Introduction
Lung cancer is the most common cause of
cancer-related mortality worldwide (1), with increasing incidence and mortality
rates. Non-small cell lung cancer (NSCLC) accounts for ~80% of
newly diagnosed lung cancer cases annually, and the majority are
diagnosed at an advanced stage (2).
Chemotherapy has been recommended as the first-line treatment in
patients with advanced NSCLC for the last 10 years. However, due to
the toxicity and side effects, the therapeutic effect is limited
and the clinical outcomes are poor, with a median overall survival
(OS) time of only 8–10 months and a 5-year survival rate of only
~15% (3). Recently, treatment for
patients with advanced or metastatic NSCLC has been modified.
Epidermal growth factor receptor tyrosine kinase inhibitors
(EGFR-TKIs) have revealed significant efficacy in NSCLC patients
with EGFR mutations (4,5), are associated with fewer side effects
and have improved quality of life, particularly in patients
harboring the exon 19 deletion (19-del) or exon 21 point mutation
(21-L858R).
EGFR is a member of the ErbB receptor TK family and
serves a key role in the development and progression of NSCLC
(6). Overexpression of EGFR may lead
to cell proliferation, promote angiogenesis, tumor invasion and
metastasis, and inhibit cell apoptosis, serving an important role
in the evolution of malignant tumors (7–9).
Numerous studies have confirmed that EGFRs are uniquely expressed
in tumor tissues, particularly NSCLC (10,11).
Exon 19-del and 21-L858R mutations are common, accounting for 85%
of all EGFR mutations in NSCLC (12–14).
With the extensive use of TKIs in patients with NSCLC harboring
EGFR mutations, accumulating evidence has demonstrated that exon
19-del and 21-L858R mutations are associated with distinguishing
clinical characteristics (15). The
aim of the present study was to further investigate whether these
two mutations result in different prognoses in patients with
NSCLC.
Materials and methods
Patient characteristics
The clinical and follow-up data of 137 patients
treated at the Zhongnan Hospital of Wuhan University (Wuhan, Hubei,
China) between August 2012 and August 2016, who were diagnosed with
stage IIIB-IV NSCLC and harboring either the exon 19-del or
21-L858R mutations, were collected. Patient sex, age, smoking
status, primary site, disease stage, type of EGFR-TKI administered
(icotinib, gefitinib or erlotinib) and treatment protocol
(first-line TKIs, first-line chemotherapy or second-line TKIs) were
recorded during a retrospective chart review.
The inclusion criteria for the present study were as
follows: i) Patients with histologically or cytologically confirmed
stage IIIB-IV NSCLC according to the 7th American Joint Committee
on Cancer (AJCC) staging manual (16); ii) patients harboring either the exon
19-del or 21-L858R mutation which was detected with PCR (17); iii) no serious cardiovascular disease
or other diseases precluding patients from receiving chemotherapy
or EGFR-TKI therapy; iv) presence of at least 1 measurable lesion
assessable by computed tomography (CT) or magnetic resonance
imaging (MRI); and v) Karnofsky performance status score >70 and
a life expectancy ≥3 months.
The exclusion criteria were as follows: i) Patients
with small cell or mixed small cell histology; ii) unknown EGFR
mutation type; iii) absence of measurable lesions according to the
Response Evaluation Criteria in Solid Tumors (RECIST) v1.1
(18); and iv) life expectancy <3
months.
Treatment and patient follow-up
The 137 patients were divided into first-line TKI
treatment, first-line chemotherapy and second-line TKI treatment
groups. A total of 89 patients were treated with first-line TKIs.
First-line chemotherapy was administered to 48 patients, among who
27 received TKIs as second-line treatment following disease
progression.
The oral TKIs gefitinib (250 mg/day), erlotinib (150
mg/day) or icotinib (375 mg/day) were administered as the
first-line or second-line treatment for the collected patients with
NSCLC until disease progression or development of intolerable
adverse effects, such as severe rash, diarrhea, liver and kidney
toxicity. The chemotherapy consisted of a platinum-based
combination regimen, including pemetrexed (500 mg/m2;
day 1) plus cisplatin or nedaplatin (75 mg/m2; day 1),
gemcitabine (1,000 mg/m2; days 1 and 8) plus cisplatin
or nedaplatin (75 mg/m2; day 1), docetaxel (75
mg/m2; day 1) plus cisplatin or nedaplatin (75
mg/m2; day 1), and taxol (175 mg/m2; day 1)
plus cisplatin or nedaplatin (75 mg/m2; day 1), once
every 21 days. A dose reduction to 80% was allowed in case of
treatment-associated grade 3 or selected lengthy grade 2
toxicities.
Disease evaluation was initiated 4 weeks
post-treatment and was performed every 8 weeks thereafter until
disease progression or the start of new anticancer therapies.
Evaluation tools included chest CT scans, brain MRI with and
without contrast, abdominal CT scans, bone emission CT scans and
positron emission tomography-CT scans if necessary.
Statistical analysis
Progression-free survival (PFS) was defined as the
time from the first administration of first-line EGFR-TKIs,
first-line chemotherapy or second-line EGFR-TKI treatment to the
confirmation of disease progression or mortality from any cause.
The tumor response to EGFR-TKI treatment or chemotherapy was
assessed according to RECIST v1.1. Objective response rate (ORR)
was defined as the sum of patients with a complete response or
partial response divided by the total number of treated patients
with measurable disease. Disease control rate (DCR) was defined as
the number of patients with a complete response, partial response
or stable disease divided by the total number of patients treated.
