Introduction
Ovarian cancer adversely affects female health
worldwide and is one of the major causes of mortality of women with
severe gynecological issues (1).
Ovarian cancer primarily presents as tumors of the epithelial cells
that grow from the surface epithelial cells of the ovary, and are
histologically classified into four main subtypes: Mucinous,
endometrioid, serous and clear cells. The most common histologic
subtype in epithelial ovarian cancer is the OSC, representing
75–80% of all cases worldwide (2,3). OSC is
a common female genital cancer. These types of cancer are either
asymptomatic or have similar symptoms to other benign gynecological
diseases, until the tumor has metastasized on the surface of the
peritoneum and then it will be diagnosed. Thus, the majority of
patients are diagnosed when the disease has reached an advanced
stage. Furthermore, due to the current absence of effective
treatment options, patients with the disease experience extremely
poor overall survival rate (OS), and only 45% 5-year relative
survival rate of all stages (4).
Therefore, identification and validation of prognostic biomarkers
to predict OSC outcomes are of high clinical value.
There is an urgent need to identify new, highly
sensitive and specific biomarkers for improved diagnosis and
targeted therapies (5). The
biomarkers bestow early detection as well as predict a poor
prognosis (6,7). A number of studies have used certain
genes as cancer prognostic biomarkers with significant success in
patients with ovarian cancer. For instance, Liu et al
(8) reported overexpression of
TRIM44 in ovarian cancer, and revealed a close association with
lower rates of overall- and disease-free survival. TRIM44 can be
used as an independent marker to predict poor prognosis in ovarian
cancer. Lee et al (9)
discovered that another protein, CENPK, was overexpressed in
ovarian cancer cells, and proved its direct association with a poor
prognosis in patients with ovarian cancer. Furthermore,
incorporating CENPK with the tumor markers CA125 or HE4 can
increase the sensitivity of CA125 or HE4 for predicting ECO
outcomes (9). In addition, ASAP1,
MAGE-A9 and keratin 17 have been linked to poor prognosis and,
hence, their utility as a prognostic indicator in human ovarian
cancer (10–12). Thus, while the biomarkers are of
great clinical value for predicting outcomes for patients with
ovarian cancer, there is very limited prognostic value to a single
candidate biomarker. This could be attributed to inconsistent
sample collection, detection methods and small sample sizes
(11).
Despite an increasing number of studies focusing on
malignant tumors, the exact underlying molecular mechanisms remain
unclear. For the majority of tumors, the treatment effect and
prognosis are not ideal. In clinical practice, the histological
grade provides an important prognosis for tumors, which aids in
assessing the tumor behavior (13)
and is most commonly used for the prognosis of hepatocellular
carcinoma, breast cancer and mucinous appendiceal adenocarcinoma
(13–15). The aggressive potential of the tumor
is defined by histological grades as: G1, well-differentiated and
the least aggressive (slow growing); G2, moderately differentiated;
G3, highly proliferative and most aggressive but poorly
differentiated; G4, undifferentiated (16). A statistically significant difference
was observed by Overman et al (17) between the apparent diffusion
coefficient and pure diffusion coefficient among the different
histological grades. They also found that in the mucinous
appendiceal adenocarcinoma with peritoneal metastasis, the G3 group
values were lower than those of the G2 group (17). While studying hepatocellular
carcinoma (HCC), Granata et al (18) demonstrated that within the HCC
groups, the perfusion fraction values of G1, G2 and G3 histological
grades were significantly different (18). Similarly, Grotz et al
(16) demonstrated that in mucinous
appendiceal adenocarcinoma (MAA), the G2 clinical behavior is
distinctly different from that in G1 and G3, with remarkably
different cancer-specific survival in stage IV G2 and G3 of MAA.
Although OSC is an extremely common form of ovarian cancer, no
article has reported the utility of multiple biomarkers in
determining the associated histological grade to the best of our
knowledge. With this aim, the current study investigated the
utility of multiple biomarkers to assess the OS rate of OSC and
determined the prognostic biomarkers for assessing poor prognosis
and disease progression.
Materials and methods
Dataset for patients with OSC
Patient clinical and cognate data for pre-processed
transcript mRNA (as of September 2018) were sourced from The Cancer
Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). The information
includes intact data on mRNA expression and clinical
characteristics (including age, ethnicity, sex, tumor type and
histological grade, and status and time of survival).
Screening of differentially expressed
mRNAs between G2 and G3 of OSC
Data from a total of 364 patients with OSC were
downloaded from TCGA, as per screening criteria, transcript data
and basic clinical data (Table I),
including 42 in G2 and 322 in G3. The differentially expressed
mRNAs in G2 and G3 grades were distinguished using edgeR version
3.22.5 (bioinf.wehi.edu.au/edgeR) in R (19), and the threshold values were |log
2-fold change |≥2 and false discovery rate <0.05. The edgeR
package automatically deletes outliers when analyzing differences,
thus reducing the impact of sample differences and making the
analytical data more reliable.
 | Table I.Summary of clinical characteristics
of the patients with ovarian serous cystadenocarcinoma included in
the present study. |
Table I.
