Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common malignant tumor worldwide and one of the most common causes
of cancer-associated mortality worldwide (1,2).
Ultrasonography, computed tomography examination and serum
α-fetoprotein (AFP) levels are well-characterized approaches for
the diagnosis of early HCC (3,4);
however, prognosis of patients with HCC remains poor (5). Surgical resection is the preferred
treatment for HCC (6). However,
postoperative recurrence is the main cause of early mortality in
patients with HCC following radical resection (7,8). Poon
et al (9) reported that the
prognosis of patients with HCC and early recurrence (ER; <1 year
after surgical resection) is worse than that of patients with late
recurrence (LR; >1 year), indicating that the shorter the
interval time prior to recurrence, the worse the prognosis.
Portolani et al (10)
reported that the disease-free survival rate within 1 year
following surgery is only 68.0%. As a result, the prediction of
postoperative ER is particularly important, and therefore it was
the main aim of the present study.
Ultrasound is considered the first choice for
screening HCC due to its noninvasive, convenient and inexpensive
imaging characteristics (11).
Ultrasound can evaluate the size, number, position and boundary of
tumor nodules, as well as monitoring hepatic hemodynamic changes.
Cirrhosis is a serious liver disease, and advanced cirrhosis can
easily develop into HCC (12).
Doppler ultrasound has been used to detect hemodynamic changes that
occur during the progression and occurrence of hepatic fibrogenesis
associated with cirrhosis (13).
Previous research has demonstrated that Doppler ultrasound may be
reliably used to assess hemodynamic changes in different stages of
liver fibrosis (14). Furthermore, a
study conducted by Suk et al (15) demonstrated that changes in hepatic
hemodynamics may serve as a predictor for the clinical stages of
HCC. Yang et al (16)
proposed that the occurrence of HCC is associated with abnormal
liver hemodynamics. Combined measurement of AFP levels and Doppler
ultrasound has a greater diagnostic accuracy for HCC compared with
ultrasonography alone. It has also been recognized that the
diagnostic role of AFP together with abdominal ultrasound may
effectively predict the risk of HCC development (17). However, to the best of our knowledge,
the potential diagnostic value of preoperative Doppler ultrasound
for ER of AFP-positive HCC has not been fully investigated thus
far.
In the present study, the predicted value of hepatic
hemodynamics for the ER of AFP-positive HCC was retrospectively
assessed using Doppler ultrasound. Doppler ultrasound parameters
were specifically evaluated in AFP-positive HCC and control groups.
Furthermore, the present study investigated whether the hepatic
artery and portal vein blood flow parameters were associated with
postoperative ER of AFP-positive HCC.
Materials and methods
Clinical data
The present study was performed with approval from
the Ethics Committee of the Guangxi Medical University Affiliated
Tumor Hospital and written informed consent was obtained from all
participants. All the 150 patients with HCC received surgical
treatment and were diagnosed by pathologists for the first time in
the Guangxi Medical University Affiliated Tumor Hospital between
September 2015 and December 2016. In total, the 137 AFP-positive
patients with HCC (threshold, 400 ng/ml) were followed-up for 1
year following surgical treatment. The patients included 115 males
and 22 females with a mean age of 47.82±11.43 years (range, 20–71
years). A total of 13 patients with AFP-positive HCC were lost to
follow-up. Subsequently, the patients were divided into different
groups according to their ER status, an ER group and an ER free
group. The 35 healthy controls were enrolled from the outpatients
department of the Guangxi Medical University Affiliated Tumor
Hospital between September 2015 and December 2016 and consented to
an ultrasound examination of their liver. The healthy controls
included 20 males and 15 females, with a mean age of 53.26±8.75
years (range, 35–67 years).
Ultrasound examination
A standard ultrasonographic study was performed
utilizing standard equipment (GE Logiq9 color Doppler; GE
Healthcare Life Sciences) and a transducer ultrasound probe (2.5 or
3.5 MHz with low acoustic power). The enrolled subjects did not use
drugs that affect hemodynamics (including nonselective β-blockers
and vasopressin analogues) 5 days prior to the examination,
Subjects were fasted for 6–8 h and allowed to rest for 30 min prior
to the examination. Two-dimensional ultrasound images were used to
evaluate the size, location, boundary, internal echo and blood flow
of the liver-occupying lesions. The blood flow parameters of the
hepatic artery and portal vein were measured by color Doppler
ultrasound. All of the subjects were laid flat and the ultrasonic
probe was placed intercostally. The hepatic blood flow of the
portal and hepatic arteries was detected between the neck of the
gallbladder and the inferior vena cava. The following Doppler
ultrasound parameters were measured: i) Portal vein blood flow of
the mean flow velocity; ii) portal venous blood flow volume; iii)
portal vein diameter; iv) hepatic artery pulsation index (PI); and
v) hepatic artery resistance index (RI). To ensure reliability, all
parameters were measured ≥3 times simultaneously and the mean value
was calculated. When patients with HCC were followed up for 3
months following the operation, results were similar to the
pre-operative exam. The tests were performed by two experienced
ultrasound physicians, and conflicting opinions were discussed and
resolved by a third physician.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.). The results are presented as
the mean ± standard deviation. An independent Student's t-test was
used for comparisons between two groups. A paired Student's t-test
was utilized to compare hepatic hemodynamics changes in the
preoperative and postoperative stages in the same patient. The
Chi-squared test was used to analyze the qualitative data including
sex, tumor number, tumor thrombi, liver cirrhosis and hepatitis.