The last follow-up date was August 31, 2017. The baseline
characteristics of patients were compared between the EGFR exon
19-del and 21-L858R genotype groups using Pearson's χ2
test or Fisher's exact tests (when there were <5 expected counts
in the contingency table) for categorical variables. Kaplan-Meier
analysis was applied to evaluate PFS. Univariate and multivariate
Cox proportional hazards analyses were conducted to identify
factors associated with increased risk of disease progression. All
statistical analyses were performed using SPSS (v17.0; SPSS, Inc.,
Chicago, IL, USA). Tests were 2-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
Baseline patient characteristics
Table I presents the
baseline characteristics of the involved patients. The median age
was 58 years (range, 32–93 years) and there were 69 men and 68
women, of whom 39 were smokers and 98 were non-smokers. There were
71 patients with lesions in the left lung and 66 in the right lung.
According to the 7th edition of the AJCC staging manual (16), there were 2 cases with stage IIIB and
135 cases with stage IV cancer. A total of 79 cases with exon
19-del mutation and 58 cases with 21-L858R mutation were
identified.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | n | % |
|---|
| Age, years |
|
|
| ≤60 | 82 | 59.9 |
|
>60 | 55 | 40.1 |
| Sex |
|
|
| Male | 69 | 50.4 |
|
Female | 68 | 49.6 |
| Stage |
|
|
| IIIB | 2 | 1.5 |
| IV | 135 | 98.5 |
| Smoking |
|
|
|
Ever | 39 | 28.5 |
|
Never | 98 | 71.5 |
| Primary site |
|
|
| Left
side | 71 | 51.8 |
| Right
side | 66 | 48.2 |
| EGFR |
|
|
|
19-Del | 79 | 57.7 |
|
21-L858R | 58 | 42.3 |
The characteristics of patients harboring either the
exon 19-del or 21-L858R mutations treated with different treatment
protocols are presented in Table
II. The percentages of patients harboring exon 19-del and
21-L858R mutations were 58.4% (52/89) and 41.6% (37/89) in the
first-line EGFR-TKI treatment group, 56.3% (27/48) and 43.8%
(21/48) in the first-line chemotherapy group, and 48.1% (13/27) and
51.9% (14/27) in the second-line EGFR-TKI treatment group,
respectively. The patients with either the exon 19-del or 21-L858R
mutations were proportionate in terms of sex, age, smoking status,
primary tumor sites and stage. In the first-line and second-line
EGFR-TKI treatment groups, patients with exon 19-del and 21-L858R
mutations received similar types of EGFR-TKI treatment. The cycles
of chemotherapy were comparable between patients with exon 19-del
and 21-L858R mutations in the first-line chemotherapy group.
 | Table II.Characteristics of the patients with
either the exon 19-del or 21-L858R mutation receiving different
treatments. |
Table II.
Characteristics of the patients with
either the exon 19-del or 21-L858R mutation receiving different
treatments.
|
| First-line
EGFR-TKIs | First-line
chemotherapy | Second-line
EGFR-TKIs |
|---|
|
|
|
|
|
|---|
|
Characteristics | Total, n (%) | 19-del, n (%) | 21-L858R, n
(%) | P-value | Total, (%) | 19-del, n (%) | 21-L858R, n
(%) | P-value | Total, n (%) | 19-del, n (%) | 21-L858R, n
(%) | P-value |
|---|
| All patients | 89 (100.0) | 52 (58.4) | 37 (41.6) |
| 48 (100.0) | 27 (56.3) | 21 (43.7) |
| 27 (100.0) | 13 (48.1) | 14 (51.9) |
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
| >0.999 |
|
≤60 | 51 (57.3) | 34 (65.4) | 17 (45.9) | 0.068 | 31 (64.6) | 18 (66.7) | 13 (61.9) | 0.732 | 18 (66.7) | 9 (69.2) | 9 (64.3) |
|
|
>60 | 38 (42.7) | 18 (34.6) | 20 (54.1) |
| 17 (35.4) | 9 (33.3) | 8 (38.1) |
| 9 (33.3) | 4 (30.8) | 5 (35.7) |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
| >0.999 |
|
Male | 41 (46.1) | 25 (48.1) | 16 (43.2) | 0.652 | 28 (58.3) | 18 (66.7) | 10 (47.6) | 0.184 | 15 (55.6) | 7 (53.8) | 8 (57.1) |
|
|
Female | 48 (53.9) | 27 (51.9) | 21 (56.8) |
| 20 (41.7) | 9 (33.3) | 11 (52.4) |
| 12 (44.4) | 6 (46.2) | 6 (42.9) |
|
| Stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
IIIB | 1 (1.1) | 0 (0.0) | 1 (2.7) |
| 1 (2.1) | 1 (3.7) | 0 (0.0) |
| 1 (3.7) | 1 (7.7) | 0 (0.0) |
|
| IV | 88 (98.9) | 52 (100.0) | 36 (97.3) |
| 47 (97.9) | 26 (96.3) | 21 (100.0) |
| 26 (96.3) | 12 (92.3) | 14 (100.0) |
|
| Smoking |
|
|
|
|
|
|
|
|
|
|
| 0.695 |
|
Ever | 22 (24.7) | 13 (25.0) | 9 (24.3) | 0.942 | 17 (35.4) | 10 (37.0) | 7 (33.3) | 0.790 | 10 (37.0) | 4 (30.8) | 6 (42.9) |
|
|
Never | 67 (75.3) | 39 (75.0) | 28 (75.7) |
| 31 (64.6) | 17 (63.0) | 14 (66.7) |
| 17 (63.0) | 9 (69.2) | 8 (57.1) |
|
| Primary site |
|
|
|
|
|
|
|
|
|
|
| >0.999 |
| Left
lung | 45 (50.6) | 27 (51.9) | 18 (48.6) | 0.761 | 26 (54.2) | 15 (55.6) | 11 (52.4) | 0.827 | 15 (55.6) | 7 (53.8) | 8 (57.1) |
|
| Right
lung | 44 (49.4) | 25 (48.1) | 19 (51.4) |
| 22 (45.8) | 12 (44.4) | 10 (47.6) |
| 12 (44.4) | 6 (46.2) | 6 (42.9) |
|
| Drug
categories |
|
|
|
|
|
|
|
|
|
|
| 0.660 |
|
Gefitinib | 58 (65.2) | 34 (65.4) | 24 (64.9) | 0.815 |
|
|
|
| 16 (59.3) | 8 (61.5) | 8 (57.1) |
|
|
Icotinib | 17 (19.1) | 9 (17.3) | 8 (21.6) |
|
|
|
|
| 6 (22.2) | 2 (15.4) | 4 (28.6) |
|
|
Erlotinib | 14 (15.7) | 9 (17.3) | 5 (13.5) |
|
|
|
|
| 5 (18.5) | 3 (23.1) | 2 (14.3) |
|
| Cycles of
chemotherapy |
|
|
|
|
|
|
|
|
|
|
| 0.999 |
| ≤4 |
|
|
|
| 28 (58.3) | 15 (55.6) | 13 (61.9) | 0.658 | 16 (59.3) | 8 (61.5) | 8 (57.1) |
|
|
>4 |
|
|
|
| 20 (41.7) | 12 (44.4) | 8 (38.1) |
| 11 (40.7) | 5 (38.5) | 6 (42.9) |
|
ORR and DCR
The objective response of patients harboring either
the exon 19-del or 21-L858R mutations to the first-line EGFR-TKI,
first-line chemotherapy and second-line EGFR-TKI treatments is
presented in Table III. The ORR
and DCR for patients with the exon 19-del and 21-L858R mutations
were 51.9 vs. 27.0% (P=0.019) and 96.2 vs. 83.8% (P=0.102)
following first-line EGFR-TKI treatment, 18.5 vs. 4.8% (P=0.322)
and 77.8 vs. 52.4% (P=0.064) following first-line chemotherapy, and
15.4 vs. 42.9% (P=0.209) and 76.9 vs. 92.9% (P=0.326) following
second-line EGFR-TKI treatment, respectively.
 | Table III.Disease response to the different
treatments in patients with either the exon 19-del or the 21-L858R
mutation type. |
Table III.
Disease response to the different
treatments in patients with either the exon 19-del or the 21-L858R
mutation type.
|
| First-line
EGFR-TKIs | First-line
chemotherapy | Second-line
EGFR-TKIs |
|---|
|
|
|
|
|
|---|
| Response | 19-del | 21-L858R | P-value | 19-del | 21-L858R | P-value | 19-del | 21-L858R | P-value |
|---|
| D, n | 2 | 6 |
| 6 | 10 |
| 3 | 1 |
|
| SD, n | 23 | 21 |
| 16 | 10 |
| 8 | 7 |
|
| PR, n | 25 | 10 |
| 5 | 1 |
| 2 | 6 |
|
| CR, n | 2 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| ORR, n (%) | 27 (51.9) | 10 (27.0) | 0.019 | 5 (18.5) | 1 (4.8) | 0.322 | 2 (15.4) | 6 (42.9) | 0.209 |
| DCR, n (%) | 50 (96.2) | 31 (83.8) | 0.102 | 21 (77.8) | 11 (52.4) | 0.064 | 10 (76.9) | 13 (92.9) | 0.326 |
Regardless of the mutation type, the differences
between ORR and DCR in each treatment method were compared in the
present study. It was revealed that the ORR and DCR were 41.6%
(37/89) and 91.0% (81/89) following first-line EGFR-TKI treatment,
12.5% (6/48) and 66.7% (32/48) following first-line chemotherapy,
and 29.6% (8/27) and 85.2% (23/27) following second-line TKI
treatment, respectively. Compared with first-line chemotherapy
treatment, first-line EGFR-TKI treatment resulted in an increased
ORR (41.6 vs. 12.5%; P<0.05) and DCR (91.0 vs. 66.7%;
P<0.05). Specifically, in patients with the exon 19-del
mutation, first-line TKI treatment resulted in an increased ORR
(51.9 vs. 18.5%; P=0.004) and DCR (96.2 vs. 77.8%; P=0.03) compared
with first-line chemotherapy. Similarly, in patients with the
21-L858R mutation, first-line TKI treatment also resulted in an
increased ORR (27.0 vs. 4.8%; P=0.044) and DCR (83.8 vs. 52.4%;
P=0.01) compared with first-line chemotherapy.
