Extranodal natural killer/T-cell lymphoma (ENKTCL)
is a highly aggressive type of lymphoma strongly associated with
Epstein-Barr virus infection that has a poor clinical outcome, and
the incidence of ENKTCL is higher in Asian and Latin American
countries compared with Western countries (1,2). Most
studies on ENKTCL are therefore based on the populations of eastern
Asia, and Central and South America countries, and relatively
little information about this disease in people from Western
countries is available. Neoplastic cells express cytoplasmic-CD3ε,
CD56 and cytotoxic molecules. Most studies have reported a 5-year
survival rate of <50% (3–7). The Ann Arbor staging system, which was
originally used in Hodgkin lymphoma, is useful to assess prognosis
(8). Most ENKTCL cases comprise
local lesions and ~80% cases involve stage IE and IIE (7,9,10). The treatment options and clinical
characteristics of this disease have always been an important issue
(11–13). Previously, anthracycline-containing
chemotherapy regimens, including the cyclophosphamide, doxorubicin,
vincristine and prednisolone regimen, were commonly used in
advanced ENKTL treatment; however, they did not achieve
satisfactory results (9). Currently,
L-asparaginase-based regimens are the main treatment option
(1). ENKTCL mainly originates from
the upper aerodigestive tract (UADT), which usually involves the
nasal cavity, hypopharynx, Waldeyer's ring, larynx and oral cavity
(14–16). The involvement of extra-UADT sites is
relatively rare in ENKTCL. Previous studies reported that
UADT-ENKTCL and extra-UADT-ENKTCL have different clinical features
and prognoses (17–19). The present study aimed therefore to
investigate and analyze the characteristics and survival outcomes
of patients from the United States (US) with stage IE and IIE
primary UADT-ENKTCL of the nasal cavity by using the Surveillance,
Epidemiology, and End Results (SEER) program database. In addition,
by combining multiple independent factors, including age, stage,
radiation and primary site, a nomogram based on patients overall
survival (OS) was developed. This nomogram may be used to predict
the OS and prognosis of patients with ENKTCL.
Cases based on the third edition of the
International Classification of Disease for Oncology (ICD-O-3) were
selected (8). In this study,
patients with ENKTCL were identified using the ICD-O-3 code for
histology (9719, ENKTCL). In particular, patients with stage IE and
IIE UADT-ENKTCL were selected; however, patients with unknown or
higher stage UADT-ENKTCL and with stage I and II non-UADT-ENKTCL
were excluded (Fig. 1). A total of
396 patients were eventually enrolled in the present study and were
