Introduction
Colorectal cancer is the most common type of
gastrointestinal malignancy and is the third most commonly
diagnosed cancer and fourth leading cause of cancer-associated
mortality worldwide (1). Colorectal
adenocarcinoma is the most common cause of colorectal cancer, with
1.2 million new cases and 0.6 million mortalities per year
worldwide (2). Surgical resection is
the main treatment of primary colorectal adenocarcinoma.
Approximately 90% of patients with colorectal adenocarcinoma live
>5 years following appropriate surgical intervention. However,
the 5-year survival rate for those with distant tumor metastasis
remains as low as 10% even after detection of lymph node metastases
(3). Therefore, early diagnosis and
treatment is key for the survival of patients with colorectal
adenocarcinoma.
Transforming growth factor β (TGF-β) binding to the
cell surface triggers activation of multiple signal transduction
pathways that are connected in intricate ways with each other, and
with other response networks involved in sensing cellular
information input. Recent data have indicated that changes in the
signal intensity and connectivity of these pathways may underlie
the complex transition of the TGF-β pathway from tumor suppressor
to oncogene during tumorigenesis (4). TGF-β and its signaling effectors act as
key determinants of carcinoma cell behavior. The autocrine and
paracrine effects of TGF-β on tumor cells and the tumor
micro-environment exert both positive and negative influences on
cancer development (5). Accordingly,
the TGF-β signaling pathway has been considered as both a tumor
suppressor pathway and a promoter of tumor progression and
invasion.
Besides messenger RNAs that encode protein products,
the human genome also encodes a vast population of non-coding RNAs
that have critical functions in both normal physiological processes
and pathological changes (6). Long
non-coding RNAs (lncRNAs) are a subgroup of non-coding RNAs
composed of >200 nucleotides and have been revealed to serve
important roles in the pathogenesis of human diseases including
various types of malignancies (7,8). Long
non-coding RNA associated with poor prognosis of hepatocellular
carcinoma (lncRNA-AWPPH) is newly discovered lncRNA that has been
shown to be involved in the pathogenesis of hepatocellular
carcinoma (9) and bladder cancer
(10). However, the function of
lncRNA-AWPPH in other human diseases as well as in normal
physiological processes is unknown. In this study, lncRNA-AWPPH
promoted the growth of colorectal adenocarcinoma and was shown to
have diagnostic and prognostic value in the disease.
Materials and methods
Subjects
A total of 86 patients with colorectal
adenocarcinoma diagnosed via pathological examination were selected
at the Qingdao Central Hospital (Qingdao, China) between January
2012 and January 2013. The patients included 49 males and 37
females, and the age ranged between 26 to 72 years, with a mean age
of 50.2±7.2 years. The inclusion criteria were as follows: i)
Pathologically diagnosed as colorectal adenocarcinoma; ii) patients
signed informed consent; and iii) patients willing to cooperate
with researchers. The exclusion criteria were as follows: i)
patients with other types of malignant tumors; ii) patients with
severe coagulation dysfunction; and iii) patients with other
colorectal diseases. There were 10 patients in American Joint
Committee on Cancer stage II, 16 in stage III and 60 in stage IV.
At the same time, a total of 56 healthy individuals were selected
at the Qingdao Central Hospital (Qingdao, China) between January
2012 and January 2013 to serve as a control group. The patients
included 29 males and 27 females, with a mean age of 51.2±6.4 years
(range, 26–72 years). The inclusion criteria were as follows: i)
Healthy individuals were not diagnosed pathologically with
colorectal adenocarcinoma; ii) healthy individuals provided written
informed consent; and iii) healthy individuals willing to cooperate
with researchers. The exclusion criteria were as follows: i)
Individuals with other types of malignant tumors; ii) healthy
individuals with severe coagulation dysfunction; and iii) healthy
individuals with other colorectal diseases. All participants were
Han Chinese. All participants signed informed consent and the
present study was approved by the Ethics Committee of Qingdao
Central Hospital. After discharge, patients were followed up for 5
years (until January 2018) or until their mortality.
Specimen collection
Whole blood (20 ml) was obtained from the 86
patients with colorectal adenocarcinoma and the 56 healthy
individuals on the day of admission. Blood samples were kept at
room temperature for 1.5 h, followed by centrifugation at 1,800 × g
for 20 min to collect serum. All patients received surgical
resection of the primary tumors, and tumor tissues and adjacent
healthy tissues within 5 cm of the tumor were collected from 3
different sites and preserved in liquid nitrogen before use. All
tissue samples were confirmed by pathological examination.