Summary of clinical characteristics
of the patients with ovarian serous cystadenocarcinoma included in
the present study.
|
| Patients
(n=364) |
|---|
|
|
|
|---|
| Characteristic | n | % |
|---|
| Age, years |
|
30–59 | 191 | 52.47 |
|
60–87 | 173 | 47.53 |
| Sex |
|
Female | 364 | 100 |
| Ethnicity |
|
Caucasian | 316 | 86.81 |
| Black
or African American | 23 |
6.32 |
|
Asian | 11 |
3.02 |
|
Unknown | 14 |
3.85 |
| Tumor grade |
| G2 | 42 | 11.54 |
| G3 | 322 | 88.46 |
| Patient status |
|
Alive | 166 | 45.60 |
|
Succumbed | 198 | 54.40 |
Analysis of survival and the
prognostic model based on mRNA status
The association between patient OS rate and
differentially expressed genes was assessed using the univariate
Cox package for univariate Cox proportional hazard regression
analysis, and those with P<0.01 were used as candidate
variables. A multi-gene prediction model was then established
simultaneously, and appropriate prognostic information for
validation comparisons was downloaded from Kaplan-Meier plotter
(kmplot.com/analysis/). Based on the mean
of expression, the cases were divided into a high and low
expression groups. Furthermore, the multivariate Cox regression
model was performed to assess the prognostic value of the
differentially expressed mRNAs (DEMs). Based on the mean expression
levels of the gene, the cases were classified into high and low
expression groups. A score for prognosis risk was established to
predict OS based on a linear combination of the expression level
multiplied by the multivariate Cox regression model (β) derived
regression coefficient, applying the formula: Risk score=exp DEM1 ×
β DEM1 + exp DEM2 × β DEM2 + … exp DEMn × β DEMn).
Risk classification and receiver
operating characteristic (ROC) curve
The risk scores of 364 patients were determined as
per the multi-gene model, and then grouped into high- and low-risk
groups based on the median value. The survival status and risk
scores of high- and low-risk patients were compared using the
Survival_Graph and Point_Graph ggplot version 2.3.0.0 (ggplot2.tidyverse.org). The areas under the curve
(AUC) of the predictive model were evaluated computationally to
estimate the sensitivity and specificity of mRNA prognosis, and
plotted using the Survival_ROC package version 1.0.3 (cran.r-project.org/web/packages/survivalROC/index.html).
Prediction of independent survival
time based on the 3-mRNA signature prognosis from other clinical
variables
Multivariate Cox analyses were performed to
determine the independence of the 3-mRNA signature from other
clinical factors (age, race and grade) of patients with OSC, using
OS as the dependent variable. Furthermore, stratification analysis
was carried out on clinical features mentioned in Cox regression
analysis, to assess if there is any prognostic value of the 3-mRNA
signature within the same clinical factor.
Statistical analysis
The data were derived from TCGA, processed by edgeR
package to obtain differentially expressed genes, and the
integration of differential genes with clinical data was performed
in the command prompt of Windows 10 (Microsoft Corporation). The
multivariate Cox package was used to establish a multi-gene
survival model and generate the model's survival curve and the ROC
package was utilized to predict the survival rate of patients with
G2 and G3 grade OSC. Following the construction of the 3-gene
model, the 3-gene data (selected from the differentially expressed
genes) were entered into GraphPad Prism software (version 5.0;
GraphPad Software, Inc.) to determine whether the three mRNAs were
prognostically significant for the G2 and G3 grades of OSC and a
Mann-Whitney U test was used to assess the data. The univariate Cox
package Survival version 2.43–3 (github.com/therneau/survival) was used to obtain the
survival curve of each gene. The heat map was generated using the
pheatmap package version 1.0.10 (cran.r-project.org/web/packages/pheatmap/), and
the Survival_Graph package was used to draw the survival scatter
plot and the Point_Graph package to plot the survival score curve.
The relevant gene survival curves were downloaded from the KM
plotter website. The editing and splicing of images in the text was
performed using Photoshop CS6 (Adobe Systems Europe, Ltd.), and the
data processing and data packet applications were based on R.
Language. P<0.05 was considered to indicate a statistically
significant result.
Results
OSC in G2 and G3 exhibit
differentially expressed mRNAs
As per the selection criteria, a total of 144
differentially expressed genes (104 upregulated and 40
downregulated) were analyzed in order to identify the OSC G2 and G3
grades.
Association between the 3-mRNA
signature with comprehensive survival of patients with OSC
To shortlist prognosis-associated mRNA from
differential genes, the association between the expression of each
differentially expressed gene and overall patient survival was
analyzed via univariate Cox regression analysis, and 3 mRNAs were
identified (P<0.01; Table II).