divided into training (n=280) and validation (n=116) cohorts.
A total of 396 patients with UADT-ENKTCL identified
in the SEER database were included in the present study. In the
entire study cohort, median follow-up time was ~18 months (range,
0–152 months). Median age at diagnosis was ~52 years (range, 8–93
years). There were 178 deaths among the 396 patients at 2–140
months, accounting for 44.9% of the cohort. The clinical
characteristics of patients in the training and validation cohorts
are listed in Table I.
In the training cohort, the variables in univariate
Cox regression analysis were included as follows: Sex, ethnicity,
stage, primary site, radiation therapy, surgery and age. Among
these variables, stage, primary site, radiation therapy and age
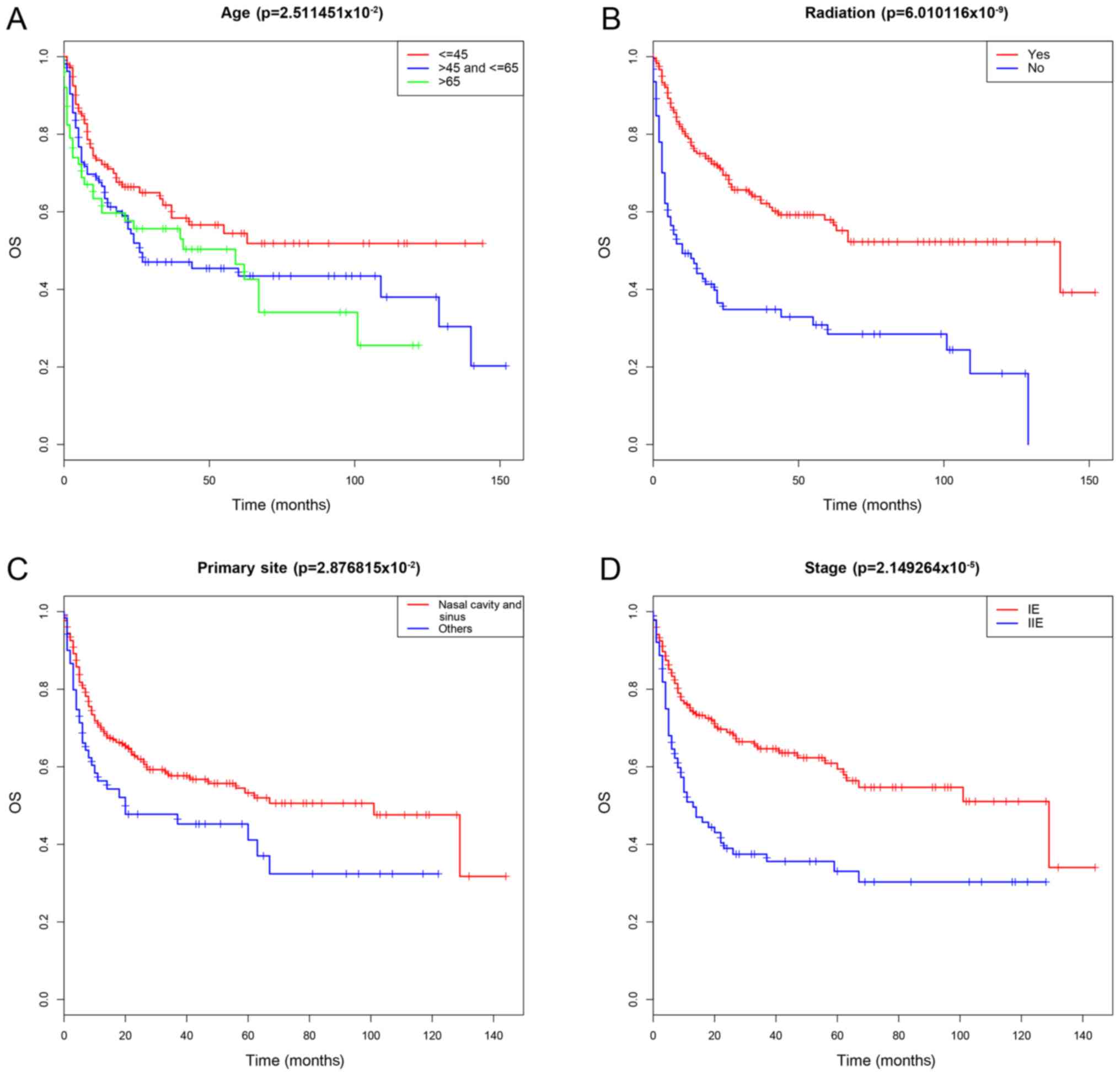
were significantly correlated with OS (P<0.05; Table II). The survival curves are
presented in Fig. 2. Due to the
limited availability of chemotherapy data, this treatment variable
was not included in the analysis. In the multivariate analyses,
factors with P<0.1, including age, stage, primary site,
radiation therapy and stage primary site, which is defined as the
interaction of two variables, were included for the Cox regression
analysis. The results demonstrated that age, stage, primary site
and radiation therapy were independently associated with
unfavorable OS (Table II).
A nomogram was established based on the results of
multivariate analyses. Age, stage, primary site and radiation
therapy were included in the nomogram. A weighted total score was
calculated for each factor and was then used to calculate and
estimate the 3-year OS and the probability for 5-year OS (Fig. 3). Harrell's C statistic of the
nomogram was calculated to be 0.717 in the training cohort and
0.737 in the validation cohort, which indicated that that the
discriminatory capacity of the nomogram was relatively clear and
accurate. In addition, the calibration curves revealed excellent
consistency between predicted OS and actual OS (Fig. 4).
A subgroup analysis was performed involving 264
patients receiving chemotherapy. The subgroup analysis results are
presented in Table III. Univariate
analysis demonstrated that stage, primary site and radiation
therapy were significant factors in the chemotherapy group.
Variables with P<0.1 on univariate analysis were included in
multivariate analysis. Age, which is generally considered as a
clinically important variable, was included in the Cox regression
analysis. Stage and radiation therapy remained independent
prognostic indicators. This result indicated that, based on
chemotherapy, radiation therapy was beneficial for increasing
OS.