Receiver operating characteristic (ROC) curves were generated to
evaluate the diagnostic accuracy of Doppler ultrasonography for ER
in patients with HCC. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
A total of 150 patients (including 128 males and 22
females) with AFP-positive HCC and 35 healthy controls were
recruited at the Affiliated Tumor Hospital of Guangxi Medical
University (Nanning, China) between September 2015 and December
2016 (Table I). In total, 13
patients were loss to follow-up and 137 patients with AFP-positive
HCC (mean age, 48.360±11.482 years) completed the follow-up. Among
these patients, 41 cases were diagnosed with postoperative ER (ER
group) and 96 cases were free of postoperative ER (ER-free group).
The patients were classified into a liver cirrhosis group (n=61)
and a non-liver cirrhosis group (n=76), according to their
cirrhosis status. In the healthy control group, 20 males and 15
females were included. The characteristics of the patients are
summarized in Table II.
 | Table I.Baseline pre-operative clinical
characteristics of the enrolled patients. |
Table I.
Baseline pre-operative clinical
characteristics of the enrolled patients.
| Variables | n |
|---|
| Sex |
|
|
Male | 128 |
|
Female | 22 |
| Age, years |
|
| Mean ±
SD | 48.36±11.48 |
| Tumor number |
|
|
Multiple | 47 |
|
Single | 102 |
| Tumor thrombi |
|
|
Yes | 86 |
| No | 64 |
| Tumor diameter
(cm) |
|
| Mean ±
SD | 7.58±3.92 |
| Liver
cirrhosis |
|
|
Yes | 68 |
| No | 82 |
| Hepatitis |
|
|
Yes | 143 |
| No | 7 |
| Early
recurrence |
|
|
Yes | 41 |
| No | 96 |
 | Table II.Clinical characteristics of ER and
ER-free patients. |
Table II.
Clinical characteristics of ER and
ER-free patients.
| Variables | ER group, n | ER-free group,
n | P-value |
|---|
| Sex |
|
|
|
|
Male | 37 | 78 | 0.216 |
|
Female | 4 | 18 |
|
| Age, years |
|
|
|
| Mean ±
SD | 46.34±11.410 | 48.45±11.439 | 0.325 |
| Tumor number |
|
|
|
|
Multiple | 15 | 28 | 0.419 |
|
Single | 25 | 68 |
|
| Tumor thrombi |
|
|
|
|
Yes | 28 | 51 | 0.131 |
| No | 13 | 45 |
|
| Tumor diameter
(cm) |
|
|
|
| Mean ±
SD | 9.25±3.621 | 6.82±3.628 |
<0.05a |
| Liver
cirrhosis |
|
|
|
|
Yes | 22 | 39 | 0.190 |
| No | 19 | 57 |
|
| Hepatitis |
|
|
|
|
Yes | 41 | 90 | 0.178 |
| No | 0 | 6 |
|
Change in hepatic hemodynamics between
preoperative patients with HCC and healthy controls
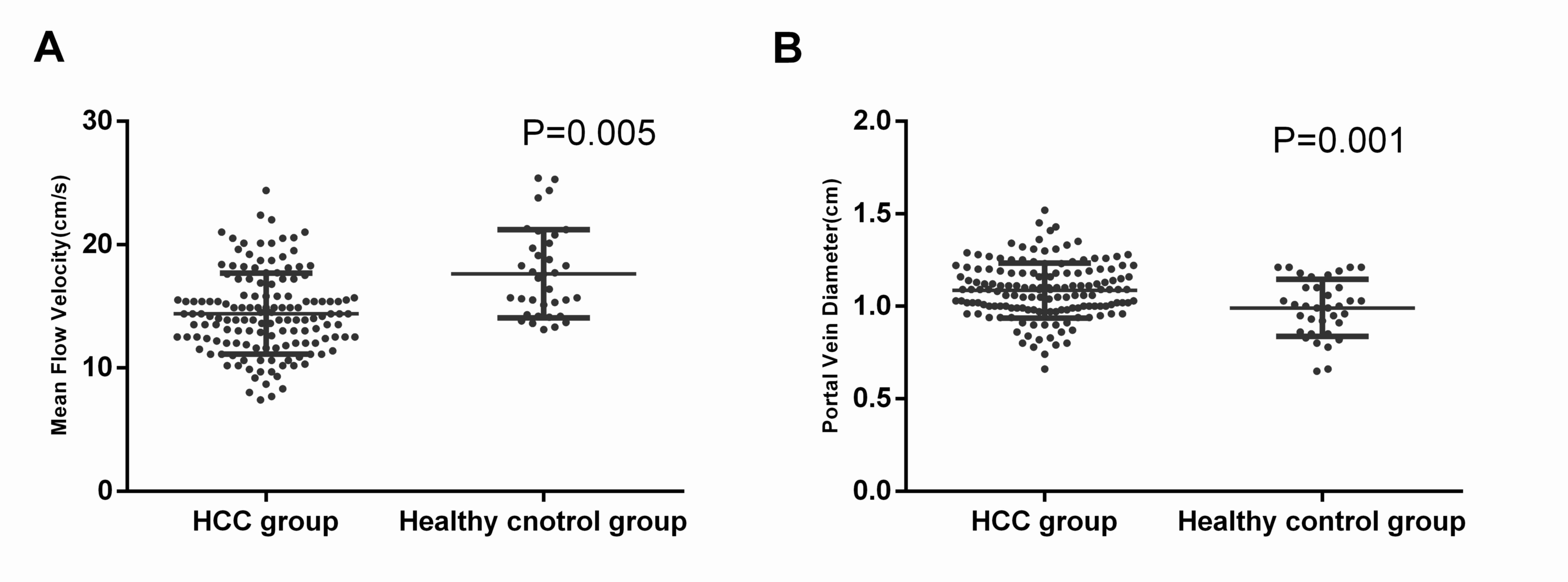
The change in hepatic hemodynamics between patients
with HCC and the control group was evaluated (Fig. 1). The mean flow velocity
(14.686±5.873 cm/sec) in patients with HCC was significantly lower
compared with that observed in healthy subjects (17.631±3.569
cm/sec; P=0.005; Fig. 2A).