Then, through multivariate Cox regression analysis, these 3 mRNAs
(Table II) were used as a
predictive model (P<0.015), which is linearly associated with
the level of corresponding mRNA expression. For the gene predictive
model in the multivariate Cox regression analysis, the risk score
was calculated as 0.0817 × NPFFR2 + 0.0976 × XPNPEP2-0.1304 ×
CELA3B. The risk scores were positively associated with NPFFR2 and
XPNPEP2, indicating that enhanced expression of these two mRNAs in
patients with OSC have shorter OS. In addition, CELA3B was
inversely associated with risk scores, suggesting that it may be a
potential protective gene for patients with OSC, and its high
expression indicates longer OS.
 | Table II.3-mRNA risk score signature. |
Table II.
3-mRNA risk score signature.
| Gene symbol | Coefficient | Univariate
P-value | Multivariate
P-value |
|---|
| NPFFR2 |
0.0817 | 0.009221 | 0.011 |
| XPNPEP2 |
0.0976 | 0.007264 | 0.010 |
| CELA3B | −0.1304 | 0.009284 | 0.013 |
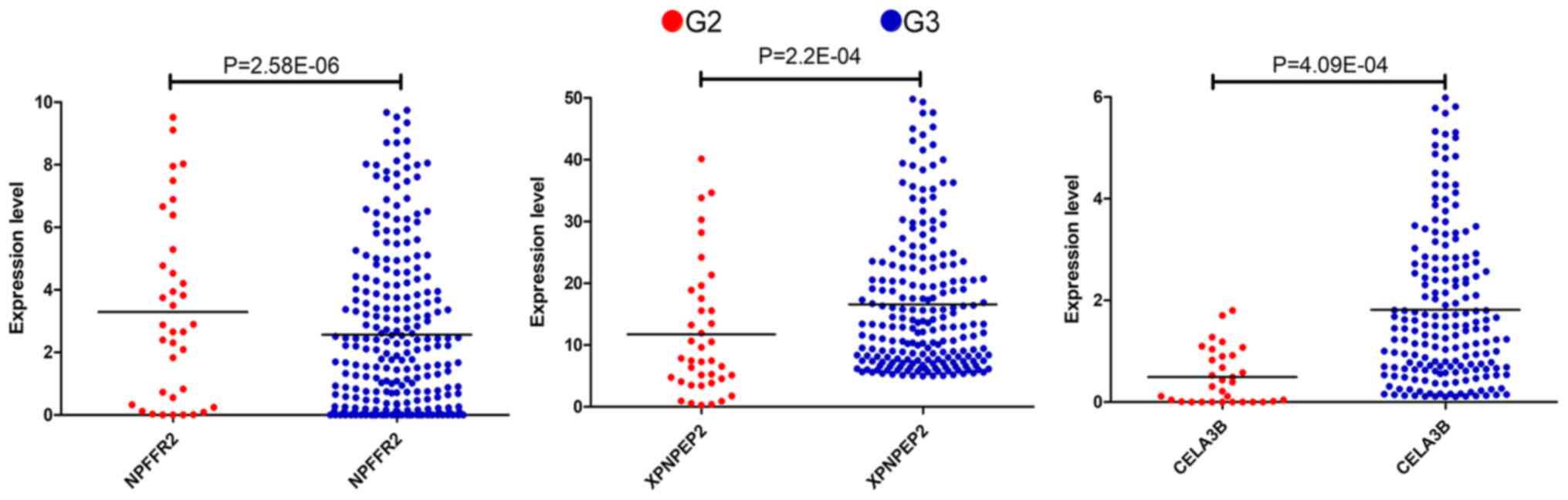
Thus, according to the Cox regression analysis, the
three mRNAs were prognostically significant for the G2 and G3
grades of OSC (Fig. 1). The enhanced
expression of NPFFR2 in G2 phase and a reduced expression of
XPNPEP2 and CELA3B in the sample were statistically significant
(P<0.001). NPFFR2 and CELA3B determined to be independent
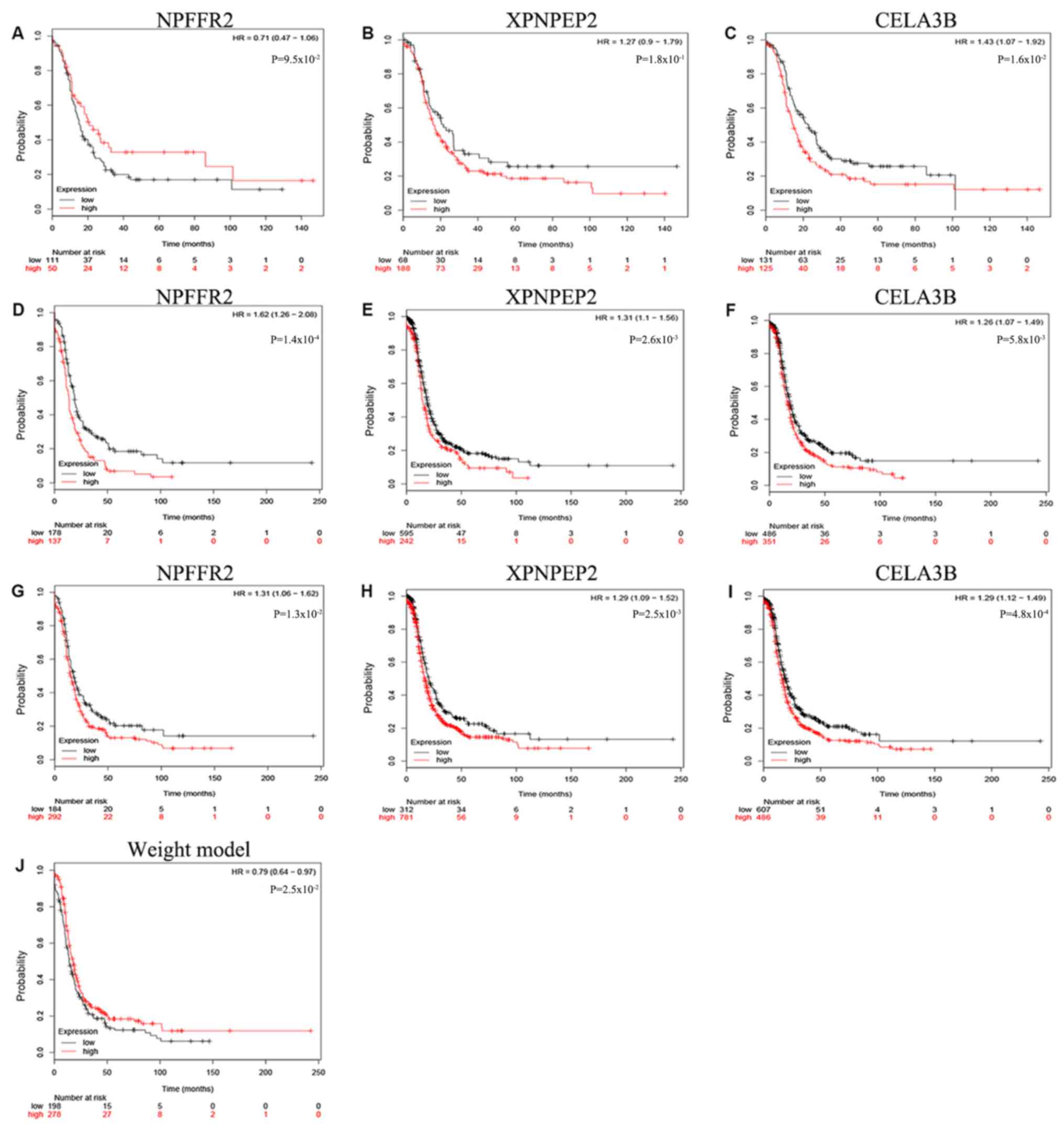
prognostic factors, whereas XPNPEP2 was not. Furthermore, the
Kaplan-Meier curves demonstrate statistically significant
differences in OS (P<0.05) of NPFFR2 and CELA3B (Fig. 2). High expression of NPFFR2 (Fig. 2A, D and G), and XPNPEP2 (Fig. 2B, E and H) was associated with a