Cell lines and cell culture
Homo sapiens colorectal adenocarcinoma cell
lines HT-29 [Caucasian American Type Culture Collection
(ATCC)-formulated McCoy's 5a Medium Modified; cat. no. 30-2007 add
10% fetal bovine serum, ATCC 30-2020 and 1%
Penicillin-Streptomycin, GBICO15140-22], Hs 698.T (American Indian;
ATCC-formulated Dulbecco's Modified Eagle's Medium; cat. no.
30-2002 add 10% fetal bovine serum, ATCC 30-2020 and 1%
Penicillin-Streptomycin, GBICO15140-22) and SNU-C1 (Asian; add 10%
fetal bovine serum; ATCC 30-2020 and 1% Penicillin-Streptomycin;
GBICO15140-22) were purchased from the American Type Culture
Collection. Cells were cultured under the conditions recommended by
the ATCC in an incubator at 37°C and 5% CO2. Cells were
harvested during logarithmic growth phase for subsequent
experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Tumor tissues and adjacent healthy tissues were
ground in liquid nitrogen. TRIzol® reagent (Thermo
Fisher Scientific, Inc.) was used to extract total RNA by mixing
directly with in vitro cultured cells. SuperScript IV
reverse transcriptase kit (Thermo Fisher Scientific, Inc.) was used
to reverse transcribe total RNA into cDNA. Reverse transcription
conditions were: 10 min at 25°C, 35 min at 50°C and 15 min at 80°C.
SYBR® Green Real-Time PCR Master Mix (Thermo Fisher
Scientific, Inc.) was used to perform RT-qPCR using a CFX96 Touch™
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The following primer pairs were used:
5′-CTGGATGGTCGCTGCTTTTTA-3′ (forward) and
5′-AGGGGGATGAGTCGTGATTT-3′ (reverse) for lncRNA-AWPPH;
5′-CCCACTCCTCCACCTTTGAC-3′ (forward) and 5′-ATGAGGTCCACCACCCTGTT-3′
(reverse) for human GAPDH. The following thermocycling conditions
were used: 95°C for 15 sec, followed by 40 cycles of 95°C for 14
sec and 50°C for 42 sec. The 2−∆∆Cq method was used to
process all data (11), and relative
expression level of lncRNA-AWPPH was normalized to endogenous
control GAPDH.
Construction of the lncRNA AWPPH
vector and silencing cell lines
A lncRNA-AWPPH expression vector was constructed by
inserting an EcoRI-EcoRI fragment containing full
length lncRNA-AWPPH cDNA into a pIRES2-EGFP vector (Clontech
Laboratories, Inc.). Negative control short hairpin (shRNA)
targeting sequence 5′-GACTTCATAAGGCGCATGC-3′ and shRNA lncRNA-AWPPH
targeting sequence 5′-GGAATGCAGCTGAAAGATTCC-3′ were synthesized by
Chang Jing Bio-Tech, Ltd. Lipofectamine® 2000 (cat. no.
11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) was used to
transfect 10 nM vector or 50 mM shRNA into 5×106 cells
for 4 h. Transfections with empty pIRES2-EGFP vector or negative
control shRNA were used as negative controls. The control group
cells were untransfected.
Cell proliferation assay
A total of 4×104 cells in 100 µl cell
suspension were added into each well of 96-well plates. Cells were
cultured in an incubator (37°C, 5% CO2), and 10 µl Cell
Counting Kit-8 solution (Beijing Solarbio Science & Technology
Co., Ltd.; cat. no. CA1210-100) was added into each well 24, 48, 72
and 96 h later. Cells were cultured at 37°C for another 4 h, and
optical density (OD) values at 450 nm were measured using an
accuSkan™ GO UV/Vis microplate spectrophotometer (Thermo Fisher
Scientific, Inc.). The sample with the largest OD value was set to
100 and all cell proliferation values were normalized to this
sample.
MTT assay
The cell suspension was diluted using culture medium
(ATCC) to obtain a final cell density of 4×104 cells/ml.
A total of 10 mM tetraethylammonium was then added to the cell
suspension. A total of 4×103 cells in 100 µl cell
suspension were added into each well of a 96-well plate. Cells were
allowed to incubate at 37°C and 5% CO2 for 6 h. This was
followed by a 4-h incubation period with 10 µl MTT (DMSO).