Additionally, the results revealed that patients with HCC presented
with significantly higher portal vein diameters (1.085±0.149 cm)
compared with the healthy control group (0.991±0.155 cm; P=0.001;
Fig. 2B). There was no statistical
significance difference in the other parameters, including portal
venous flow velocity, portal venous volume, hepatic artery RI and
PI, between the preoperative HCC group and the healthy control
group (Table III).
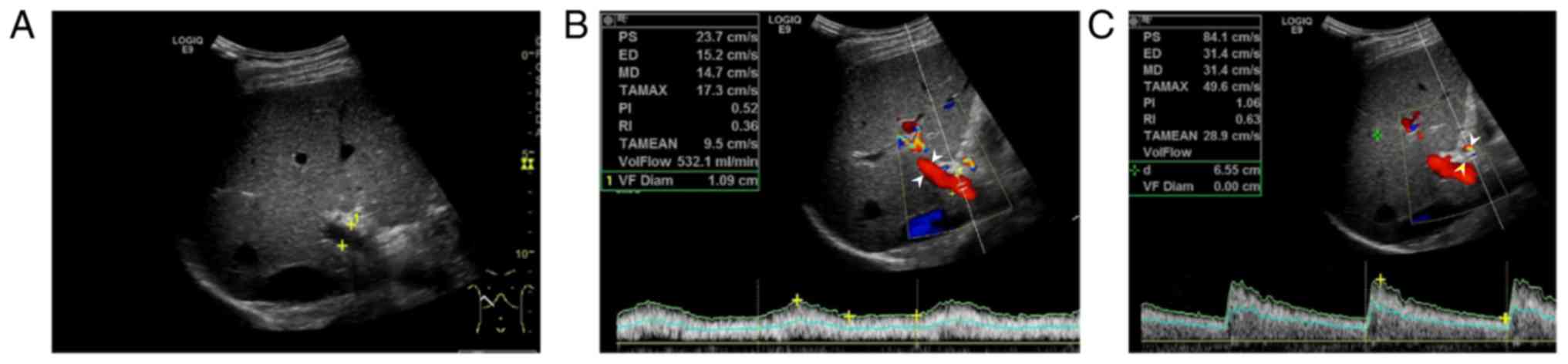 | Figure 1.Results from a 50-year-old healthy
control. (A) Two-dimensional ultrasound image. (B) Color Doppler
blood flow diagram of the portal vein (D, 1.09 cm; V, 14.7 cm/sec;
Q, 532.1 ml/min). (C) Color Doppler blood flow diagram of the
hepatic artery (white arrowheads; PI, 1.06; RI, 0.63). PS, peak
systolic velocity; ED, end-diastolic velocity; VF Diam, portal vein
diameter; MD, average flow velocity; VolFlow, portal venous blood
flow volume; TAMEAN, time averaged velocity; TAMAX, mean time
maximum velocity. |
 | Table III.Blood flow parameters of the portal
vein and hepatic artery in health controls and patients with
HCC. |
Table III.
Blood flow parameters of the portal
vein and hepatic artery in health controls and patients with
HCC.
|
| Healthy control
group | HCC group |
|
|---|
|
|
|
|
|
|---|
| Variable | n | Mean | SD | n | Mean | SD | P-value |
|---|
| Hepatic artery |
|
|
|
|
|
|
|
| RI | 35 | 0.663 | 0.048 | 86 | 0.654 | 0.077 | 0.524 |
| PI | 35 | 1.185 | 0.217 | 86 | 1.1116 | 0.306 | 0.442 |
| Portal vein |
|
|
|
|
|
|
|
| V,
cm/s | 35 | 17.631 | 3.569 | 146 | 14.686 | 5.873 | 0.005a |
| Q,
ml/min | 35 | 466.349 | 165.623 | 150 | 535.635 | 229.640 | 0.094 |
| D,
cm | 35 | 0.991 | 0.155 | 150 | 1.085 | 0.149 | 0.001a |
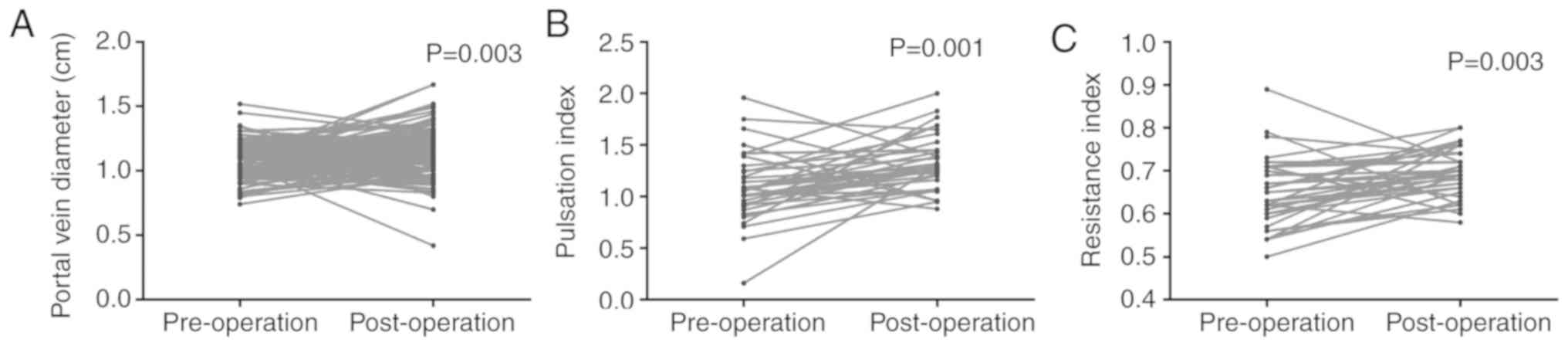
Effect of surgery on the hemodynamics
of patients with HCC
The hemodynamic changes prior to and following the
surgical procedure in patients with HCC were compared using paired
Student's t-test. The portal vein diameter significantly increased
upon operation (pre-operative, 1.07±0.14 cm vs. post-operative,
1.14±0.19 cm; P=0.003; Fig. 3A). The
effect of surgery on the hepatic artery was evaluated. The results
revealed that the PI presented the same trend in the post-operative
group (pre-operative group, 1.095±36.331 cm; post-operative group,
1.330±0.260 cm; P=0.001; Fig. 3B).