Stage IE and IIE UADT-ENKTCL account for the largest
proportion of ENKTCL cases and have unique clinical features, such
as tumors usually infiltrating the nasopharynx or surrounding areas
(15). Compared with other ENKTCL
cases, stage IE and IIE UADT-ENKTCL cases usually exhibit lower
lactate dehydrogenase levels and better performance status
(25). In recent years, radiotherapy
has been considered to be the most effective treatment (25). In the present study, a novel nomogram
was built for this particular patient group based on the
characteristics of the US population. This nomogram was validated
in a validation cohort.
A nomogram is a simple and intuitive statistical
tool used to calculate survival probability by incorporating
relevant determinants of a disease. It may therefore be a useful
tool for clinicians to make clinical decisions (26,27). In
particular, in the field of oncology, nomograms can serve in
designing various prediction models for survival, recurrence and
metastasis (28–32).
The present study demonstrated that age, primary
site, radiation therapy and stage were independent prognostic
factors for OS. Previous studies reported that age is an important
prognostic factor for OS (33,34). In
order to further stratify the nomogram, two cut-off points for age
(45 and 65 years) were determined. The results demonstrated that HR
of OS increased with age; however, older patients had worsen
survival, notably patients >65 year old. The primary site was
also an important prognostic variable for ENKTCL. It has been
demonstrated that patients with extra-nasal UADT-ENKTCL are more
likely to have worsen OS compared with those with ENKTCL (25,35,36).
Data about chemotherapy treatment were divided into ‘receiving
chemotherapy’ and ‘No/Unknown’ groups; however, due to the
uncertainty of certain data, this information was unusable for
univariate or multivariate analysis. Subsequently, the analysis
focused on the efficacy of additional radiotherapy in patients who
were receiving chemotherapy. Previous studies reported that initial
treatment with radiation therapy and subsequent chemotherapy is
superior to chemotherapy alone, and to chemotherapy followed by
radiation (4,37). The present study also confirmed that
radiation therapy was still an independent prognostic factor in the
chemotherapy subgroup, which was consistent with the results of
previous studies (38,39). Avilés et al (38) and You et al (39) reported that radiation therapy, as
complementary treatment for chemotherapy or as palliative treatment
following chemotherapy, is superior to chemotherapy alone. With
regards to the stage, numerous studies have compared the prognoses
of stage IE and IIE ENKTCL with that of stage IIIE and IVE ENKTCL
(35,40,41), and
these studies reported that stage IIIE and IVE ENKTCL have worse
prognostic results. However, only a few studies compared the
prognoses of stage IE ENKTCL with that of stage IIE ENKTCL,
including the studies by Wang et al (42) and Dai et al (43), which revealed that patients with
stage IE ENKTCL have a longer OS, which was consistent with the
results of the present study. In addition, the analysis of the
subgroup of the chemotherapy group revealed that stage remained an
important prognostic variable. Notably, studies from Wang et
al (42) and Dai et al
(43) reported that in early-stage
ENKTCL, B symptoms, including fever, weight loss and night sweats,
are not associated with prognosis. The results from the present
study were consistent with those from these previous studies
(41,42), and were also similar to those from
studies on some early-stage lymphomas (44,45).
The present study presented certain limitations.
Firstly, data selection from the SEER database may potentially
cause selection bias, since this is prevalent in all
non-prospective, nonrandomized studies. Secondly, SEER database
does not include clinically meaningful variables, including the
expression levels of Ki-67, lactate dehydrogenase, β2-microglobulin
and local tumor invasiveness. Although the sample size was large,
the present study is a retrospective study and needs to be
validated in a cohort study. Thirdly, since information about
patient treatment in the SEER database was incomplete, the present
study did not include information on specific modalities and doses
of radiotherapy and chemotherapy.
In conclusion, the present study analyzed prognostic
data of stage IE and IIE UADT-ENKTCL from the SEER database and
developed a prognostic nomogram. This nomogram may predict the OS
of patients with ENKTCL by using some clinically common variables.
This nomogram may guide clinicians in better decision-making and
have crucial clinical applications.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (grant no. 81570203).
The datasets generated and/or analyzed during the
present study are available in SEER stat 8.3.5 software (https://seer.cancer.gov/data/).