lower overall survival rate, and similarly, lower CELA3B expression
was associated with a lower overall survival rate (Fig. 2F and I), in agreement with the
results of univariate analysis.
The risk scores of these patients with OSC could be
divided into low- and high-risk groups according to the median
value of the risk score which was 0.9580745 (Fig. 3C). This group visualizes the survival
score and survival status of patients with OSC (Fig. 3A and B). Patients with higher risk
scores were observed to have higher NPFFR2 and XPNPEP2 and lower
CELA3B expressions. The majority of the risk scores were <2.5,
and their survival time was <7.5 months (Fig. 3A and B); however, as the risk score
increased, the survival time and the survival rate decreased
(Fig. 3A and B).
Based on the 5-year ROC AUC of 0.628 (Fig. 4A), the risk score was used to predict
the survival rate of G2 and G3 grade patients with OSC.
Furthermore, the Kaplan-Meier curve of the 3-mRNA signature further
validated that the high-risk group had notably shorter survival
times compared with the low-risk group (P=0.00149, Fig. 4B).
The Kaplan-Meier curve for NPFFR2 indicated that
there was no significant difference when the OS rate of the
patients with G2 grade were compared with others (P>0.05;
Fig. 5). In addition, the
Kaplan-Meier curve of the 3-mRNA signature further validated that
patients in the high-risk group had significantly shorter survival
times compared with the low-risk group (P=0.025; Fig. 5J). The role of NPFFR2 appears to
change from being a protective factor in G2 to a risk factor in G3,
making it a risk factor overall (Fig.