Additionally, the RI in the post-operative group (0.690±0.060) was
significantly higher compared with that observed in the
pre-operative group (0.650±0.080; P=0.003; Table IV; Fig.
3C).
 | Table IV.Changes of the blood flow parameters
of the portal vein pre- and post-surgery in patients with
hepatocellular carcinoma. |
Table IV.
Changes of the blood flow parameters
of the portal vein pre- and post-surgery in patients with
hepatocellular carcinoma.
|
| Pre-operative | Post-operative |
|
|---|
|
|
|
|
|
|---|
| Variable | n | Mean | SD | n | Mean | SD | P-value |
|---|
| Portal vein |
|
|
|
|
|
|
|
| V,
cm/s | 107 | 14.680 | 6.540 | 107 | 13.988 | 3.748862749 | 0.287 |
| Q,
ml/min | 107 | 526.190 | 245.810 | 107 | 531.600 | 230.780 | 0.868 |
| D,
cm | 107 | 1.070 | 0.140 | 107 | 1.140 | 0.190 | 0.003a |
| Hepatic artery |
|
|
|
|
|
|
|
| PI | 36 | 1.100 | 0.330 | 36 | 1.330 | 0.260 | 0.001a |
| RI | 36 | 0.650 | 0.080 | 36 | 0.690 | 0.060 | 0.003a |
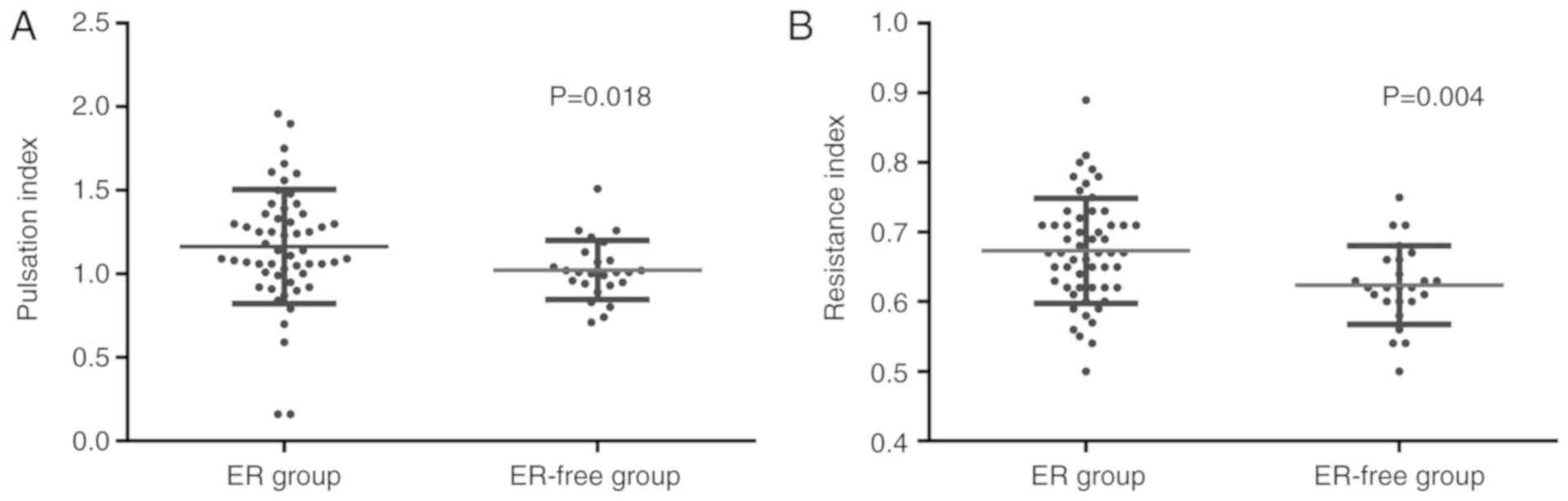
Comparison of hemodynamic changes
between patients with HCC and postoperative ER and ER-free patients
with HCC
The hemodynamic changes between the ER group
(Fig. 4) and the ER-free group
(Fig. 5) were compared. Notably, the
postoperative ER group exhibited a significantly increased hepatic
artery PI (0.673±0.075 vs. 0.624±0.056; P=0.018; Fig. 6A; Table
V) compared with the ER-free group. Furthermore, a
statistically significant difference in RI was observed between the
ER and ER-free groups, indicating that the RI in the ER group
(1.163±0.342) was significantly lower than that of the ER-free
group (1.023±0.176; P=0.004; Fig.
