y and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev Oncol Hematol. 99:141–149.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalkat M, Resetca D, Lourenco C, Chan P,
Wei Y, Shiah Y, Vitkin N, Tong Y, Sunnerhagen M, Done SJ, et al:
MYC protein interactome profiling reveals functionally distinct
regions that cooperate to drive tumorigenesis. Mol Cell.
72:836–848.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stine ZE, Walton ZE, Altman BJ, Hsieh AL
and Dang CV: MYC, metabolism, and cancer. Cancer Discov.
5:1024–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Cheng M, Wu M, Boriboun C, Jujo K,
Xu S, Zhao TC, Tang Y, Kishore R and Qin G: Contrasting roles of
E2F2 and E2F3 in endothelial cell growth and ischemic angiogenesis.
J Mol Cell Cardiol. 60:68–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Y, Feng B, Lu L, Han S, Chu X, Chen L
and Wang R: MiRNAs and E2F3: A complex network of reciprocal
regulations in human cancers. Oncotarget. 8:60624–60639.
2017.PubMed/NCBI
|
|
29
|
Lee SW, Li CF, Jin G, Cai Z, Han F, Chan
CH, Yang WL, Li BK, Rezaeian AH, Li HY, et al: Skp2-dependent
ubiquitination and activation of LKB1 is essential for cancer cell
survival under energy stress. Mol Cell. 57:1022–1033. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao R, Han D, Sun X, Xie Y, Wu Q, Fu C,
Yao Y, Li H, Li Z and Xu K: Scriptaid inhibits cell survival, cell
cycle, and promotes apoptosis in multiple myeloma via epigenetic
regulation of p21. Exp Hematol. 60:63–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wall SJ, Zhong Z and DeClerck YA: The
Cyclin-dependent kinase inhibitors p15INK4B and p21CIP are critical
regulators of fibrillar collagen-induced tumor cell cycle arrest. J
Biol Chem. 282:24471–24476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ansems M, Søndergaard JN, Sieuwerts AM,
Looman MW, Smid M, de Graaf AM, de Weerd V, Zuidscherwoude M,
Foekens JA, Martens JW and Adema GJ: DC-SCRIPT is a novel regulator
of the tumor suppressor gene CDKN2B and induces cell cycle arrest
in ERα-positive breast cancer cells. Breast Cancer Res Treat.
149:693–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Han S, Peng R, Jiao C, Wang X,
Yang X, Yang R and Li X: Depletion of histone demethylase KDM5B
inhibits cell proliferation of hepatocellular carcinoma by
regulation of cell cycle checkpoint proteins p15 and p27. J Exp
Clin Cancer Res. 35:372016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park J, Park M, Oh EH, Soung N, Lee SJ,
Jung J, Lee O, Yun SJ, Kim W, Shin E and Kim EG: The p21-activated
kinase 4-Slug transcription factor axis promotes
epithelial-mesenchymal transition and worsens prognosis in prostate
cancer. Oncogene. 37:5147–5159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dutto I, Tillhon M, Cazzalini O, Stivala
LA and Prosperi E: Biology of the cell cycle inhibitor p21CDKN1A:
Molecular mechanisms and relevance in chemical toxicology. Arch
Toxicol. 89:155–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Basudan A, Priedigkeit N, Hartmaier RJ,
Sokol ES, Bahreini A, Watters RJ, Boisen MM, Bhargava R, Weiss KR,
Karsten MM, et al: Frequent ESR1 and CDK pathway copy number
alterations in metastatic breast cancer. Mol Cancer Res.
17:457–468. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Z, Qiu R, Qiu X and Tian T: SNHG6
promotes tumor growth via repression of P21 in colorectal cancer.
Cell Physiol Biochem. 49:463–478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang Z, Li J, Huang Y, Peng W, Qian W, Gu
J, Wang Q, Hu T, Ji D, Ji B, et al: Upregulated miR-1258 regulates
cell cycle and inhibits cell proliferation by directly targeting
E2F8 in CRC. Cell Prolif. 51:e125052018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Z, Hou R, Gao S, Song D and Feng Y:
Baicalein inhibits proliferation activity of human colorectal
cancer cells HCT116 through downregulation of Ezrin. Cell Physiol
Biochem. 49:2035–2046. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Del CS, Sayagues JM, Bengoechea O, Anduaga
MF, Alcazar JA, Gervas R, Garcia J, Orfao A, Bellvis LM, Sarasquete
ME and Del Mar Abad M: Spatio-temporal tumor heterogeneity in
metastatic CRC tumors: A mutational-based approach. Oncotarget.
9:34279–34288. 2018.PubMed/NCBI
|
|
41
|
Xie XN, Yu J, Zhang LH, Luo ZY, Ouyang DS,
Zheng LJ, Wang CY, Yang L, Chen L and Tan ZR: Relationship between
polymorphisms of the lipid metabolism-related gene PLA2G16 and risk
of colorectal cancer in the Chinese population. Funct Integr
Genomic. 19:227–236. 2019. View Article : Google Scholar
|
|
42
|
Yu C, Hong H, Lu J, Zhao X, Hu W, Zhang S,
Zong Y, Mao Z, Li J, Wang M, et al: Prediction of target genes and
pathways associated with cetuximab insensitivity in colorectal
cancer. Technol Cancer Res Treat. 17:2018.doi:
10.1177/1533033818806905. View Article : Google Scholar
|
|
43
|
Xue W, Li J, Wang F, Han P, Liu Y and Cui
B: A long non-coding RNA expression signature to predict survival
of patients with colon adenocarcinoma. Oncotarget. 8:101298–101308.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin Y, Wang X, Yu Y, Liu W, Xie F, Ouyang
X and Huang Q: Expression and prognostic significance of
cyclin-dependent kinase inhibitor 1A in patients with resected
gastric adenocarcinoma. Oncol Lett. 14:7473–7482. 2017.PubMed/NCBI
|