5A, D and G); although the other two biomarkers, XPNPEP2 and
CELA3B, were risk factors in both G2 and G3 (Fig. 5B, E and H, and C, F and I), thus
confirming the 3-mRNA model.
3-mRNA signature is prognostically
independent of other clinical factors
The 3-mRNA risk scoring model exhibited predictive
power independent of other clinical factors [hazard ratio (HR),
1.442; 95% confidence interval [CI] 1.081–1.924; P=0.013; Table III]. Age (P=0.119) and histological
grade (P=0.128) were not considered significant factors. It was
observed that in terms of age, ethnicity, histological grade and
risk score HR, values for age, histological grade and risk scores
corresponded with greater susceptibility to cancer (HR can help
determine if the factor is protective or detrimental; Table III). Among different races,
Caucasian individuals may have been less susceptible, as the
P-value was close to 0.05, and the HR value is 0.669. Therefore,
race may be a protective factor. The histological grades and the
corresponding age were considered risk factors (HR>1), which
authenticates this scoring model. Although, whether ethnicity
serves as a protective factor requires further investigation.
P<0.05 for each of the 3 mRNAs in the signature, suggested that
this model was highly specific. Thus, according to these results,
the 3-mRNA signature used in the present study may serve as a
predictor of other clinical factors.
 | Table III.Patient overall survival in terms of
multivariate Cox regression analysis. |
Table III.
Patient overall survival in terms of
multivariate Cox regression analysis.
| Variables | HR | 95% CI | P-value |
|---|
| Age, years (≤60 vs.
>60) | 1.251 | 0.944–1.658 | 0.119 |
| Ethnicity
(Caucasian vs. others) | 0.669 | 0.436–1.026 | 0.066 |
| Grade (G2 vs.
G3) | 1.386 | 0.911–2.108 | 0.128 |
| RS (Low vs.
high) | 1.442 | 1.081–1.924 | 0.013 |
Discussion
Nearly 90% of all ovarian cancers present as OSC,
making it the most common type of epithelial ovarian cancer
(20). OSC results in greater
mortality rates than any other type of cancer of the female
reproductive system (21). According
to the Global Cancer Statistics, in 2016 there were ~230,000 cases
of female ovarian cancer diagnosed, and 150,000 women succumbed to
the disease (21). While studying
ovarian cancer, focus should be paid to the differences in gene
expression between normal and tumor tissues (22), characterizing differences between
histological subtypes (23,24) and marking differences between tumors
with invasive and low malignant potential (25,26). The
present study focuses on the moderate differentiation of G2 tumors
while transitioning from G2 to G3, which are the most invasive and
poorly differentiated tumor grades. To achieve good curative
effect, the most important prerequisite is accurate tumor staging
in the patients with ovarian cancer. A number of studies have used
gene expression for the prognosis of OSC, which exhibit high
sensitivity and specificity, and may be clinically significant
(27,28). The G2 and G3 grades are crucial
indicators for prognosis in OSC as the metastatic rate of ovarian
cancer is closely associated with histological grade. A cancer
tissue with a lower degree of differentiation is more likely to
metastasize, leading to worse prognosis. Hence, the differentiation
between G2 and G3 is helpful in evaluating patient prognosis. Owing
to similarly hypothesized prognoses, the majority of studies
combine G2 and G3, whereas others do not support this amalgamation,
suggesting that the differentiated G2 have distinct clinical
behavior and outcome from that of G1 and G3. The data from the
present study demonstrate differential clinical manifestations of
G2 and G3, with different prognoses and different treatment
options, including the scope of surgery and the course of
chemotherapy.
In the present study, three different genes (NPFFR2,
XPNPEP2 and CELA3B) were obtained that distinguished G2 and G3
grades of patients with OSC, and established a 3-mRNA signature.
The OS rate of patients with G2 and G3 grade OSC was predicted
based on the high-differential low-risk group. To the best of our
knowledge, this is the first genetic prediction model based on the
histological grading of patients with OSC. The differentially
expressed mRNAs were obtained from 364 patients with OSC whose
clinical information was available on TCGA (42 cases in G2 stage
and 322 cases in G3 stage). These differentially expressed mRNAs
were assessed through single factor and stepwise multivariate Cox
analyses and a linear prediction model was built. It was observed
that the high- and low-risk patient groups were significantly
different, in addition to high sensitivity and specificity as
determined through the ROC curve (AUC, 0.628; Fig. 4A). The multivariate Cox regression
analysis indicated that the 3-mRNA signature can aid in prognosis,
independent of the traditional clinical factors such as age,
ethnicity and histological grade. Furthermore, the KM plotter
confirmed that OS based on NPFFR2 and CELA3B were significant in
all patients (G2+G3) and that the 3-mRNA signature was therefore
greatly different from the gene weight model. The expression of
NPFFR2 is inversely proportional to cancer malignancy. A similar
pattern is observed in the pathological grading and staging of head
and neck squamous cell carcinoma; the degree of malignancy was
directly proportional to that of NPFFR2 methylation (29). NPFFR2 is also associated with the
activity of hypothalamic-pituitary-adrenal axis (30). In terms of stress-associated hormones
and released neurotransmitters, quality has an adverse effect on
stress-induced tumor progression and cancer treatment (31), which indirectly indicate that the
progression of cancer (increased histological grade, etc.) is
indirectly affected by NPFFR2. As a proline hydrolase, XPNPEP2,
also known as aminopeptidase P or APP2, hydrolyzes numerous
biologically active peptides, such as XPNPEP2, which inactivates
bradykinin (32). XPNPEP2 serves a
vital role in the ovarian breakdown and follicular dysplasia
induced by hexavalent chromium in rat germ cells (33). XPNPEP2 overexpression is also
observed in cervical cancer tissues, and is associated with
pathological staging, lymph node metastasis and poor OS (34), which coincides with the results from
the present study findings in the XPNPEP2 study in OSC, in renal
clear cell carcinoma (CCRCC) (35)
and in advanced gastric cancer (36).