6B, Table V). For the other
parameters measured, including average flow velocity, portal venous
blood flow volume and portal vein diameter, no statistically
significant differences were observed between the ER group and the
ER-free group (Table V).
 | Figure 4.Results from a 45-year-old patient
with hepatocellular carcinoma, positive α-fetoprotein, liver
cirrhosis, Child-Pugh A, tumor pathological stage of intermediate
differentiation, Barcelona clinic liver cancer stage B and
postoperative early recurrence. (A) Conventional ultrasound image
revealing a 14 cm-diameter lesion tumor (white arrowhead). (B)
Color Doppler blood flow diagram of the portal vein (white
arrowheads; D, 1.17 cm; V, 12.0 cm/sec; Q, 516.5 ml/min). (C) Color
Doppler blood flow diagram of the hepatic artery (white arrowheads;
PI, 1.18; RI, 0.68). PS, peak systolic velocity; ED, end-diastolic
velocity; MD, average flow velocity; VolFlow, portal venous blood
flow volume; VF Diam, portal vein diameter; PI, pulsation index;
RI, resistance index; TAMEAN, time averaged velocity; TAMAX, mean
time maximum velocity. |
 | Figure 5.Results from a 65-year-old patient
with hepatocellular carcinoma, positive α-fetoprotein, no liver
cirrhosis, Child-Pugh A, tumor pathological stage of intermediate
differentiation, tumor emboli, Barcelona clinic liver cancer stage
C and no early recurrence following surgery. (A) Conventional
ultrasound image revealing no early recurrence following surgery.
(B) Color Doppler blood flow diagram of the portal vein (white
arrowheads; D, 1.24 cm; V, 19.6 cm/sec; Q, 788.6 ml/min). (C) Color
Doppler blood flow diagram of the hepatic artery (white arrowheads;
PI, 1.30; RI, 0.71). PS, peak Systolic velocity; ED, end-diastolic
velocity; MD, average flow velocity; Vloflow, portal venous blood
flow volume; VF Diam, portal vein diameter; PI, pulsation index;
RI, resistance index, TAMEAN, time averaged velocity; TAMAX, mean
time maximum velocity. |
 | Table V.Blood flow parameters of the portal
vein and hepatic artery in the ER-free group and ER group of
patients with hepatocellular carcinoma. |
Table V.
Blood flow parameters of the portal
vein and hepatic artery in the ER-free group and ER group of
patients with hepatocellular carcinoma.
|
| ER-free group | ER group |
|
|---|
|
|
|
|
|
|---|
| Variables | n | Mean | SD | n | Mean | SD | P-value |
|---|
| Hepatic artery |
|
|
|
|
|
|
|
| RI | 25 | 1.023 | 0.176 | 55 | 1.163 | 0.342 | 0.004a |
| PI | 25 | 0.624 | 0.056 | 55 | 0.673 | 0.075 | 0.018a |
| Portal vein |
|
|
|
|
|
|
|
| V,
cm/s | 96 | 14.725 | 6.827 | 37 | 14.284 | 3.261 | 0.687 |
| Q,
ml/min | 96 | 520.685 | 244.144 | 41 | 538.344 | 210.213 | 0.708 |
| D,
cm | 96 | 1.067 | 0.15 | 41 | 1.1 | 0.14 | 0.233 |
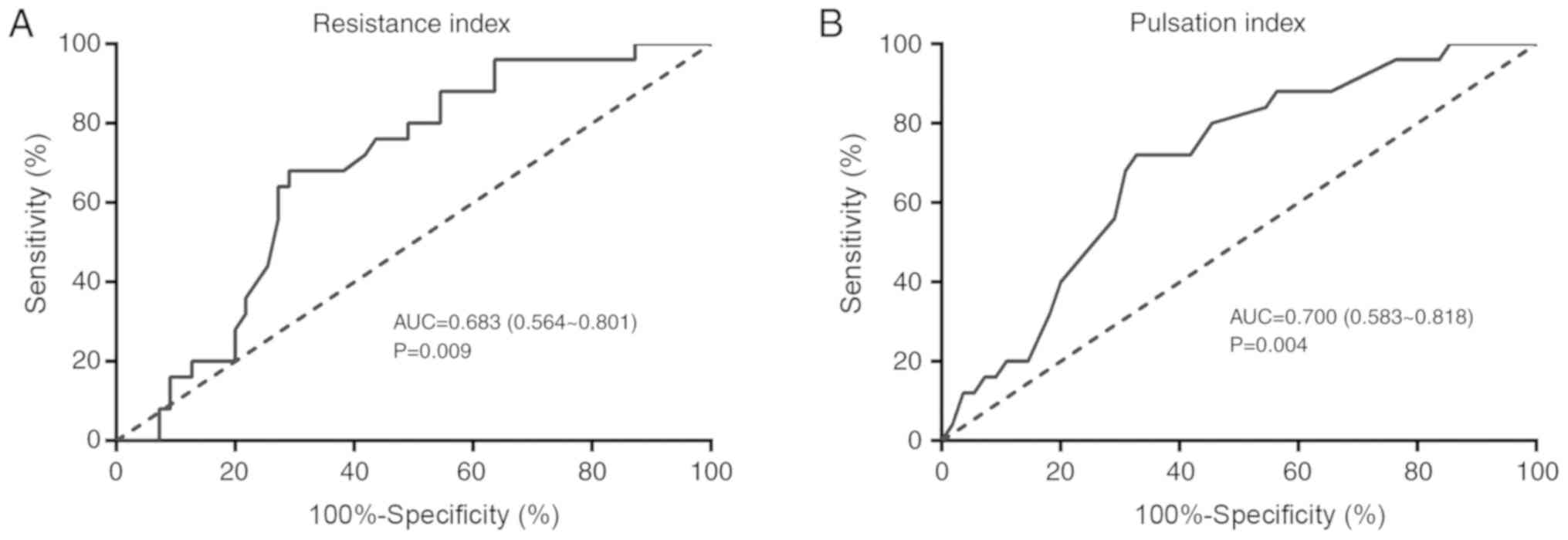
Diagnostic accuracy of Doppler
ultrasonography for the diagnosis of ER in HCC
ROC curve analysis was performed to assess the
diagnostic accuracy of Doppler ultrasonography for the diagnosis of
ER in patients with HCC. The area under the ROC curve (AUC) of
preoperative RI value was 0.683 (range, 0.564–0.801; P=0.009). An
AUC value for RI of 0.645 corresponded with the highest diagnostic
value for ER with a sensitivity of 68% and a specificity of 70.91%
(Fig. 7A). As for the preoperative
PI, an AUC of 0.700 suggested a high diagnostic accuracy of
preoperative PI for the ER of patients with HCC (range,
0.583–0.818; P=0.004). The best cut-off value of pre-operative PI
was 1.045, with a sensitivity of 72.00% and a specificity of 67.27%
(Table VI; Fig 7B).