There was a similar ORR (41.6 vs. 29.6%; P>0.05)
and DCR (91.0 vs. 85.2%; P>0.05) between the first-line and
second-line TKI treatment groups. According to the EGFR mutation
status, in patients with the exon 19-del mutation, first-line TKI
treatment resulted in an increased ORR (51.9 vs. 15.4%; P=0.018)
and a similar DCR (96.2 vs. 76.9%; P=0.081) compared with
second-line TKI treatment. However, in patients with the 21-L858R
mutation, the first-line and second-line TKI treatment resulted in
a similar ORR (27 vs. 42.9%; P=0.322) and DCR (83.8 vs. 92.9%;
P=0.657).
PFS time
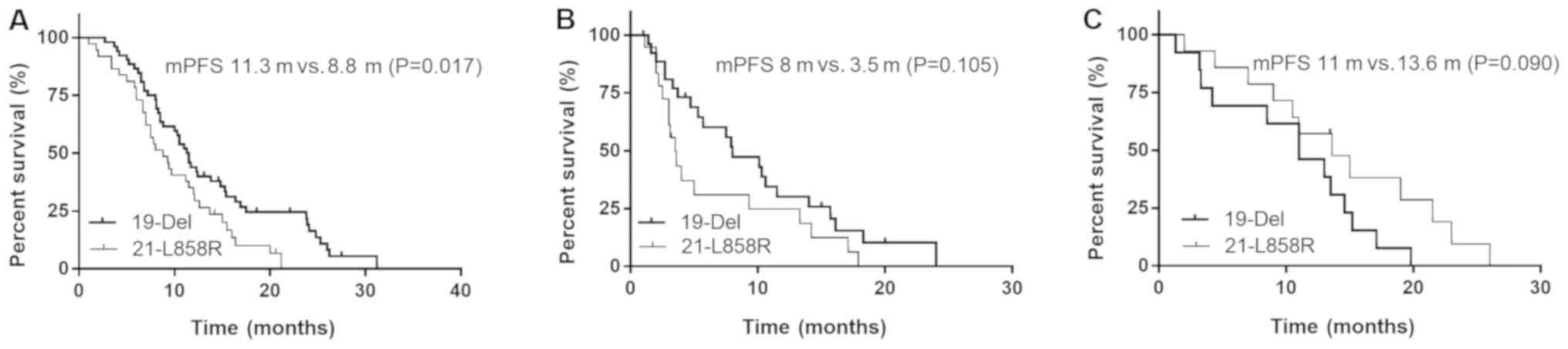
The median PFS time for patients with the exon
19-del and 21-L858R mutations was 11.3 vs. 8.8 months (P=0.017)
following first-line EGFR-TKIs, 8.0 vs. 3.5 months (P=0.105)
following first-line chemotherapy and 11.0 vs. 13.6 months
(P=0.090) following second-line EGFR-TKIs, respectively (Fig. 1).
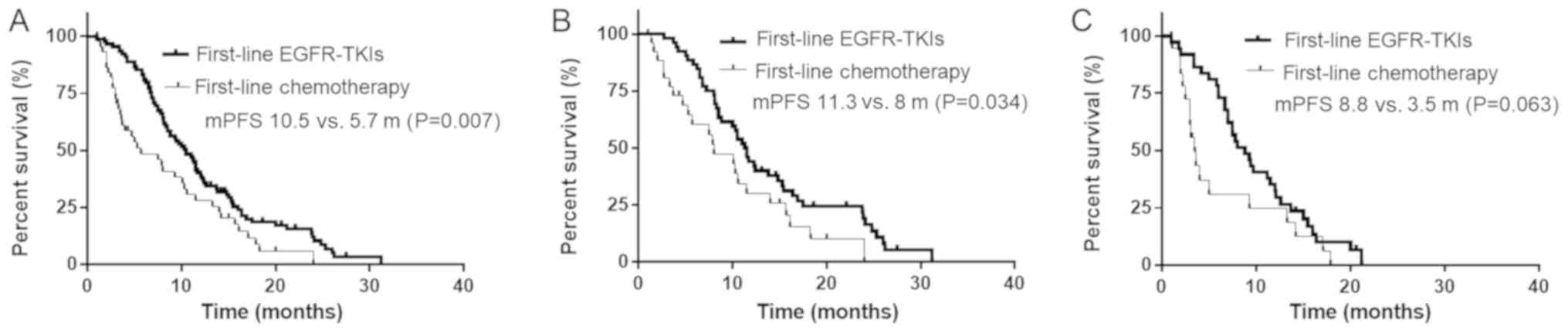
The median PFS time of the enrolled patients with
NSCLC was 10.5 months following first-line TKI treatment, 5.7
months following first-line chemotherapy and 13.0 months following
second-line TKI treatment. The median PFS time of patients treated
with first-line TKIs was significantly improved compared with that
of patients treated with first-line chemotherapy (10.5 vs. 5.7
months; P=0.007; Fig. 2A).
Specifically, in patients harboring the exon 19-del, first-line TKI
treatment led to the prolongation of the median PFS time (11.3 vs.
8.0 months; P=0.034) compared with that of first-line chemotherapy
(Fig. 2B). However, in patients
harboring the 21-L858R mutation, there was no significant
difference in the median PFS time (8.8 vs. 3.5 months; P=0.063)
between the exon 19-del and 21-L858R treatment groups (Fig. 2C).