Absorbance at a wavelength of 570 nm was measured using an
accuSkan™ GO UV/Vis microplate spectrophotometer (Thermo Fisher
Scientific, Inc.). The percentage of viable cells was calculated
according to the following formula: % cell viability=(absorbance
sample-absorbance blank)/(absorbance control-absorbance blank)
×100. The sample with the highest cell viability was set to 100 and
all other samples were normalized to this sample.
Western blot analysis
Total protein was extracted from in vitro
cultured cells using radioimmunoprecipitation assay buffer (Thermo
Fisher Scientific, Inc.). Total protein was quantified using a
bicinchoninic acid assay. Following boiling at 100°C for 5 min for
the denaturation, 20 µg protein/lane was separated via SDS-PAGE on
a 10% gel. The separated proteins were subsequently transferred
onto a polyvinylidene fluoride membrane (Thermo Fisher Scientific,
Inc.). Membranes were washed with TBST buffer 3 times, 15 min each
time. The membranes were incubated with primary antibodies TGF-β1
(1:2,000; cat. no. ab92486; Abcam) and GAPDH (1:2,000; cat. no.
ab181602; Abcam) on the shaker at 4°C overnight. Membranes were
washed with TBST buffer 3 times, 15 min each time. Membranes were
incubated with a horseradish peroxidase-labeled secondary antibody
(1:1,000; cat. no. MBS435036; MyBioSource) for 2 h at room
temperature. Membranes were washed with TBST buffer 3 times, 15 min
each time. The chemiluminescence method was used to detect the
protein bands (EMD Millipore). Relative expression level of TGF-β1
was normalized to the endogenous control GAPDH using Image J
software (version 1.48; National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism Software (version 6; GraphPad Software, Inc.). Gene
expression and cell proliferation and viability data are presented
as the mean ± standard deviation. Comparisons between two groups
were performed using a paired or unpaired Student's t-test, as
appropriate, while comparisons between multiple groups were
performed using the one-way analysis of variance followed by the
Least Significant Difference test. Receiver operating
characteristic (ROC) curve analysis was performed to evaluate the
diagnostic value of serum AWPPH for colorectal adenocarcinoma, with
patients with colorectal adenocarcinoma as true positive cases and
healthy controls as true negative cases. The 86 patients with
colorectal adenocarcinoma were divided into high expression group
(n=43) and low expression group (n=43) according to the median
serum level of AWPPH. The Kaplan-Meier method was used to plot
survival curves for both groups, and survival curves were compared
by log-rank test. Figures were plotted using Origin software
(version 9.1; OriginLab, Northampton, MA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of lncRNA-AWPPH is
significantly higher in tumor tissues compared with adjacent
healthy tissues in the majority of patients with colorectal
adenocarcinoma
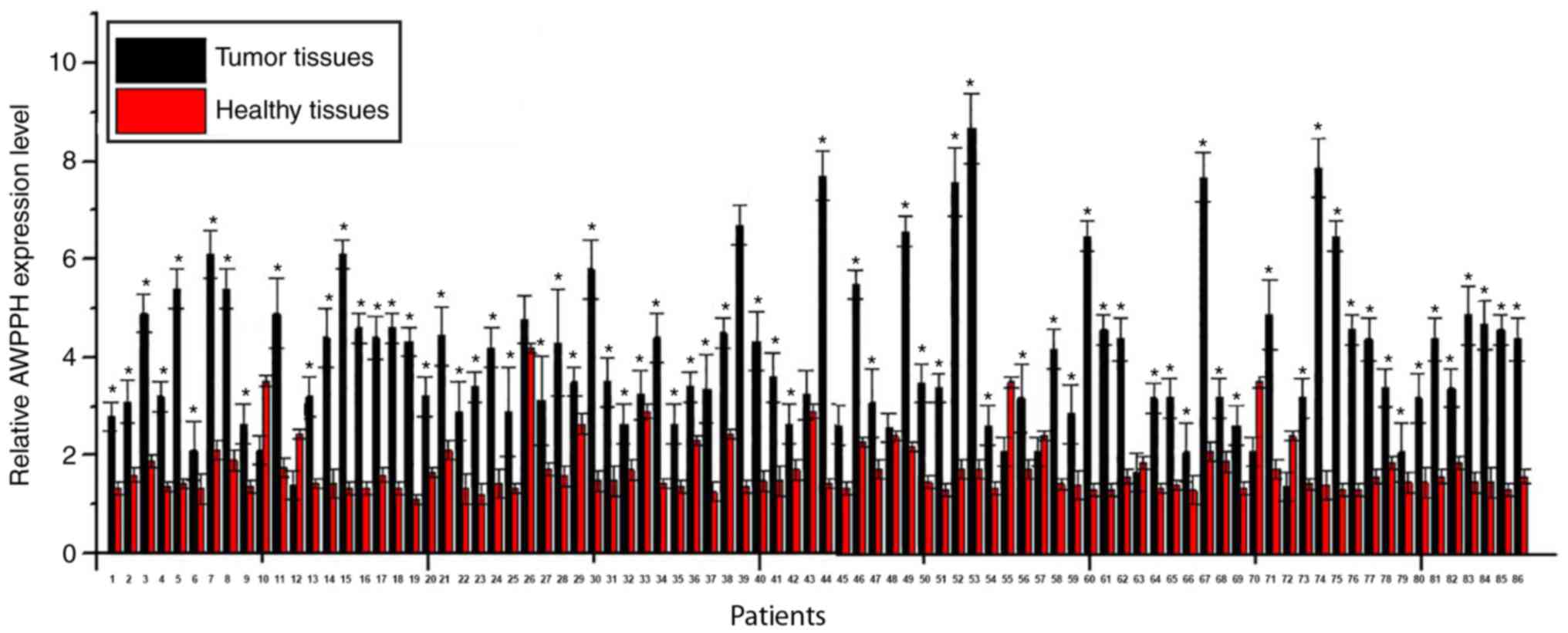