 | Table VI.Receiver operating characteristic
curve analysis of PI and RI for the diagnosis of ER in patients
with hepatocellular carcinoma. |
Table VI.
Receiver operating characteristic
curve analysis of PI and RI for the diagnosis of ER in patients
with hepatocellular carcinoma.
| Variables | Area under the
curve (range) | P-value | Cut-off | Sensitivity, % | Specificity, % |
|---|
| RI | 0.683
(0.564–0.801) | 0.009 | <0.6450 | 68.00 | 70.91 |
| PI | 0.700
(0.583–0.818) | 0.004 | <1.0450 | 72.00 | 67.27 |
Discussion
Surgical resection is the first choice for the
treatment of HCC. However, recurrence subsequent to resection is
one of the main factors affecting the prognosis of HCC (18). It has become evident that
post-operative recurrence of HCC can be classified into ER and LR
(8,9). In particular, ER presents challenges
for the treatment of liver cancer. Colecchia et al (19) demonstrated that ER may originate from
the liver metastasis of primary tumors, which is mainly associated
with biological factors of the tumor, including tumor size, number
of lesions and vascular invasion. LR is caused by the residual
liver state, and is affected by the history of HCC prior to
treatment and the presence of hepatitis and cirrhosis (20). A reliable and effective prediction
method to assess the risk of HCC recurrence may shed light on
future treatment strategies.
The examination of blood vessels morphology and
hemodynamics may directly or indirectly reflect the inherent
biological characteristics of tumors (21). As a noninvasive imaging modality,
ultrasonography is widely used in routine clinical practice for
diagnosis, treatment planning or monitoring of cancer, particularly
for detecting changes in hemodynamics of the portal vein and
hepatic artery (22–25). Therefore, it was speculated that the
hemodynamics of ultrasound detection may be a valuable predictor
for the ER of HCC following resection. It has been demonstrated
that the combination of ultrasonography and measurement of AFP
levels confers the efficient surveillance strategy for early HCC
detection (26). Previous studies
have demonstrated that the factors affecting the recurrence of HCC
include incomplete or absent tumor capsule, preoperative AFP levels
>400 ng/l, lesion size and positive pathological margin
(25,27–30).
Clinically, the size and capsule of HCC tumors may be assessed by
various imaging methods prior to surgery, while the AFP levels can
be detected through laboratory examination. However, pathological
grading of tumors and microvascular invasion cannot be obtained
prior to pathological examination. Therefore, the prediction of ER
of HCC requires future investigation to improve patient prognosis
(3). The present study focused on
patients with AFP-positive HCC to investigate the diagnostic value
of ultrasonography for the recurrence of HCC (31).
Comparison of the hemodynamics between patients with
HCC and healthy controls revealed that the pre-operative portal
venous blood flow volume and portal vein diameter in patients with
HCC were significantly increased compared with the control group,
while the preoperative hepatic artery PI in patients with HCC was
significantly decreased compared with the control group. The
present study demonstrated that monitoring of the hepatic
hemodynamics is important for the early diagnosis of HCC
recurrence. To the best of our knowledge, no studies on this topic
have been conducted thus far. The results obtained in the present
study revealed that pre-operative hepatic artery RI and PI in the
ER group were significantly lower compared with in the ER-free
group. In addition, the ROC curve revealed that pre-operative PI
<1.05 and RI <0.645 may be used as reference values for
predicting the occurrence of ER in postoperative HCC. Bonnin et
al (32) revealed that the
hepatic artery Doppler perfusion index was established earlier
during the diagnosis of liver cancer when compared with
conventional ultrasonography (37.6±12.7 days), and that increased
blood flow in the hepatic artery may be used to monitor the growth
of liver tumors in mice, leading to a diagnosis of liver cancer
4–20 weeks earlier.