Human CELA3B is a product of gene duplication. In
CELA3B, the 241st residue is polymorphic (p.A241 G), and the
differences may lead to the risk of chronic pancreatitis (37) and capsular fibrosis islets (38). While capsular fibrosis also conforms
to histological changes in OSC, in type 2 diabetes, CELA3B has been
revealed to be associated with microvascular ischemia (39), which may explain the cause of OSC
rupture. Therefore, NPFFR2, XPNPEP2 and CELA3B can be associated
with the occurrence of OSC and the associated prognosis.
There were also some limitations to the present
study. The number of patients in G2 and G3 were not equal. The
pathological grades of OSC are G1, G2, G3 and G4. However, limited
data were available on TCGA for the G1 and G4 stages of OSC (<5
cases each) and no data for adjacent tissues. If the sample size is
too small, errors occur in the statistical analyses, and thus
should not be included within the study. Updates to the OSC data on
TCGA will be assessed in future studies once they become available.
The clinical data of OSC are incomplete, and the
Tumor-Node-Metastasis (TNM) stages were unknown. While TNM is the
standardized staging method for malignant tumor progression
(4), it also plays an important role
in guiding the prognosis and treatment of tumors. In the
multi-factor analysis, if the influence of TNM staging on OS is
also assessed then, through database verification, testing the
feasibility of the gene model can be improved. Another high-risk
factor for ovarian cancer is age, and it should be assessed in
greater detail; the small amount of age groups in the present study
may be one of the most important shortcomings of the present study.
Due to the small number of age groups, the results may have
demonstrated larger errors, therefore age should be divided into
additional groups for more accurate comparisons. Furthermore, there
are large inconsistencies between the case survival and the
database survival analyses. More than 3,000 patients with ovarian
cancer are usually included in the KM plotter database, but the
number of cases in the present study, obtained from TCGA, was much
lower, causing inconsistencies in the results. The fact that some
of the data in Figs. 2 and 5 do not match may have been due these
restrictions. While the model is feasible according to the moderate
area of the AUC curve, it is not highly sensitive and specific, and
so may only be suitable for the initial screening of disease.
Whilst the sensitivity was high compared to other factors (age,
histological grade and ethnicity), when the data was compared
independently (amongst itself), the absolute sensitivity was not
high. To the best of our knowledge, the genetic model involving the
three genes (NPFFR2, XPNPEP2 and CELA3B) signature has not been
studied in other tumors, making it difficult to prove that they
have roles in OSC. Thus, a combination of clinical experience and
other auxiliary examinations is required in order to make a correct
diagnosis.
The present study established a genetic model using
three genes, NPFFR2, XPNPEP2 and CELA3B, for identifying G2 and G3
grades of OSC through statistical analysis. The model is different
from traditional prognosis and clinical identification methods. The
role of these three genes was further analyzed and compared against
the published literature. This newly established genetic model has
the potential for prognosis of patients with OSC.
Acknowledgements
The authors would like to acknowledge Professor Hong
Wang (Hong Wang, Pediatric Surgery II Ward, First Affiliated
Hospital of Guangxi Medical University, Nanning, Guangxi) for her
support.
Funding
The present study was supported by the Scientific
Research Project of Guangxi Provincial Health and Family Planning
Commission (grant no. Z20180900).
Availability of data and materials
The datasets generated and analyzed during the
current study are available in the TCGA repository, https://portal.gdc.cancer.gov/.
Authors' contributions
JZ designed the study. CS and YL revised the
experimental design. JZ and YY acquired, analyzed and interpreted
the data. CW validated the experimental data. CS and YL verified
the results of the experiment. JZ, YY and CW wrote the manuscript
and revised it for important intellectual content.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Escalona RM, Chan E, Kannourakis G,
Findlay JK and Ahmed N: The many facets of metzincins and their
endogenous inhibitors: Perspectives on ovarian cancer progression.
