Introduction
Although refined chemotherapy, including rituximab
(an anti-CD20 monoclonal antibody), and autologous stem cell
transplantation have improved the prognosis for B-cell non-Hodgkin
lymphoma (B-NHL), patients with refractory B-NHL still have a poor
prognosis (1,2). Approximately 19–26% of patients with
follicular lymphoma (FL) receiving first-line immunochemotherapy
with rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisone (R-CHOP) experienced progression of disease within 24
months (2). Chimeric antigen
receptor (CAR) T cells are a remedial treatment for these patients.
Anti-CD19 CAR T cell (CAR-T 19) therapies have exhibited potent
activity against numerous subtypes of B-NHL, including FL (3). Nivolumab, the human immunoglobulin G4
programmed death-1 (PD-1) immune checkpoint inhibitor antibody with
high affinity to PD-1 receptors on T cells, could block their
interaction with PD ligands 1 and 2 (PD-L1/PD-L2) and restore
T-cell function (4). A significant
association between the expression levels of PD-1 on T cells and
the immunosuppression of T cells has previously been reported
(5). A study that focused on the use
of Nivolumab in a cohort of 10 patients with relapsed or refractory
FL, reported the overall response rate was four patients (40%), and
one achieved complete response (6).
Meanwhile, PD-1 inhibitors may lead to an imbalance in immune
tolerance and uncontrolled immune response, even fatal myocarditis
(7). The present study describes a
patient with successfully treated refractory FL, who received
anti-CD19 CAR-T cells combined with decreased dose PD-1 inhibitor
regimen.
Case report
A 70-year-old female patient was admitted to the
Tianjin First Central Hospital (Tianjin, China) in July 2017,
presenting with fever and lower back pain, which had persisted for
10 days. A number of inpatient laboratory tests were performed. A
bone marrow biopsy revealed lymphocyte dysplasia, small cell
bodies, slightly irregular nuclei and thicker chromatin. The biopsy
was fixed for 16–18 h with 10% formaldehyde, which was composed of
90 ml distilled water and 40% formaldehyde 10 ml and incubated for
18–20 h in Richman decalcification solution at room temperature
which composed of 8% hydrochloric acid 40 ml and 10% formaldehyde
60 ml and embedded in paraffin. Subsequently, the 3-µm thin
sections were cut and incubated with the following blocking
reagents: Peroxide Block (ready to use; cat. no. DS9263; Leica
Microsystems, Inc.) or EnVsion FLEX Peroxidase-Blocking Reagent
(ready to use; cat. no. DM821; Dako; Agilent Technologies, Inc.),
at room temperature for 3–5 min. Immunohistochemical staining was
performed using commercially available primary antibodies at room
temperature for 20–30 min according to the manufacturer's protocol
to the following antigens: CD20 (1:100; cat. no. RTU-CD20-7D1-QH;
Beijing Xin'ao Medical Technology Co., Ltd.), CD138 (1:100; cat.
no. RTU-CD138-QH; Beijing Xin'ao Medical Technology Co., Ltd.),
PAX-5 (1:100; cat. no. PM-0111; Shanghai Ruijing Biotechnology Co.,
Ltd.), CD10 (1:100; cat. no. CRM-0191; Shanghai Ruijing
Biotechnology Co., Ltd.) and Bcl-2 (1:100; cat. no.
RTU-BCL-2-486-QH; Beijing Xin'ao Medical Technology Co., Ltd.). All
antibodies were observed under an Olympus light microscope at ×100,
×400 and ×1,000 magnification. Abnormal lymphocytes were observed
in the bone marrow morphology (Fig.
1A-C). The results of the immunohistochemistry revealed that
samples were CD20+, PAX5+, CD138+,
CD10 partially positive and BCL2+. Approximately
107 nucleated cells were resuspended per 100 µl of
buffer. The nucleated cells were incubated with the human FcR
Blocking Reagent (dilution, ready to use; cat. no. 130-059-901,
Miltenyi Biotec GmbH) in the dark at 2–8°C for 10 min No fixative
was used for the bone marrow fluid samples. Staining was performed
using commercially available antibodies at room temperature for 15
min according to the manufacturer's protocol: Anti-Skappa (ready to
use; cat. no. 349516; BD Biosciences), anti-Slambda (ready to use;
cat. no. 349516; BD Biosciences), CD19 (ready to use; cat. no.