Blood to the liver is supplied by the hepatic artery
and portal vein. Advanced liver cirrhosis is associated with portal
hypertension and portal vein widening, with the majority of cases
progressing into HCC. The histopathology of HCC revealed the
presence of neovasculature and blood pooling in the tumor. Compared
with the same diameter hepatic artery, the lower number of elastic
fibers in patients with HCC contributes to a lower hepatic artery
blood flow resistance and faster flow rate. In conclusion, the
pathological changes of HCC are accompanied by changes in the
hemodynamics of the hepatic artery and portal vein (33). Therefore, the early dynamic changes
of the hepatic artery and portal vein may be used to predict
recurrence in HCC. The application of color Doppler ultrasound for
hepatic artery and portal venous hemodynamics is particularly
useful for the early diagnosis of HCC recurrence.
HCC tumor blood is mainly supplied by the hepatic
artery, while the portal vein is involved in the peripheral part of
the liver and extends to the center with a small branch (34). When the tumor size is >5 cm, the
hepatic artery and portal vein are intertwined to form a vascular
lake around the tumor, whereas the tumor blood supply is partially
or completely derived from the portal vein when the tumor size is
<5 cm (35–37). The portal venous system has multiple
collateral circulations and may exhibit a small blood flow in HCC
with ER (37,38). Therefore, the hepatic artery affects
HCC tumor recurrence more than the portal vein, resulting in no
significant changes when the latter is monitored by current
ultrasound instrumentation. Hepatic artery PI and RI are the main
parameters to reflect the blood flow of the hepatic artery. The
hepatic artery hemodynamic changes in liver cancer were previously
evaluated by RI and PI in the literature. The PI and RI
measurements of the hepatic artery are highly reproducible, easily
measured and not affected by the changes in respiration or body
position (39). In addition, the
ultrasonic detection of PI and RI can be fully quantified.
Therefore, the PI and RI of the hepatic artery may be predictors of
ER following surgery in HCC.
The present study had several limitations. Firstly,
The PI value is affected by heart rate, hemangiectasis ability and
the total resistance of peripheral blood vessels (40). Secondly, the limits of the instrument
and the operator may lead to discrepancy in the observations.
Finally, the retrospective nature and small sample size of the
present study may lead to statistical type-II errors. Overall, the
sensitivity and specificity of ultrasonic monitoring require
improvement. In conclusion, the present study indicated that color
Doppler ultrasound detection of hepatic hemodynamics may have
significant value for the diagnosis of ER in HCC. It may
significantly improve the accuracy of predicting the occurrence of
preoperative ER, which may assist clinicians in selecting optimal
treatment strategies for patients with HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Young
Scientists Fund of the National Natural Science Foundation of China
(grant no. 81701721), the Guangxi Key Project of Science and
Technology (grant no. 2017AB48027) and the Guangxi Natural Science
Foundation (grant no. 2016XNSFRA380194).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HL designed the study and revised the manuscript. JL
participated in designing and conducting the research. MC conceived
the research and contributed to manuscript writing. DW and DL
collected and analyzed the data. YZ and HL analyzed the data and
interpreted the results.
Ethics approval and consent to
participate
The present study was performed with approval of the
Ethics Committee of the Guangxi Medical University Affiliated Tumor
Hospital and written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
AFP
|
α-fetoprotein
|
|
ER
|
early recurrence
|
|
LR
|
late recurrence
|
|
ROC
|
receiver operating characteristic
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang A, Hallouch O, Chernyak V, Kamaya A
and Sirlin CB: Epidemiology of hepatocellular carcinoma: Target
population for surveillance and diagnosis. Abdom Radiol (NY).
43:13–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ayuso C, Rimola J, Vilana R, Burrel M,
Darnell A, García-Criado Á, Bianchi L, Belmonte E, Caparroz C,
Barrufet M, et al: Diagnosis and staging of hepatocellular
carcinoma (HCC): Current guidelines. Eur J Radiol. 101:72–81. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colombo F, Baldan F, Mazzucchelli S,
Martin-Padura I, Marighetti P, Cattaneo A, Foglieni B, Spreafico M,
Guerneri S, Baccarin M, et al: Evidence of distinct
tumour-propagating cell populations with different properties in
primary human hepatocellular carcinoma. PLoS One. 6:e213692011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual report to the nation on the status of cancer, 1975–2014,
featuring survival. J Natl Cancer Inst. 109:2017. View Article : Google Scholar
|
|
6
|
He W, Peng B, Tang Y, Yang J, Zheng Y, Qiu
J, Zou R, Shen J, Li B and Yuan Y: Nomogram to predict survival of
patients with recurrence of hepatocellular carcinoma after surgery.
Clin Gastroenterol Hepatol. 16:756–764.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shu T, Zhao D, Li B, Wang Y, Liu S, Li P,
Zuo J, Bai P, Zhang R and Wu L: Prognostic evaluation of
postoperative adjuvant therapy for operable cervical cancer: 10
years' experience of National Cancer Center in China. Chin J Cancer
Res. 29:510–520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Liao J, Qi W, Xie L and Li Y:
Predictive value of conventional ultrasound and contrast-enhanced
ultrasound in early recurrence of hepatocellular carcinoma after
surgical resection. Ultrasound Med Biol. 42:1042–1048. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poon RT, Fan ST, Ng IO, Lo CM, Liu CL and
Wong J: Different risk factors and prognosis for early and late
intrahepatic recurrence after resection of hepatocellular
carcinoma. Cancer. 89:500–507. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Portolani N, Coniglio A, Ghidoni S,
Giovanelli M, Benetti A, Tiberio GA and Giulini SM: Early and late
recurrence after liver resection for hepatocellular carcinoma:
Prognostic and therapeutic implications. Ann Surg. 243:229–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Y, Zheng DY, Cui Z, Ma Y, Liu YZ and
Zhang W: Predictive value of quantitative contrast-enhanced
ultrasound in hepatocellular carcinoma recurrence after ablation.