Int J Mol Sci. 19(pii): E4502018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang S: Gastric metastasis of ovarian
serous cystadenocarcinoma. Int Med Case Rep J. 11:201–204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cress RD, Chen YS, Morris CR, Petersen M
and Leiserowitz GS: Characteristics of long-term survivors of
epithelial ovarian cancer. Obstet Gynecol. 126:491–497. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Qiu J, Ye C, Yang D, Gao L, Su Y,
Tang X, Xu N, Zhang D, Xiong L, et al: ROR1 expression correlated
with poor clinical outcome in human ovarian cancer. Sci Rep.
4:58112014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pal SK, Figlin RA and Reckamp K: Targeted
therapies for non-small cell lung cancer: An evolving landscape.
Mol Cancer Ther. 9:1931–1944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelly K and Huang C: Biological agents in
non-small cell lung cancer: A review of recent advances and
clinical results with a focus on epidermal growth factor receptor
and vascular endothelial growth factor. J Thorac Oncol. 3:664–673.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Yin H, Ji H, Zhu J and Ma R:
Overexpression of TRIM44 is an independent marker for predicting
poor prognosis in epithelial ovarian cancer. Exp Ther Med.
16:3034–3040. 2018.PubMed/NCBI
|
|
9
|
Lee YC, Huang CC, Lin DY, Chang WC and Lee
KH: Overexpression of centromere protein K (CENPK) in ovarian
cancer is correlated with poor patient survival and associated with
predictive and prognostic relevance. PeerJ. 3:e13862015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Y, Wang C, Zhang Y, Jia L and Huang J:
Overexpression of MAGE-A9 is predictive of poor prognosis in
epithelial ovarian cancer. Sci Rep. 5:121042015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou T, Yang C, Tong C, Zhang H, Xiao J and
Li J: Overexpression of ASAP1 is associated with poor prognosis in
epithelial ovarian cancer. Int J Clin Exp Pathol. 7:280–287.
2013.PubMed/NCBI
|
|
12
|
Wang YF, Lang HY, Yuan J, Wang J, Wang R,
Zhang XH, Zhang J, Zhao T, Li YR, Liu JY, et al: Overexpression of
keratin 17 is associated with poor prognosis in epithelial ovarian
cancer. Tumour Biol. 34:1685–1689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rakha EA, Reis-Filho JS, Baehner F, Dabbs
DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani
SR, et al: Breast cancer prognostic classification in the molecular
era: The role of histological grade. Breast Cancer Res. 12:2072010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Skoog P, Ohlsson M, Fernö M, Rydén L,
Borrebaeck CAK and Wingren C: Tumor tissue protein signatures
reflect histological grade of breast cancer. PLoS One.
12:e01797752017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu SC, Liu YH, Wei Y, Li LL, Dou SW, Sun
TY and Shi DP: Intravoxel incoherent motion diffusion-weighted
magnetic resonance imaging for predicting histological grade of
hepatocellular carcinoma: Comparison with conventional
diffusion-weighted imaging. World J Gastroenterol. 24:929–940.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grotz TE, Royal RE, Mansfield PF, Overman
MJ, Mann GN, Robinson KA, Beaty KA, Rafeeq S, Matamoros A, Taggart
MW and Fournier KF: Stratification of outcomes for mucinous
appendiceal adenocarcinoma with peritoneal metastasis by
histological grade. World J Gastrointest Oncol. 9:354–362. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Overman MJ, Fournier K, Hu CY, Eng C,
Taggart M, Royal R, Mansfield P and Chang GJ: Improving the
AJCC/TNM staging for adenocarcinomas of the appendix: The
prognostic impact of histological grade. Ann Surg. 257:1072–1078.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Granata V, Fusco R, Catalano O, Guarino B,
Granata F, Tatangelo F, Avallone A, Piccirillo M, Palaia R, Izzo F
and Petrillo A: Intravoxel incoherent motion (IVIM) in
diffusion-weighted imaging (DWI) for Hepatocellular carcinoma:
Correlation with histologic grade. Oncotarget. 7:79357–79364. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
R Core Team 2012. R, . A language and
environment for statistical computing. R Foundation for Statistical
Computing. (Vienna, Austria). ISBN 3-900051-07-0. http://www.R-project.org/
|
|
20
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Welsh JB, Zarrinkar PP, Sapinoso LM, Kern
SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA and Hampton GM:
Analysis of gene expression profiles in normal and neoplastic
ovarian tissue samples identifies candidate molecular markers of
epithelial ovarian cancer. Proc Natl Acad Sci USA. 98:1176–1181.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwartz DR, Kardia SL, Shedden KA, Kuick
R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, et
al: Gene expression in ovarian cancer reflects both morphology and
biological behavior, distinguishing clear cell from other
poor-prognosis ovarian carcinomas. Cancer Res. 62:4722–4729.