130-113-647; Miltenyi Biotec GmbH), anti-CD34 (ready to use; cat.
no. 348053; BD Biosciences), anti-CD10 (ready to use; cat. no.
IM3633; Beckman Coulter, Inc.), anti-CD45 (ready to use; cat. no.
347463; BD Biosciences), anti-CD20 (ready to use; cat. no.
130-113-371; Miltenyi Biotec GmbH), anti-CD5 (ready to use; cat.
no. 651154; BD Biosciences), anti-FMC7 (ready to use; cat. no.
340919; BD Biosciences), anti-CD23 (ready to use; cat. no. 341007;
BD Biosciences), anti-CD71 (ready to use; cat. no. 347513, BD
Biosciences), and anti-CD22 (ready to use; cat. no. 347573; BD
Biosciences). Flow cytometry was performed using the Beckman
Coulter cell telescopic flow cytometer and cells were assessed
using CytExpert software (version 2.3.0.84; Beckman Coulter, Inc.).
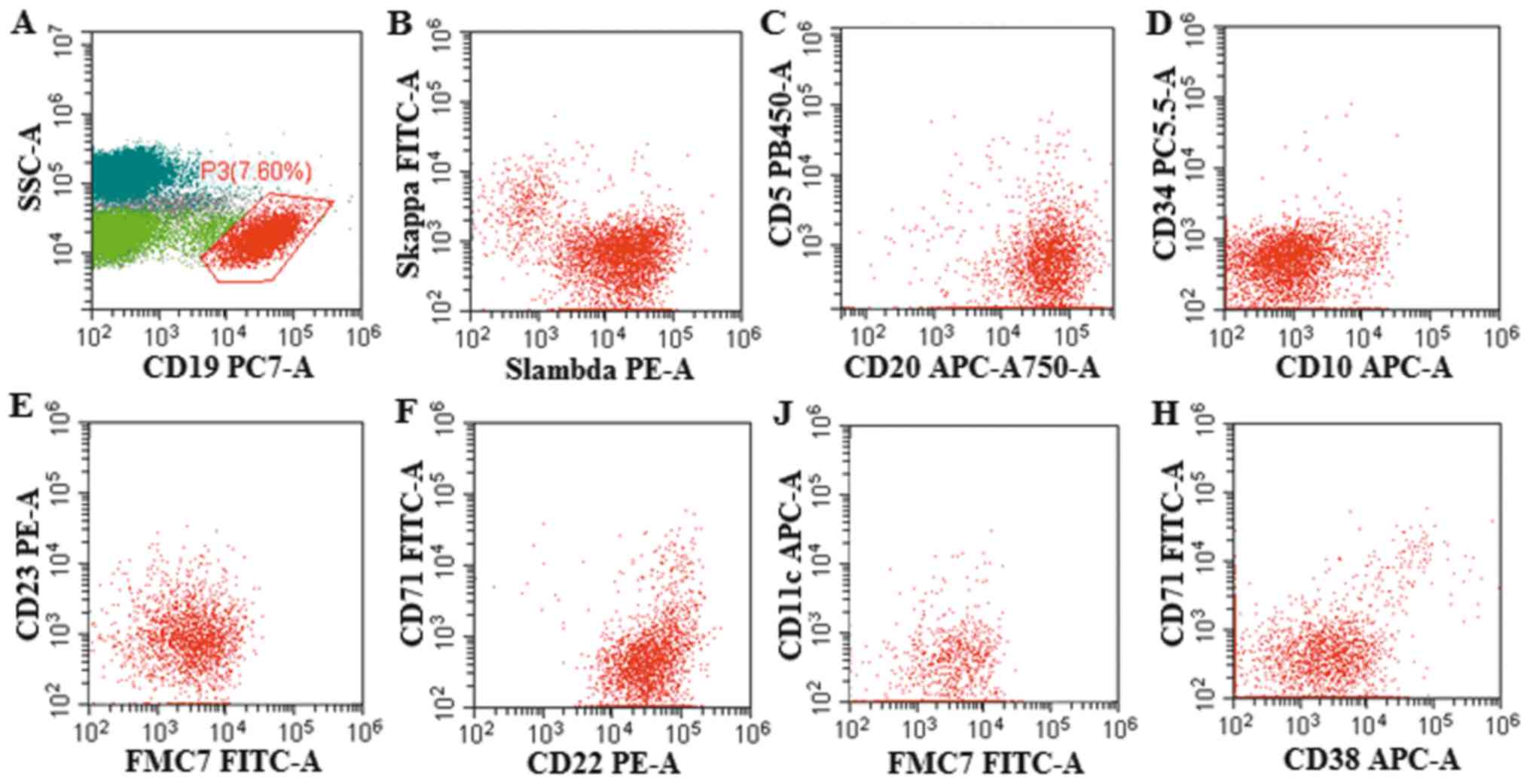
There were 7.6% abnormal monoclonal small B lymphocytes expressing
CD19+, CD20+, CD22+,
FMC7+, CD23−, CD38−,
CD71−, CD5−, CD10−,
CD11c− detected by flow cytometry in the bone marrow
(Fig. 2). The IgH/BCL2 gene
rearrange was 4.8% detected by fluorescence in situ
hybridization (FISH), as analyzed by Tianjin Sino-US-Diagnostics
Technology Co., Ltd. in bone marrow (Fig. 1D). MYD88-L265P gene examination was
negative, detected by direct sequencing as performed by Tianjin
Sino-US-Diagnostics Technology Co., Ltd.) (Fig. 1D). Positron emission
tomography-computed tomography (PET-CT) examination revealed that
bone density was not uniform, with partial bone destruction.
Additionally, PET-CT revealed that bone metabolism was increased
[L3 vertebral body local maximum standardized uptake value
(SUVmax), 28.20; bilateral neck, right supraclavicular fossa
enlarged lymph node SUVmax, 8.59] and that the metabolism of a soft
tissue mass in the left mesenteric region of lumbar 2–3 disc level
was increased (SUVmax, 8.57; Fig.
3A). The patient was diagnosed with advanced-stage IV FL, and
treated with a combination regimen of rituximab, cyclophosphamide,
doxorubicin, vincristine and prednisolone (R-CHOP). Following six
cycles of R-CHOP treatment, the patient presented with generalized
weakness, lower back pain and intermittent abdominal pain. No
abnormal phenotype B lymphocytes were observed in the bone marrow
at this time. A PET-CT examination revealed that the increased
metabolism of the bones and the soft tissue mass in the left
mesenteric region was decreased; however, the density of mesenteric