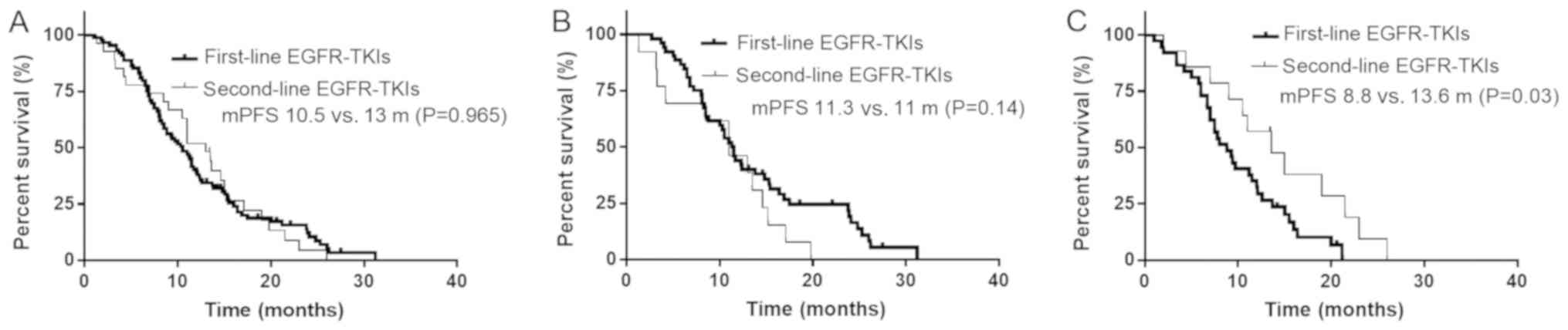
There was no significant difference in the median
PFS time between patients treated with first- and second-line TKIs
(13.0 vs. 10.5 months, respectively; P=0.965; Fig. 3A). In patients with the exon 19-del
mutation, first- and second-line TKI treatment also led to a
similar median PFS time (11.3 vs. 11.0 months; P=0.140; Fig. 3B). However, in patients with the
21-L858R mutation, second-line TKI treatment resulted in a longer
median PFS time compared with that in patients with the first-line
TKI treatment (8.8 vs. 13.6 months, respectively; P=0.030; Fig. 3C).
Univariate and multivariate
analyses
The results of the univariate and multivariate
analyses of PFS time for patients with NSCLC treated with
first-line EGFR-TKIs, first-line chemotherapy and second-line
EGFR-TKIs indicated that the type of EGFR mutation was an
independent predictor of PFS time for patients with NSCLC treated
with first-line TKIs [hazard ratio (HR), 2.071; 95% confidence
interval (CI), 1.120–3.480; P=0.006; Table IV]. First-line chemotherapy of >4
cycles was also associated with a longer PFS time (HR, 0.444; 95%
CI, 0.214–0.921; P=0.029).
 | Table IV.Univariate and multivariate analysis
on PFS for NSCLC patients with either the exon 19-del or 21-L858R
mutation type. |
Table IV.
Univariate and multivariate analysis
on PFS for NSCLC patients with either the exon 19-del or 21-L858R
mutation type.
|
| First-line
EGFR-TKIs | First-line
chemotherapy | Second-line
EGFR-TKIs |
|---|
|
|
|
|
|
|---|
|
|
| Univariate | Multivariate |
| Univariate | Multivariate |
| Univariate | Multivariate |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | n | P-value | HR (95% CI) | P-value | n | P-value | HR (95% CI) | P-value | n | P-value | HR (95% CI) | P-value |
|---|
| EGFR |
|
|
|
|
|
|
|
|
|
|
|
|
|
19-Del | 52 | 0.018 | 2.071
(1.120–3.480) | 0.006 | 27 | 0.105 | 2.086
(0.988–4.402) | 0.054 | 13 | 0.090 | 0.766
(0.275–2.587) | 0.843 |
|
21-L858R | 37 |
|
|
| 21 |
|
|
| 14 |
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
|
|
≤60 | 41 | 0.629 | 0.684
(0.418–1.119) | 0.131 | 31 | 0.688 | 0.751
(0.378–1.491) | 0.423 | 18 | 0.288 | 0.777
(0.195–3.090) | 0.720 |
|
>60 | 48 |
|
|
| 17 |
|
|
| 9 |
|
|
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
|
Male | 41 | 0.421 | 0.891
(0.525–1.511) | 0.668 | 28 | 0.350 | 1.145
(0.48–2.732) | 0.760 | 15 | 0.786 | 1.191
(0.243–5.830) | 0.830 |
|
Female | 48 |
|
|
| 20 |
|
|
| 12 |
|
|
|
| Smoking |
|
|
|
|
|
|
|
|
|
|
|
|
|
Ever | 22 | 0.646 | 1.141
(0.604–2.155) | 0.685 | 17 | 0.715 | 0.938
(0.394–2.234) | 0.886 | 10 | 0.891 | 1.057
(0.195–5.735) | 0.948 |
|
Never | 67 |
|
|
| 31 |
|
|
| 17 |
|
|
|
| Primary site |
|
|
|
|
|
|
|
|
|
|
|
|
| Left
lung | 45 | 0.138 | 0.721
(0.452–1.151) | 0.170 | 26 | 0.804 | 1.143
0.577–2.266 | 0.701 | 15 | 0.105 | 1.28
0.334–4.893 | 0.720 |
| Right
lung | 44 |
|
|
| 22 |
|
|
| 12 |
|
|
|
| Drug
categories |
|
|
|
|
|
|
|
|
|
|
|
|
|
Gefitinib | 58 | 0.517 | 0.990
(0.720–1.361) | 0.951 |
|
|
|
| 16 | 0.261 | 0.789
(0.381–1.672) | 0.550 |
|
Icotinib | 17 |
|
|
|
|
|
|
| 6 |
|
|
|
|
Erlotinib | 14 |
|
|
|
|
|
|
| 5 |
|
|
|
| Cycles of
chemotherapy |
|
|
|
|
|
|
|
|
|
|
|
|
| ≤4 |
|
|
|
| 28 | 0.070 | 0.444
(0.214–0.921) | 0.029 | 16 | 0.707 | 0.905
(0.246–3.329) | 0.881 |
|
>4 |
|
|
|
| 20 |
|
|
| 11 |
|
|
|
Discussion
Lung cancer is one of the leading causes of
mortality worldwide (19). Due to
their tumor-targeting properties and significant therapeutic
efficacy, EGFR-TKIs have been a focus of investigation and their
application has lead to major advances in the treatment of NSCLC,
particularly for patients with either the EGFR exon 19 del or
21-L858R mutations (10,20).