MZ and GW designed the study, wrote the original
draft and revised the manuscript. YC, XW, XL and LL contributed to
the collection and analysis of the data and the preparation of
figures and tables. All authors read and approved the final
manuscript
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X,
Wu J, Fu X, Ma W, Zhang X, et al: DDGP versus SMILE in newly
diagnosed advanced natural killer/T-Cell lymphoma: A randomized
controlled, multicenter, open-label study in China. Clin Cancer
Res. 22:5223–5228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aozasa K and Zaki MA: Epidemiology and
pathogenesis of nasal NK/T-cell lymphoma: A mini-review.
ScientificWorldJournal. 11:422–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ai WZ, Chang ET, Fish K, Fu K,
Weisenburger DD and Keegan TH: Racial patterns of extranodal
natural killer/T-cell lymphoma, nasal type, in California: A
population-based study. Br J Haematol. 156:626–632. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Au WY, Weisenburger DD, Intragumtornchai
T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO and Liang R;
International Peripheral T-Cell Lymphoma Project, : Clinical
differences between nasal and extranasal natural killer/T-cell
lymphoma: A study of 136 cases from the International Peripheral
T-Cell Lymphoma Project. Blood. 113:3931–3937. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki R, Suzumiya J, Yamaguchi M,
Nakamura S, Kameoka J, Kojima H, Abe M, Kinoshita T, Yoshino T,
Iwatsuki K, et al: Prognostic factors for mature natural killer
(NK) cell neoplasms: Aggressive NK cell leukemia and extranodal NK
cell lymphoma, nasal type. Ann Oncol. 21:1032–1040. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim TM, Lee SY, Jeon YK, Ryoo BY, Cho GJ,
Hong YS, Kim HJ, Kim SY, Kim CS, Kim S, et al: Clinical
heterogeneity of extranodal NK/T-cell lymphoma, nasal type: A
national survey of the Korean Cancer Study Group. Ann Oncol.
19:1477–1484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vazquez A, Khan MN, Blake DM, Sanghvi S,
Baredes S and Eloy JA: Extranodal natural killer/T-Cell lymphoma: A
population-based comparison of sinonasal and extranasal disease.
Laryngoscope. 124:888–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fritz A: ICD-O-3 terminology approved for
use with cases diagnosed January 1, 2014 and after. J Registry
Manag. 40:140–143. 2013.PubMed/NCBI
|
|
9
|
Liu X, Huang E, Wang Y, He Y, Luo H, Zhong
M, Qiu D, Li C, Yang H, He G, et al: Dosimetric comparison of
helical tomotherapy, VMAT, fixed-field IMRT and 3D-conformal
radiotherapy for stage I–II nasal natural killer T-cell lymphoma.
Radiat Oncol. 12:762017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma X, Guo Y, Pang Z, Wang B, Lu H, Gu YJ
and Guo X: A randomized phase II study of CEOP with or without
semustine as induction chemotherapy in patients with stage IE/IIE
extranodal NK/T-cell lymphoma, nasal type in the upper
aerodigestive tract. Radiother Oncol. 93:492–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SJ, Yang DH, Kim JS, Kwak JY, Eom HS,
Hong DS, Won JH, Lee JH, Yoon DH, Cho J, et al: Concurrent
chemoradiotherapy followed by L-asparaginase-containing
chemotherapy, VIDL, for localized nasal extranodal NK/T cell
lymphoma: CISL08-01 phase II study. Ann Hematol. 93:1895–1901.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim WS, Song SY, Ahn YC, Ko YH, Baek CH,
Kim DY, Yoon SS, Lee HG, Kang WK, Lee HJ, et al: CHOP followed by
involved field radiation: Is it optimal for localized nasal natural
killer/T-cell lymphoma? Ann Oncol. 12:349–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh
J, Lee SW, Kim JS, Cho J, Lee GW, et al: Phase II trial of
concurrent radiation and weekly cisplatin followed by VIPD
chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal
NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma
study. J Clin Oncol. 27:6027–6032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song MK, Chung JS, Yhim HY, Lim SN, Kim
SJ, Han YH, Shim HK, Jung SH, Lee JJ and Yang DH: Tumor necrosis
and complete resection has significant impacts on survival in
patients with limited-stage upper aerodigestive tract NK/T cell
lymphoma. Oncotarget. 8:79337–79346. 2017.PubMed/NCBI
|
|
15
|
Li YX, Fang H, Liu QF, Lu J, Qi SN, Wang
H, Jin J, Wang WH, Liu YP, Song YW, et al: Clinical features and
treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer
ring. Blood. 112:3057–3064. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao DL, Fu QQ, Zhang TT, Li SL, Pan Y and
Zhai QL: Analysis of clinicopathological characteristics and
prognosis of 112 patients with primary Waldeyer's ring lymphoma.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 23:1301–1308. 2015.(In
Chinese). PubMed/NCBI
|
|
17
|
Huang JJ, Zhu YJ, Xia Y, Zhao W, Lin TY,
Jiang WQ, Huang HQ and Li ZM: A novel prognostic model for
extranodal natural killer/T-cell lymphoma. Med Oncol. 29:2183–2190.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jo JC, Yoon DH, Kim S, Lee BJ, Jang YJ,
Park CS, Huh J, Lee SW, Ryu JS and Suh C: Clinical features and
prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol.