lymph nodes at the lumbar 1–5 level was increased (SUVmax, 6.70;
Fig. 3B). PET-CT examination
revealed a maximum probability that the mesenteric lymph nodes were
new lymphoma lesions. However, the patient had comorbid
hypertension and diabetes, and was therefore unable to undergo a
further biopsy to confirm this result. In addition, high expression
of PD-1 in CD3+ T cells (80.76%) was detected in the
peripheral blood samples from this patient by flow cytometry.
The patient was diagnosed as having refractory
lymphoma. The Eastern Cooperative Oncology Group Performance Status
condition of the patient was 2 points with hypertension and
diabetes (8). Following the initial
R-CHOP, the patient was subsequently enrolled in a clinical trial
at the Department of Hematology, Tianjin First Center Hospital with
autologous anti-CD19 CAR-T cell expressing murine anti-CD19 single
chain fragment variable and 4-1BB-CD3ζ costimulatory-activation
domains (ChiCTR-ONN-16009862) in Jan 2018. The patient received
lymphodepleting chemotherapy with fludarabine (30 mg/m2)
and cyclophosphamide (400 mg/m2) daily from day −4 to
day −2. Autologous anti-CD19 CAR-T cells were infused
(1.3×107 cells/kg) on day 0. Because of the high
expression of PD-1 in her peripheral blood, a combination of PD-1
blockade therapy and anti-CD19 CAR-T cell treatment was used to
improve the efficacy of CAR-T cell therapy and to avoid anti-CD19
CAR-T cell treatment failure for the high expression of PD-1. As
PD-1 blockade therapy may result in adverse effects especially for
a 70-year-old patient, following the infusion of CAR-T 19 cells, a
reduced dose of the PD-1 inhibitor Opdivo® (Nivolumab;
1.5 mg/kg) was administered on day 1. Other than fever and chills,
a slight headache, mild low blood pressure and low blood oxygen,
the combination therapy was well tolerated (Table I). These adverse effects abated after
14 days. No severe adverse effects of the PD-1 inhibitor were
observed during the treatment period. The levels of PD-1 expression
on T cells were monitored in peripheral blood samples from the
patient and PD-1 expression was maintained at <5% over the
following 60 days. The patient did not receive Nivolumab infusion
in order to avoid adverse effects in the following therapy. The
expression levels of PD-1 remained low for 6 months after the
salvage treatment and the patient received no further treatment
with a PD-1 inhibitor in the next round of therapy.