Several studies indicated that treatment with
EGFR-TKIs resulted in significant improvements in PFS time, quality
of life and tolerance to treatment (6,21–24). The
LUX-Lung6 study (25) demonstrated
that treatment with EGFR-TKIs led to a significantly longer PFS
time (11.0 vs. 5.6 months; P<0.001) and increased ORR (66.9 vs.
23%; P<0.001) and DCR (92.6 vs. 76.2%; P<0.001) compared with
those obtained using standard chemotherapy. As in the
aforementioned studies, the present results suggested that the PFS
time (10.5 vs. 5.7 months; P=0.007), ORR (41.6 vs. 12.5%;
P<0.05) and DCR (91.0 vs. 66.7%; P<0.05) of patients with
NSCLC harboring EGFR mutations treated with first-line TKIs were
improved compared with those in patients treated with
chemotherapy.
Wu et al (26)
analyzed the data of 152 patients with advanced NSCLC harboring
EGFR mutations in Taiwan, among whom 91 were treated with
first-line gefitinib and 61 were treated with second-line
gefitinib. Similar PFS and OS times were observed between the
first-line and second-line gefitinib treatment groups. In addition,
a Spanish Lung Cancer Group trial (27) demonstrated no significant differences
in PFS and OS times between first- and second-line erlotinib
treatments. Similarly, in the present study, the PFS time, ORR and
DCR of patients harboring the exon 19-del and 21-L858R mutations
were 10.5 vs. 13.0 months, 41.6 vs. 29.6% and 91.0 vs. 85.2%
(P>0.05), respectively.
Several previous studies indicated that patients
with the EGFR exon 19-del and 21-L858R mutations exhibited
distinguishing clinical characteristics and different prognoses. A
subgroup analysis of the IPASS (21)
study indicated that, among patients with the EGFR exon 19-del, the
ORR in the gefitinib group increased significantly compared with
that in the chemotherapy group (84.8 vs. 43.2%). However, among
patients with the 21-L858R mutation, there was no significant
difference between the exon 19-del and 21-L858R mutation groups.
Other studies also reported that the exon 19-del mutation was
associated with an improved prognosis compared with the 21-L858R
mutation in patients with stage IIIB-IV NSCLC (28,29).
When treated with first-line TKIs, patients with the exon 19-del
mutation exhibited an increased ORR (51.9 vs. 27%; P=0.019) and
longer PFS time (11.3 vs. 8.8 months; P=0.017) compared with that
in patients with the 21-L858R mutation. Accordingly, the ORR (73.0
vs. 50%; P=0.025) and PFS time (24.0 vs. 10 months; P=0.04) of
patients with the exon 19-del mutation in the present study was
also improved compared with those in patients with the 21-L858R
mutation.
The mechanism underlying the different sensitivities
to EGFR-TKI treatment between the exon 19-del and 21-L858R
mutations remains to be elucidated. Previous studies suggested
that, following treatment with gefitinib, the levels of
G1 arrest increased in cells with the exon 19-del
mutation compared with that in cells with the 21-L858R mutation
(30). Sordella et al
(31) found that the exon 19-del and
21-L858R mutations altered the EGFR autophosphorylation and
downstream signaling pathways. For example, Y845 was highly
phosphorylated in cells harboring the 21-L858R mutation compared
with cells harboring the exon 19-del mutation, which may contribute
to the differential sensitivities to EGFR-TKI treatments. An
additional explanation may be that the exon 19-del and 21-L858R
mutations may exhibit different intrinsic sensitivities to the
EGFR-TKIs (11). Finally, the
21-L858R mutation is more likely to occur in combination with other
rare mutations, such as 21-L861Q, 18-G719X, 18-G719X and 20-Ins,
which affects the sensitivity to EGFR-TKI treatment (32).