Expression levels of lncRNA-AWPPH in tumor tissues
and adjacent healthy tissues (collected from 3 different sites) of
86 patients with colorectal adenocarcinoma were detected by
RT-qPCR. A significantly higher expression level of lncRNA-AWPPH
was observed in tumor tissues compared with adjacent healthy
tissues in 75 out of 86 patients, accounting for 87.2% (Fig. 1; all P<0.05). By contrast, a
significantly lower expression level of lncRNA-AWPPH in tumor
tissues compared to adjacent healthy tissues was found in 6 out of
86 patients, accounting for 7%. No significant difference was found
in 5 cases, accounting for 5.8%. These data suggested that
upregulation of lncRNA-AWPPH is likely to be involved in the
pathogenesis of colorectal adenocarcinoma.
Expression of lncRNA-AWPPH in serum is
higher in patients with colorectal adenocarcinoma
Compared with healthy controls, expression of
lncRNA-AWPPH in the serum of the 56 healthy individuals and the 86
patients with colorectal adenocarcinoma was also detected by
RT-qPCR. Serum levels of lncRNA-AWPPH were significantly higher in
patients with colorectal adenocarcinoma compared with healthy
controls (P<0.05; Fig. 2).
Serum lncRNA-AWPPH has a diagnostic
and prognostic value for colorectal adenocarcinoma
ROC curve analysis was performed to evaluate the
diagnostic value of serum lncRNA-AWPPH for colorectal
adenocarcinoma. The area under the curve was 0.9065, with a 95%
confidence interval of 0.8590–0.9539 (P<0.0001; Fig. 3A). The 86 patients with colorectal
adenocarcinoma were divided into a high expression group (n=43) and
a low expression group (n=43) according to the median serum level
of lncRNA-AWPPH. The Kaplan-Meier method was used to plot survival
curves for both groups, and the curves were compared by log rank
test. Results revealed that the overall survival of patients with a
high serum level of lncRNA-AWPPH was significantly worse than that
of patients with a low serum level of lncRNA-AWPPH (P<0.001;
Fig. 3B). These data suggested that
serum lncRNA-AWPPH may serve as a promising diagnostic and
prognostic biomarker for colorectal adenocarcinoma.
Effects of lncRNA-AWPPH overexpression
and silencing on colorectal adenocarcinoma cell proliferation and
viability
In the current study, three colorectal
adenocarcinoma cell lines derived from patients with different
ethnic backgrounds, namely HT-29 (Caucasian), Hs 698.T (American
Indian) and SNU-C1 (Asian), were investigated (Fig. 4). LncRNA-AWPPH overexpression and
silencing in the cell lines were confirmed by RT-qPCR (Fig. 5). lncRNA-AWPPH overexpression and
silencing promoted and inhibited the proliferation of colorectal
adenocarcinoma cells respectively, compared with the cells
transfected with negative control constructs (Fig. 4A). In addition, lncRNA-AWPPH
overexpression and silencing increased and decreased the viability
of colorectal adenocarcinoma cells respectively, compared with
their respective control groups.