 | Table I.Adverse effects following salvage
treatment with anti-CD19 CAR-T cell combined with reduced-dose
programmed cell death 1 blockade therapy. |
Table I.
Adverse effects following salvage
treatment with anti-CD19 CAR-T cell combined with reduced-dose
programmed cell death 1 blockade therapy.
|
| Time point
(days) |
|---|
|
|
|
|---|
| Variable | −12 | 0 | 4 | 7 | 14 | 21 |
|---|
| Temperature (°C) | 36.7 | 38.0 | 37.6 | 38.8 | 36.4 | 36.5 |
| Chills | No | Yes (twice) | No | No | No | No |
| Blood pressure
(mmHg) | 100/60 | 110/74 | 156/89 | 89/52 | 125/76 | 105/70 |
| Blood oxygen (%) | 95 | 93 | 91 | 84 | 89 | 94 |
| Neurological
symptoms | – | N | Headache | Headache | N | N |
| White blood cell
count (×109/l) | 4.27 | 2.37 | 1.04 | 4.01 | 2.55 | 5.16 |
| Hemoglobin (g/l) | 114 | 106 | 94 | 89 | 105 | 109 |
| Blood platelet count
(×109/l) | 155 | 68 | 47 | 104 | 127 | 173 |
After 30 days of combined therapy, significant
clinical improvement was observed. The weakness and lower back pain
gradually abated, and the patient resumed normal activities. A
PET-CT examination was conducted and reviewed after 60 days of
combined therapy. On the PET-CT scan, the density of mesenteric
lymph nodes at the lumbar 1–5 level was decreased compared with
that of the previous scan (Fig. 3C).
The patient achieved complete response (CR) after 60 days of the
anti-CD19 CAR-T cells combined with reduced-dose PD-1 blockade
treatment. Until now, the patient maintained CR for 16 months
following the combination therapy.
During treatment, the percentage of anti-CD19 CAR-T
cells and the expression of PD-1 on CD3+ T cells in
peripheral blood samples were detected by flow cytometry. The
anti-CD19 CAR DNA was detected by the quantitative PCR in
peripheral blood. DNA was obtained from the peripheral blood of the
patient. The mRNA level of CD19 CAR was detected via reverse
transcription-quantitative PCR (RT-qPCR) analysis. After 24 or 48 h
of co-culture, total RNA was extracted from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) as the template for the reactions. cDNA was synthesized with
random primers from 10 µl total RNA with the aid of the Revert
AidTM First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.). Following the protocol of the manufacturer,
RT-qPCR was performed to characterize the mRNA levels of specific
genes using Fast SYBR-Green Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with a Biosystems StepOne Real-Time PCR
machine (Applied Biosystems; Thermo Fisher Scientific, Inc). The
primer sequences were restricted by company patents and could not
be provided. Each PCR reaction was performed in triplicate as
follows: In order to prevent any non-specific PCR, amplification
and contamination was performed at 50°C for 2 min. Denaturation was
performed at 95°C for 10 min. Annealing and elongation was
performed with 35 cycles at 95°C for 15 sec and final extension was
performed at 60°C for 1 min. The levels of interleukin-6 (IL-6) in
the serum were detected via ELISA, using the one-step sandwich
method (Shanghai Renjie Biotechnology Co., Ltd.). Other indicators
were measured, including serum ferritin using the chemiluminescence
immunoassay method (Beckman Inc.) and C-reactive protein (CRP)
using the Latex enhanced immunoturbidimetric method, performed by
the Nanjing Jiancheng Bioengineering Institute. The highest
percentage of anti-CD19 CAR-T cells among CD3+ T cells
was 9.22% on day 7 of combination therapy (day 0 was set as the day
of CD19 CAR-T cell infusion). Approximately 90 days after
combination therapy, the percentage of anti-CD19 CAR-T cells had
decreased to ~0.01% (Fig. 4A). The
highest anti-CD19 CAR DNA copy number was 3,560 copies/µg of DNA on
day 7 after combination therapy. Approximately 90 days after
therapy, it had decreased to 320 copies/µg of DNA (Fig. 4B). Notably, the expression of PD-1 in
CD3+ T cells was decreased from 80.90% prior to
combination therapy, which was 80.76% on day 0 of this therapy, to
0.05% on day 7 following combination therapy. PD-1 expression
remained <5% over the following 60 days, and <7% for 180 days
following the salvage treatment (Fig.