2002.PubMed/NCBI
|
|
24
|
Schaner ME, Ross DT, Ciaravino G, Sorlie
T, Troyanskaya O, Diehn M, Wang YC, Duran GE, Sikic TL, Caldeira S,
et al: Gene expression patterns in ovarian carcinomas. Mol Biol
Cell. 14:4376–4386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonome T, Lee JY, Park DC, Radonovich M,
Pise-Masison C, Brady J, Gardner GJ, Hao K, Wong WH, Barrett JC, et
al: Expression profiling of serous low malignant potential,
low-grade, and high-grade tumors of the ovary. Cancer Res.
65:10602–10612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilks CB, Vanderhyden BC, Zhu S, van de
Rijn M and Longacre TA: Distinction between serous tumors of low
malignant potential and serous carcinomas based on global mRNA
expression profiling. Gynecol Oncol. 96:684–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Xu M, Gao H, Guo JC, Guo YL, Zou
M and Wu XF: Two protein-coding genes act as a novel clinical
signature to predict prognosis in patients with ovarian serous
cystadenocarcinoma. Oncol Lett. 15:3669–3675. 2018.PubMed/NCBI
|
|
28
|
Liu LW, Zhang Q, Guo W, Qian K and Wang Q:
A Five-gene expression signature predicts clinical outcome of
ovarian serous cystadenocarcinoma. Biomed Res Int.
2016:69453042016.PubMed/NCBI
|
|
29
|
Misawa K, Imai A, Mochizuki D, Misawa Y,
Endo S, Hosokawa S, Ishikawa R, Mima M, Shinmura K, Kanazawa T and
Mineta H: Genes encoding neuropeptide receptors are epigenetic
markers in patients with head and neck cancer: A site-specific
analysis. Oncotarget. 8:76318–76328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin YT, Yu YL, Hong WC, Yeh TS, Chen TC
and Chen JC: NPFFR2 activates the HPA axis and induces anxiogenic
effects in rodents. Int J Mol Sci. 18(pii): E18102017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin KJ, Lee YJ, Yang YR, Park S, Suh PG,
Follo MY, Cocco L and Ryu SH: Molecular mechanisms underlying
psychological stress and cancer. Curr Pharm Des. 22:2389–2402.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hui Y, Wu Y and Tormey CA: The development
of a novel molecular assay examining the role of aminopeptidase P
polymorphisms in acute hypotensive transfusion reactions. Arch
Pathol Lab Med. 137:96–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Banu SK, Stanley JA, Sivakumar KK, Arosh
JA, Barhoumi R and Burghardt RC: Identifying a novel role for
X-prolyl aminopeptidase (Xpnpep) 2 in CrVI-induced adverse effects
on germ cell nest breakdown and follicle development in rats. Biol
Reprod. 92:672015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng T, Wei R, Jiang G, Zhou Y, Lv M, Dai
Y, Yuan Y, Luo D, Ma D, Li F and Xi L: XPNPEP2 is overexpressed in
cervical cancer and promotes cervical cancer metastasis. Tumour
Biol. 39:10104283177171222017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drendel V, Heckelmann B, Chen CY, Weisser
J, Espadas G, Schell C, Sabido E, Werner M, Jilg CA and Schilling
O: Proteome profiling of clear cell renal cell carcinoma in von
Hippel-Lindau patients highlights upregulation of Xaa-Pro
aminopeptidase-1, an anti-proliferative and anti-migratory
exoprotease. Oncotarget. 8:100066–100078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeung HC, Rha SY, Kim HK, Lim HY, Kim S,
Kim SY, Gong SJ, Park CH, Ahn JB, Noh SH and Chung HC:
Multi-institutional phase II study of S-1 monotherapy in advanced
gastric cancer with pharmacokinetic and pharmacogenomic
evaluations. Oncologist. 12:543–554. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Párniczky A, Hegyi E, Tóth AZ, Szücs Á,
Szentesi A, Vincze Á, Izbéki F, Németh BC, Hegyi P and Sahin-Tóth
M: Genetic analysis of human chymotrypsin-like Elastases 3A and 3B
(CELA3A and CELA3B) to assess the role of complex formation between
proelastases and procarboxypeptidases in chronic pancreatitis. Int
J Mol Sci. 17:21482016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun X, Yi Y, Xie W, Liang B, Winter MC, He
N, Liu X, Luo M, Yang Y, Ode KL, et al: CFTR influences beta cell
function and insulin secretion through non-cell autonomous
exocrine-derived factors. Endocrinology. 158:3325–3338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han D, Moon S, Kim H, Choi SE, Lee SJ,
Park KS, Jun H, Kang Y and Kim Y: Detection of differential
proteomes associated with the development of type 2 diabetes in the
Zucker rat model using the iTRAQ technique. J Proteome Res.
10:564–577. 2011. View Article : Google Scholar : PubMed/NCBI
|