89:103–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pongpruttipan T, Sukpanichnant S,
Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, Kayasut K,
Mitarnun W, Khuhapinant A, Bunworasate U, et al: Extranodal
NK/T-cell lymphoma, nasal type, includes cases of natural killer
cell and αβ, γδ, and αβ/γδ T-cell origin: A comprehensive
clinicopathologic and phenotypic study. Am J Surg Pathol.
36:481–499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebied A, Thanh Huan V, Makram OM, Sang TK,
Ghorab M, Ngo HT, Iraqi A, Kamel MG, Dang TN, Vuong NL, et al: The
role of primary lymph node sites in survival and mortality
prediction in Hodgkin lymphoma: A SEER population-based
retrospective study. Cancer Med. 7:953–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu PP, Wang KF, Jin JT, Bi XW, Sun P,
Wang Y, Yang H, Li ZM, Jiang WQ and Xia Y: Role of radiation
therapy in primary breast diffuse large B-cell lymphoma in the
Rituximab era: A SEER database analysis. Cancer Med. 7:1845–1851.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adams SV, Newcomb PA and Shustov AR:
Racial patterns of peripheral T-Cell lymphoma incidence and
survival in the United States. J Clin Oncol. 34:963–971. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pencina MJ and D'Agostino RB: Overall C as
a measure of discrimination in survival analysis: Model specific
population value and confidence interval estimation. Stat Med.
23:2109–2123. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harrell FE Jr, Lee KL and Mark DB:
Multivariable prognostic models: Issues in developing models,
evaluating assumptions and adequacy, and measuring and reducing
errors. Stat Med. 15:361–387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Niu SQ, Yang Y, Li YY, Wen G, Wang L, Li
ZM, Wang HY, Zhang LL, Xia YF and Zhang YJ: Primary site and
regional lymph node involvement are independent prognostic factors
for early-stage extranodal nasal-type natural killer/T cell
lymphoma. Chin J Cancer. 35:342016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balachandran VP, Gonen M, Smith JJ and
DeMatteo RP: Nomograms in oncology: More than meets the eye. Lancet
Oncol. 16:e173–e180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miao DL, Song W, Qian J, Zhu ZG, Wu Q, Lv
CG and Chen L: Development and validation of a nomogram for
predicting overall survival in pancreatic neuroendocrinetumors.
Transl Oncol. 11:1097–1103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayashi Y, Xiao L, Suzuki A, Blum MA,
Sabloff B, Taketa T, Maru DM, Welsh J, Lin SH, Weston B, et al: A
nomogram associated with high probability of malignant nodes in the
surgical specimen after trimodality therapy of patients with
oesophageal cancer. Eur J Cancer. 48:3396–3404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gold JS, Gönen M, Gutiérrez A, Broto JM,
García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF,
Antonescu CR, et al: Development and validation of a prognostic
nomogram for recurrence-free survival after complete surgical
resection of localised primary gastrointestinal stromal tumour: A
retrospective analysis. Lancet Oncol. 10:1045–1052. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang JX, Song W, Chen ZH, Wei JH, Liao
YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J, et al: Prognostic and
predictive value of a microRNA signature in stage II colon cancer:
A microRNA expression analysis. Lancet Oncol. 14:1295–1306. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kattan MW, Karpeh MS, Mazumdar M and
Brennan MF: Postoperative nomogram for disease-specific survival
after an R0 resection for gastric carcinoma. J Clin Oncol.
21:3647–3650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zivanovic O, Jacks LM, Iasonos A, Leitao
MM Jr, Soslow RA, Veras E, Chi DS, Abu-Rustum NR, Barakat RR,
Brennan MF and Hensley ML: A nomogram to predict postresection
5-year overall survival for patients with uterine leiomyosarcoma.