4C). The highest level of IL-6 in the serum was 110.2 pg/ml on
day 4 of combination therapy. The level of IL-6 in serum had
decreased to a normal level, whereas symptoms such as fever and
headache had abated on day 14 (Fig.
4D). Serum ferritin and CRP levels had similar changes to IL-6
(Fig. 4E and F). Cytokine release
syndrome following treatment with CAR-T 19 cells was grade II
(5).
Discussion
B-NHL is the most frequent hematological malignancy.
Chemotherapy, anti-CD20 monoclonal antibodies and autologous stem
cell transplantation have improved the prognosis of patients with
B-NHL in recent decades; however, the prognosis of patients with
refractory B-NHL remains poor (9).
Anti-CD19 CAR-T cell therapy may improve remission rates and
prolong survival time in patients with relapsed/refractory
B-lineage hematological malignancies (10,11).
The immunosuppressive PD-1 pathway prevents T cells
from entering the tumor area and blocks the effects of T-cell
immunotherapy (12,13). Although the effects of the PD-1
inhibitor were significant, it requires regular and repeated
administration (14). Anti-CD19
CAR-T cells cultured from PD-1 high expression T cells exhibit
decreased anti-tumor immune responses and result in failure of
CAR-T cell therapy (15). According
to the beneficial effects of PD-1 inhibitors on the clinical
treatment of tumors (16), PD-1
inhibitors may be able to partially rescue the deterioration of
anti-CD19 CAR-T cell function. Anti-CD19 CAR-T cell in combination
with PD-1 blockade may overcome the immunosuppressive effects of
high PD-1 expression on T cells, thereby improving the therapeutic
efficacy in relapsed/refractory B-NHL.
A number of adverse effects are associated with PD-1
blockade, particularly immune-associated adverse events in the
respiratory and circulatory systems (17). The mechanisms underlying these
effects remain unclear; however, they may result in mortality.
Additionally, the adverse effects of anti-CD19 CAR-T cells,
including cytokine release syndrome, must be taken into account
(18). The present study
hypothesized that CAR-T 19 cell therapy combined with
decreased-dose PD-1 blockade may be effective in treating B-NHL.
This combination decreased the adverse effects associated with PD-1
inhibitors and anti-CD19 CAR-T cell, and led to improved
therapeutic efficacy.
The present case study demonstrated the therapeutic
feasibility of decreased-dose PD-1 inhibitor in combination with
CAR-T 19 cells to overcome immunosuppression. A decreased dose of
PD-1 inhibitor may decrease its adverse effects, whilst still
ensuring efficacy. Future studies are required in order to test the
combination of decreased-dose PD-1 inhibitor and CAR-T 19 cells in
more patients with refractory lymphoma and high expression of PD-1
in peripheral blood samples. In addition, further studies are
required to elucidate the molecular mechanisms underlying this
combination therapy and to design a clinical trial to test the
efficacy of PD-1 inhibitors combined with CAR-T 19 cells in
patients with refractory B-NHL.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hospital
Funding Project (grant no. CM201805).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
QD conceptualized and designed the study. JW, YJ, HZ
and YL performed the clinical trial. RZ acquired the data. JM
analyzed and interpreted the data. All authors contributed to the
writing and revision of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Tianjin First Center Hospital and written informed
consent was obtained from the patient.