Effects of lncRNA-AWPPH overexpression
and silencing on TGF-β1 expression
TGF-β1 has been shown to promote the growth of
colorectal adenocarcinoma (12).
Therefore the effects of AWPPH overexpression and silencing on
TGF-β1 expression were investigated in the current study. AWPPH
overexpression promoted (Fig. 5A)
and silencing inhibited (Fig. 5B)
TGF-β1 expression, suggesting that AWPPH may upregulate TGF-β1
expression in colorectal adenocarcinoma.
Discussion
Several factors are involved in the growth,
development and progression of colorectal adenocarcinoma (13). lncRNAs have been implicated in the
pathogenesis of colorectal adenocarcinoma (14), however, their exact roles have not
been well studied. Colon cancer associated transcript 1 was found
to be specifically expressed in colorectal adenocarcinoma tissues
but not in normal tissue, and promoted the progression of tumor
through multiple pathways (15).
LncRNA-AWPPH is a recently discovered lncRNA that is upregulated
during the development of hepatocellular carcinoma (9) and bladder cancer (10). The involvement of lncRNA-AWPPH in
other human diseases as well as in normal physiological processes
is unknown. In the present study, lncRNA-AWPPH was found to be
significantly upregulated in colorectal adenocarcinoma compared
with adjacent healthy tissues in the majority of patients with
colorectal adenocarcinoma. In addition, the expression level of
lncRNA-AWPPH in serum was significantly increased in patients with
colorectal adenocarcinoma compared with healthy controls. These
data suggested that upregulation of lncRNA-AWPPH is likely to be
involved in the pathogenesis of colorectal adenocarcinoma.
The prognosis of patients with colorectal
adenocarcinoma is poor (3) and early
diagnosis and treatment is critical. The development of human
diseases may be accompanied by changes in blood composition, and
the detection of specific substances may be of diagnostic value
(16,17). In the current study, ROC curve
analysis revealed that serum lncRNA-AWPPH can be used to
sensitively and effectively distinguish patients with colorectal
adenocarcinoma from normal controls. In addition, higher serum
level of lncRNA-AWPPH predicted shorter survival time. Taken
together, these data suggested that serum lncRNA-AWPPH may serve as
a promising diagnostic and prognostic biomarker for colorectal
adenocarcinoma. lncRNA-AWPPH expression may be affected by multiple
malignancies (9,10), which may affect the specificity of
serum lncRNA-AWPPH as a diagnostic and prognostic marker of
colorectal adenocarcinoma. Therefore, multiple biomarkers should be
combined to increase specificity.
It is known that ethnic backgrounds affect the
pathogenesis of different types of malignancies (18–20). In
the present study, lncRNA-AWPPH was shown to promote the
proliferation and increase the viability of three colorectal
adenocarcinoma cell lines derived from patients with different
ethics backgrounds. Furthermore, it has been reported that
lncRNA-AWPPH promoted the proliferation of cells in both
hepatocellular carcinoma (9) and
bladder cancer (10).
The TGF-β1 signaling has been implicated in the
growth and metastasis of colorectal adenocarcinoma (12,21,22). In
the current study, lncRNA-AWPPH overexpression and silencing
significantly promoted and inhibited the expression of TGF-β1
protein in all three colorectal adenocarcinoma cell lines
respectively. Cancer cell stemness is critical for cancer
progression (23) and the TGF-β
signaling serves and important role in the maintenance of cancer
cell stemness (24). Therefore,
lncRNA-AWPPH may interact with the TGF-β signaling to participate
in the biological behaviors of cancer stem cells in colorectal
adenocarcinoma.
In conclusion, AWPPH expression was significantly
upregulated in tumor tissues compared with adjacent healthy tissues
in the majority of patients with colorectal adenocarcinoma in the
current study. AWPPH expression level in blood was increased in
patients with colorectal adenocarcinoma compared with healthy
controls. Blood AWPPH may therefore serve as a promising diagnostic
and prognostic biomarker for colorectal adenocarcinoma. AWPPH
increased cancer cell proliferation and viability as well as TGF-β1
expression in colorectal adenocarcinoma in the present study.
Therefore, lncRNA-AWPPH can promote the growth of colorectal
adenocarcinoma by promoting tumor growth, increasing tumor cell
viability and activating the TGF-β1 signaling.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and CY conceived and designed the study. CL and
BH performed experiments and analyzed the data. JX interpreted the
data. CY drafted the manuscript and all authors approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Qingdao Central Hospital (Qingdao, China) and informed consent was
obtained.