Previous studies indicated that patients with the
EGFR exon 19-del exhibited a prolonged PFS time following
first-line TKI treatment compared with that in patients with the
21-L858R mutation (15,29). However, data on the difference in
prognosis between the exon 19-del and 21-L858R mutations following
second-line treatment with TKIs are limited. Wang et al
(33) identified that patients
harboring the exon 19-del experienced a prolonged PFS (8.1 vs. 6.8
months; P=0.002) and OS (17.6 vs. 12.5 months; P<0.01) time, and
an increased ORR (81.1 vs. 55.6%; P=0.002) compared with those in
patients harboring the 21-L858R mutation following second-line TKI
treatment. However, the results obtained in the present study were
different. There was no significant difference in PFS time, ORR or
DCR between patients with the exon 19-del and 21-L858R mutations
following second-line TKI treatment. The reason for these
discrepancies may be associated with the fact that patients in the
study by Wang et al (33)
received at least 1 cycle of chemotherapy prior to treatment with
TKIs, and it was not indicated whether disease regression was
observed following chemotherapy. By contrast, the present study
selected patients with disease progression following at least 2
cycles of chemotherapy followed by treatment with TKIs as
second-line therapy. Further analysis indicated that, in patients
with the exon 19-del mutation, there were no significant
differences in PFS time (11.3 vs. 11.0 months) or DCR (96.2 vs.
76.9%) between the first- and second-line TKI treatment groups.
However, in patients with the 21-L858R mutation, second-line TKI
treatment significantly prolonged the PFS time (13.6 vs. 8.8
months; P=0.030) compared with that in patients with first-line TKI
treatment.
The reasons for this significant difference in PFS
following second-line TKI treatment between patients harboring the
exon 19-del and 21-L858R mutations remain to be elucidated. Some
investigators have suggested that chemotherapy and radiotherapy may
modify the tumor microenvironment and modulate the sensitivity of
the mutant cells to the same TKI treatment, thus resulting in
different response rates (34).
Furthermore, chemotherapy and radiotherapy may eliminate
chemoradiation-sensitive cells, while cells resistant to
chemoradiotherapy remain and repopulate the tumor. In addition,
chemotherapy may reduce the EGFR mutation rate of patients with
NSCLC (35), which may
differentially affect the occurrence of the exon 19-del and
21-L858R mutations. Furthermore, compared with tumors with the
21-L858R mutation, tumors in the exon 19-del group exhibited an
increased EGFR T790 mutation rate (36), which may be another reason for
patients with 21-L858R mutation to be more sensitive to second-line
TKI treatment compared with patients with the exon 19-del mutation.
Finally, the spatial and temporal intratumor heterogeneity may also
contribute to different response rates to TKI treatment (37).
In conclusion, the present study demonstrated that
the PFS time of patients harboring the exon 19-del mutation was
significantly improved compared with that in patients with the
21-L858R mutation following first-line TKI treatment, while there
was no significant difference in PFS time between the exon 19-del
and 21-L858R mutation groups following first-line chemotherapy and
second-line TKI treatment. In patients with the exon 19-del
mutation, first- and second-line TKI treatment resulted in a
similar median PFS time. However, in patients harboring the
21-L858R mutation, second-line TKI treatment resulted in a longer
median PFS time compared with first-line TKI treatment. Whether
patients harboring the exon 19-del mutation should be administered
first-line TKIs, whereas those with the 21-L858R mutation should
receive second-line TKIs, requires confirmation by large
prospective clinical trials.
Acknowledgements
The authors would like to thank Dr Qiuji Wu
(Zhongnan Hospital of Wuhan University, China) for providing useful
additions to the manuscript prior to its submission.
Funding
The present study was funded by National Natural
Science Foundation of China (grant no. 81472799).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WH, QW, JZ and YZ were responsible for the study
design, data analysis and manuscript preparation. WH wrote the
manuscript. The final manuscript draft was approved by all the
authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang H: Overview of gefitinib in
non-small cell lung cancer: An Asian perspective. Jpn J Clin Oncol.
39:137–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
NSCLC Meta-Analyses Collaborative Group, :
Chemotherapy in addition to supportive care improves survival in
advanced non-small-cell lung cancer: A systematic review and
meta-analysis of individual patient data from 16 randomized
controlled trials. J Clin Oncol. 26:4617–4625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lara-Guerra H, Waddell TK, Salvarrey MA,
Joshua AM, Chung CT, Paul N, Boerner S, Sakurada A, Ludkovski O, Ma
C, et al: Phase II study of preoperative gefitinib in clinical
stage I non-small-cell lung cancer. J Clin Oncol. 27:6229–6236.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts PJ, Stinchcombe TE, Der CJ and
Socinski MA: Personalized medicine in non-small-cell lung cancer:
Is KRAS a useful marker in selecting patients for epidermal growth
factor receptor-targeted therapy? J Clin Oncol. 28:4769–4777. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Isobe H, Gemma A, Harada M, Yoshizawa H, et
al: Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732015.
View Article : Google Scholar
|
|
8
|
Ciardicllo F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coorper WA, Lam DC, O'Toole SA and Minna
JD: Molecular biology of lung cancer. J Thorac Dis. 5 (Suppl
5):S479–S490. 2013.PubMed/NCBI
|
|
10
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gazdar AF: Activating and resistance
mutations of EGFR in non-small-cell lung cancer: Role in clinical
response to EGFR tyrosine kinase inhibitors. Oncology. 28 (Suppl
1):S24–S31. 2009.