Patient consent for publication
Informed consent for publication was obtained from
the patient.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Van Den Neste E, Schmitz N, Mounier N,
Gill D, Linch D, Trneny M, Bouadballah R, Radford J, Bargetzi M,
Ribrag V, et al: Outcomes of diffuse large B-cell lymphoma patients
relapsing after autologous stem cell transplantation: An analysis
of patients included in the CORAL study. Bone Marrow Transplant.
52:216–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jurinovic V, Kridel R, Staiger AM,
Szczepanowski M, Horn H, Dreyling MH, Rosenwald A, Ott G, Klapper
W, Zelenetz AD, et al: Clinicogenetic risk models predict early
progression of follicular lymphoma after first-line
immunochemotherapy. Blood. 128:1112–1120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuster SJ, Svoboda J, Chong EA, Nasta
SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V,
Landsburg D, et al: Chimeric antigen receptor T cells in refractory
B-cell lymphomas. N Engl J Med. 377:2545–2554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Thudium KB, Han M, Wang XT, Huang
H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al: In
vitro characterization of the anti-PD-1 antibody nivolumab,
BMS-936558, and in vivo toxicology in non-human primates. Cancer
Immunol Res. 2:846–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Annibali O, Crescenzi A, Tomarchio V,
Pagano A, Bianchi A, Grifoni A and Avvisati G: PD-1 /PD-L1
checkpoint in hematological malignancies. Leuk Res. 67:45–55. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naidoo J, Page DB, Li BT, Connell LC,
Schindler K, Lacouture ME, Postow MA and Wolchok JD: Toxicities of
the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann
Oncol. 26:2375–2391. 2015.PubMed/NCBI
|
|
8
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martelli M, Ferreri AJ, Agostinelli C, Di
Rocco A, Pfreundschuh M and Pileri SA: Diffuse large B-cell
lymphoma. Crit Rev Oncol Hematol. 87:146–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kochenderfer JN, Dudley ME, Kassim SH,
Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ,
Hughes MS, Sherry RM, et al: Chemotherapy-refractory diffuse large
B-cell lymphoma and indolent B-cell malignancies can be effectively
treated with autologous T cells expressing an anti-CD19 chimeric
antigen receptor. J Clin Oncol. 33:540–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Batlevi CL, Matsuki E, Brentjens RJ and
Younes A: Novel immunotherapies in lymphoid malignancies. Nat Rev
Clin Oncol. 13:25–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224–225. 2014. View Article : Google Scholar
|
|
13
|
Jaspers JE and Brentjens RJ: Development
of CAR T cells designed to improve antitumor efficacy and safety.
Pharmacol Ther. 178:83–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang AC, Postow MA, Orlowski RJ, Mick R,
Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al: T-cell
invigoration to tumour burden ratio associated with anti-PD-1
response. Nature. 545:60–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Siriwon N, Zhang X, Yang S, Jin T,
He F, Kim YJ, Mac J, Lu Z, Wang S, et al: Enhanced cancer
immunotherapy by chimeric antigen receptor-modified T cells
engineered to secrete checkpoint inhibitors. Clin Cancer Res.
23:6982–6992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou W, Wolchok JD and Chen L: PD-L1
(B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms,
response biomarkers, and combinations. Sci Transl Med. 8:328–324.
2016. View Article : Google Scholar
|
|
17
|
Läubli H, Balmelli C, Bossard M, Pfister
O, Glatz K and Zippelius A: Acute heart failure due to autoimmune
myocarditis under pembrolizumab treatment for metastatic melanoma.
J Immunother Cancer. 3:112015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brudno JN and Kochenderfer JN: Toxicities
of chimeric antigen receptor T cells: Recognition and management.
Blood. 127:3321–3330. 2016. View Article : Google Scholar : PubMed/NCBI
|