Patient consent for publication
All patients signed informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng JH, Liang L, He RQ, Tang RX, Cai XY,
Chen JQ, Luo DZ and Chen G: Comprehensive investigation of a novel
differentially expressed lncRNA expression profile signature to
assess the survival of patients with colorectal adenocarcinoma.
Oncotarget. 8:16811–16828. 2017.PubMed/NCBI
|
|
3
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wakefield LM and Roberts AB: TGF-beta
signaling: Positive and negative effects on tumorigenesis. Curr
Opin Genet Dev. 12:22–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Principe DR, Doll JA, Bauer J, Jung B,
Munshi HG, Bartholin L, Pasche B, Lee C and Grippo PJ: TGF-β:
duality of function between tumor prevention and carcinogenesis. J
Natl Cancer Inst. 106:djt3692014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet 15 Spec No. 1:R17–R29. 2006. View Article : Google Scholar
|
|
7
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C T method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen W, Liu G, Jin K and Hu X: TGF-β1
induces PGP9. 5 expression in CAFs to promote the growth of
colorectal cancer cells. Oncol Rep. 37:115–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Williams NS, Gaynor RB, Scoggin S, Verma
U, Gokaslan T, Simmang C, Fleming J, Tavana D, Frenkel E and
Becerra C: Identification and validation of genes involved in the
pathogenesis of colorectal cancer using cDNA microarrays and RNA
interference. Clin Cancer Res. 9:931–946. 2003.PubMed/NCBI
|
|
14
|
Thorenoor N, Faltejskova-Vychytilova P,
Hombach S, Mlcochova J, Kretz M, Svoboda M and Slaby O: Long
non-coding RNA ZFAS1 interacts with CDK1 and is involved in
p53-dependent cell cycle control and apoptosis in colorectal
cancer. Oncotarget. 7:622–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kam Y, Rubinstein A, Naik S, Djavsarov I,
Halle D, Ariel I, Gure AO, Stojadinovic A, Pan H, Tsivin V, et al:
Detection of a long non-coding RNA (CCAT1) in living cells and
human adenocarcinoma of colon tissues using FIT-PNA molecular
beacons. Cancer Lett. 352:90–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'brien PJ, Slaughter MR, Polley SR and
Kramer K: Advantages of glutamate dehydrogenase as a blood
biomarker of acute hepatic injury in rats. Lab Anim. 36:313–321.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aberg KA, McClay JL, Nerella S, Clark S,
Kumar G, Chen W, Khachane AN, Xie L, Hudson A, Gao G, et al:
Methylome-wide association study of schizophrenia: Identifying
blood biomarker signatures of environmental insults. JAMA
Psychiatry. 71:255–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Warner ET, Tamimi RM, Hughes ME, Ottesen
RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer
EP, et al: Racial and ethnic differences in breast cancer survival:
Mediating effect of tumor characteristics and sociodemographic and
treatment factors. J Clin Oncol. 33:2254–2261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SL, Tiirikainen MI, Patel YM, Wilkens
LR, Stram DO, Le Marchand L and Murphy SE: Genetic determinants of
CYP2A6 activity across racial/ethnic groups with different risks of
lung cancer and effect on their smoking intensity. Carcinogenesis.
37:269–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen A, Lin W, Chen Y, Liu L, Chen H,
Zhuang Q, Lin J, Sferra TJ and Peng J: Pien Tze Huang inhibits
metastasis of human colorectal carcinoma cells via modulation of
TGF-β1/ZEB/miR-200 signaling network. Int J Oncol. 46:685–690.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YH, Kim G, Kwon CI, Kim JW, Park PW
and Hahm KB: TWIST1 and SNAI1 as markers of poor prognosis in human
colorectal cancer are associated with the expression of ALDH1 and
TGF-β1. Oncol Rep. 31:1380–1388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aponte PM and Caicedo A: Stemness in
cancer: Stem cells, cancer stem cells, and their microenvironment.
Stem Cells Int. 2017:56194722017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malfettone A, Soukupova J, Bertran E,
Crosas-Molist E, Lastra R, Fernando J, Koudelkova P, Rani B, Fabra
Á, Serrano T, et al: Transforming growth factor-β-induced
plasticity causes a migratory stemness phenotype in hepatocellular
carcinoma. Cancer Lett. 392:39–50. 2017. View Article : Google Scholar : PubMed/NCBI
|