|
|
13
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chintala L and Kurzrock R: Epidermal
growth factor receptor mutation and diverse tumors: Case report and
concise literature review. Mol Oncol. 4:306–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riely GJ, Pao W, Pham DK, Li AR, Rizvi N,
Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M and Miller VA:
Clinical course of patients with non-small cell lung cancer and
epidermal growth factor receptor exon 19 and exon 21 mutations
treated with gefitinib or erlotinib. Clin Cancer Res. 12:839–844.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th. Chicago:
Springer; pp. 129–143. 2010
|
|
17
|
Taron M, Ichinose Y, Rosell R, Mok T,
Massuti B, Zamora L, Mate JL, Manegold C, Ono M, Queralt C, et al:
Activating mutations in the tyrosine kinase domain of the epidermal
growth factor receptor are associated with improved survival in
gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer
Res. 11:5878–5885. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisenhauer EA, Therasse P, Bogearts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugio K, Uramoto H, Onitsuka T, Mizukami
M, Ichiki Y, Sugaya M, Yasuda M, Takenoyama M, Oyama T, Hanagiri T
and Yasumoto K: Prospective phase II study of gefitinib in
non-small cell lung cancer with epidermal growth factor receptor
gene mutations. Lung Cancer. 64:314–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuler M, Yang JCH, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Massey D, Zazulina V, Shahidi M and Sequist L:
LUX-Lung 3: A randomized, open-label, phase III study of afatinib
versus pemetrexed and cisplatin as first-line treatment for
patients with advanced adenocarcinoma of the lung harboring
EGFR-activating mutations (Subgroup Analysis). Lung Cancer. 77
(Suppl 1):S25–S26. 2012. View Article : Google Scholar
|
|
21
|
Mok TS, Wu Y, Thongprasert S, Yang C, Chu
D, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou C, Wu Y, Chen G, Feng J, Liu X, Wang
C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu JY, Yu CJ, Yang CH, Wu SG, Chiu YH, Gow
CH, Chang YC, Hsu YC, Wei PF, Shih JY and Yang PC: First- or
second-line therapy with gefitinib produces equal survival in
non-small cell lung cancer. Am J Respir Crit Care Med. 178:847–853.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Massuti B, Morán T, Porta R, Queralt C,
Cardenal F, Mayo C, Camps C, Majem M, Tarón M and Rosell R:
Multicenter prospective trial of customized erlotinib for advanced
non-small cell lung cancer (NSCLC) patients (p) with epidermal
growth factor receptor (EGFR) mutations: Final results of the
Spanish lung cancer group (SLCG) trial. J Clin Oncol.
27:80232009.
|
|
28
|
Wang H, Huang J, Yu X, Han S, Yan X, Sun S
and Zhu X: Different efficacy of EGFR tyrosine kinase inhibitors
and prognosis in patients with subtypes of EGFR-mutated advanced
non-small cell lung cancer: A meta-analysis. J Cancer Res Clin
Oncol. 140:1901–1909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jackman DM, Yeap BY, Sequist LV, Lindeman
N, Holmes AJ, Joshi VA, Bell DW, Huberman MS, Halmos B, Rabin MS,
et al: Exon 19 deletion mutations of epidermal growth factor
receptor are associated with prolonged survival in non-small cell
lung cancer patients treated with gefitinib or erlotinib. Clin
Cancer Res. 12:3908–3914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang
XC, Guo AL, Zhang YF, An SJ, Mok TS and Wu YL: Better survival with
EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small
cell lung cancer patients is due to differential inhibition of
downstream signals. Cancer Lett. 265:307–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sordella R, Bell DW, Haber DA and
Settleman J: Gefitinib-sensitizing EGFR mutations in lung cancer
activate anti-apoptotic pathways. Science. 305:1163–1167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hata A, Yoshioka H, Fujita S, Kunimasa K,
Kaji R, Imai Y, Tomii K, Iwasaku M, Nishiyama A, Ishida T and
Katakami N: Complex mutations in the epidermal growth factor
receptor gene in non-small cell lung cancer. J Thorac Oncol.
5:1524–1528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Li RQ, Ai YQ, Zhang J, Zhao PZ, Li
YF, He WJ, Xia YX and Li WH: Exon 19 deletion was associated with
better survival outcomes in advanced lung adenocarcinoma with
mutant EGFR treated with EGFR-TKIs as second-line therapy after
first-line chemotherapy: A retrospective analysis of 128 patients.
Clin Transl Oncol. 17:727–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Risbud MV, Fertala J, Vresilovic EJ,
Albert TJ and Shapiro IM: Nucleus pulposus cells upregulate
PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions
and resist apoptosis induced by serum withdrawal. Spine (Phila Pa
1976). 30:882–889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai H, Wang Z, Wang Y, Zhuo M, Zhou Q,
Duan J, Yang L, Wu M, An T, Zhao J and Wang J: Detection and
clinical significance of intratumoral EGFR mutational heterogeneity
in Chinese patients with advanced non-small cell lung cancer. PLoS
One. 8:e541702013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ke EE, Zhou Q, Zhang QY, Su J, Chen ZH,
Zhang XC, Xu CR, Yang JJ, Tu HY, Yan HH, et al: A higher proportion
of the EGFR T790M mutation may contribute to the better survival of
patients with exon 19 deletions compared with those with L858R. J
Thorac Oncol. 12:1368–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tomonaga N, Nakamura Y, Yamaguchi H, Ikeda
T, Mizoguchi K, Motoshima K, Doi S, Nakatomi K, Iida T, Hayashi T,
et al: Analysis of intratumor heterogeneity of EGFR mutations in
mixed type lung adenocarcinoma. Clin Lung Cancer. 14:521–526. 2013.
View Article : Google Scholar : PubMed/NCBI
|