Cancer. 118:660–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY,
Xu LM, Wu JX, Wang W, Wu T, Lu B, et al: Prognostic nomogram for
overall survival in previously untreated patients with extranodal
NK/T-cell lymphoma, nasal-type: A multicenter study. Leukemia.
29:1571–1577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao J, Lan S, Shen L, Si H, Xiao H, Yuan
Q, Li X, Li H and Guo R: Hemoglobin level, a prognostic factor for
nasal extranodal natural killer/T-cell lymphoma patients from stage
I to IV: A validated prognostic nomogram. Sci Rep. 7:109822017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu QF, Wang WH, Wang SL, Liu YP, Huang
WT, Lu N, Zhou LQ, Ouyang H, Jin J and Li YX: Immunophenotypic and
clinical differences between the nasal and extranasal subtypes of
upper aerodigestive tract natural killer/T-cell lymphoma. Int J
Radiat Oncol Biol Phys. 88:806–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fang H, Jin J, Wang WH, Wang SL, Zhou LQ
and Li YX: Prognostic factors and treatment outcomes for patients
with stage II extranodal nasal-type natural killer/T-cell lymphoma
of the upper aerodigestive tract. Leuk Lymphoma. 55:1832–1837.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang MJ, Jiang Y, Liu WP, Li ZP, Li M,
Zhou L, Xu Y, Yu CH, Li Q, Peng F, et al: Early or up-front
radiotherapy improved survival of localized extranodal NK/T-cell
lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat
Oncol Biol Phys. 70:166–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Avilés A, Delgado S, Ruiz H, de la Torre
A, Guzman R and Talavera A: Treatment of non-Hodgkin's lymphoma of
Waldeyer's ring: Radiotherapy versus chemotherapy versus combined
therapy. Eur J Cancer B Oral Oncol 32B. 19–23. 1996. View Article : Google Scholar
|
|
39
|
You JY, Chi KH, Yang MH, Chen CC, Ho CH,
Chau WK, Hsu HC, Gau JP, Tzeng CH, Liu JH, et al: Radiation therapy
versus chemotherapy as initial treatment for localized nasal
natural killer (NK)/T-cell lymphoma: A single institute survey in
Taiwan. Ann Oncol. 15:618–625. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song G, Xiong GY, Fan Y, Huang C, Kang YM,
Ji GJ, Chen JC, Xin ZC and Zhou LQ: The role of tumor size,
ultrasonographic findings, and serum tumor markers in predicting
the likelihood of malignant testicular histology. Asian J Androl.
21:196–200. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang QS, Zhao SH, Jiang Y, Jiang TY, Yuan
SZ and Su H: Retrospective analysis of clinical features and
prognosis of 84 patients with extranodal NK/T cell lymphoma in one
center. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 25:1390–1396. 2017.(In
Chinese). PubMed/NCBI
|
|
42
|
Wang KF, Chang BY, Chen XQ, Liu PP, Wuxiao
ZJ, Wang ZH, Li S, Jiang WQ and Xia ZJ: A prognostic model based on
pretreatment platelet lymphocyte ratio for stage IE/IIE upper
aerodigestive tract extranodal NK/T cell lymphoma, nasal type. Med
Oncol. 31:3182014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai W, Jia B, Yang J, Zhou S, Liu P, He X,
Qin Y, Gui L, Zhang C, Han X, et al: Development of new prognostic
model based on pretreatment βLRI and LLRI for stage IE/IIE upper
aerodigestive tract ENKTL, nasal type. Oncotarget. 8:34787–34795.
2017.PubMed/NCBI
|
|
44
|
Wang GB, Xu GL, Luo GY, Shan HB, Li Y, Gao
XY, Li JJ and Zhang R: Primary intestinal non-Hodgkin's lymphoma: A
clinicopathologic analysis of 81 patients. World J Gastroenterol.
17:4625–4631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang S, Wang L, Yu D, Shen Y, Cheng S,
Zhang L, Qian Y, Shen Z, Li Q and Zhao W: Localized primary
gastrointestinal diffuse large B cell lymphoma received a surgical
approach: An analysis of prognostic factors and comparison of
staging systems in 101 patients from a single institution. World J
Surg Oncol. 13:2462015. View Article : Google Scholar : PubMed/NCBI
|