World J Gastroenterol. 21:10418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamin PD, Bernardino ME and Green B:
Ultrasound manifestations of hepatocellular Carcinoma. Radiology.
131:459–461. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Freitas TP, Gomes M, Fraga DB, Freitas LS,
Rezin GT, Santos PM, Silveira PC, Paula MM, Pinho RA and Streck EL:
Effect of therapeutic pulsed ultrasound on lipoperoxidation and
fibrogenesis in an animal model of wound healing. J Surg Res.
161:168–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zekanovic D, Ljubicic N, Boban M, Nikolic
M, Delic-Brkljacic D, Gacina P, Klarin I and Turcinov J: Doppler
ultrasound of hepatic and system hemodynamics in patients with
alcoholic liver cirrhosis. Dig Dis Sci. 55:458–466. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suk KT, Kim EJ, Kim DJ, Kim HS, Bang CS,
Park TY, Baik GH, Kim SE, Park JW, Park SH, et al: Prognostic
significance of hemodynamic and clinical stages in the prediction
of hepatocellular carcinoma. J Clin Gastroenterol. 51:285–293.
2017.PubMed/NCBI
|
|
16
|
Yang YL, Di L, Duan YY, Liu X, Liu J, Yang
RJ, Chen S and Yuan LJ: A prospective experimental study of liver
fibrosis with ultrasound and its correlation with hepatic reserve
function and hemodynamics. BMC Gastroenterol. 12:1682012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JD, Dai J, Singal AG, Gopal P,
Addissie BD, Nguyen MH, Befeler AS, Reddy KR, Schwartz M, Harnois
DM, et al: Improved performance of serum alpha-fetoprotein for
hepatocellular carcinoma diagnosis in HCV cirrhosis with normal
alanine transaminase. Cancer Epidemiol Biomarkers Prev.
26:1085–1092. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sangiovanni A and Colombo M: Treatment of
hepatocellular carcinoma: Beyond international guidelines. Liver
Int. 36 (Suppl 1):S124–S129. 2016. View Article : Google Scholar
|
|
19
|
Colecchia A, Schiumerini R, Cucchetti A,
Cescon M, Taddia M, Marasco G and Festi D: Prognostic factors for
hepatocellular carcinoma recurrence. World J Gastroenterol.
20:5935–5950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tong MJ, Rosinski AA, Huynh CT, Raman SS
and Lu DSK: Long-term survival after surveillance and treatment in
patients with chronic viral hepatitis and hepatocellular carcinoma.
Hepatol Commun. 1:595–608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Annet L, Materne R, Danse E, Jamart J,
Horsmans Y and Van Beers BE: Hepatic flow parameters measured with
MR imaging and Doppler US: Correlations with degree of cirrhosis
and portal hypertension. Radiology. 229:409–414. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kondo T, Maruyama H, Sekimoto T, Shimada
T, Takahashi M, Okugawa H and Yokosuka O: Impact of portal
hemodynamics on Doppler ultrasonography for predicting
decompensation and long-term outcomes in patients with cirrhosis.
Scand J Gastroenterol. 51:236–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mäkikallio K, Erkinaro T, Niemi N,
Kavasmaa T, Acharya G, Päkkilä M and Räsänen J: Fetal oxygenation
and Doppler ultrasonography of cardiovascular hemodynamics in a
chronic near-term sheep model. Am J Obstet Gynecol. 194:542–550.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maruyama H and Yokosuka O: Ultrasonography
for noninvasive assessment of portal hypertension. Gut Liver.
11:464–473. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berzigotti A, Reverter E, García-Criado Á,
Abraldes JG, Cerini F, García-Pagán JC and Bosch J: Reliability of
the estimation of total hepatic blood flow by Doppler ultrasound in
patients with cirrhotic portal hypertension. J Hepatol. 59:717–722.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biselli M, Conti F, Gramenzi A, Frigerio
M, Cucchetti A, Fatti G, D'Angelo M, Dall'Agata M, Giannini EG,
Farinati F, et al: A new approach to the use of alpha-fetoprotein
as surveillance test for hepatocellular carcinoma in patients with
cirrhosis. Br J Cancer. 112:69–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SY, Konstantinidis IT, Eaton AA, Gönen
M, Kingham TP, D'Angelica MI, Allen PJ, Fong Y, DeMatteo RP and
Jarnagin WR: Predicting recurrence patterns after resection of
hepatocellular cancer. HPB (Oxford). 16:943–953. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang P, Qiu J, Li J, Wu D, Wan X, Lau WY,
Yuan Y and Shen F: Nomograms for pre-and postoperative prediction
of long-term survival for patients who underwent hepatectomy for
multiple hepatocellular carcinomas. Ann Surg. 263:778–786. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ogihara H, Iizuka N and Hamamoto Y:
Prediction of early recurrence of liver cancer by a novel discrete
bayes decision rule for personalized medicine. Biomed Res Int.
2016:85674792016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Lai SL, Chen J, Xie D, Wu FX, Jin
GQ and Su DK: Validated preoperative computed tomography risk
estimation for postoperative hepatocellular carcinoma recurrence.
World J Gastroenterol. 23:6467–6473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehta A and Singal AG: Hepatocellular
Carcinoma surveillance: Does alpha-fetoprotein have a role.
Gastroenterology. 149:816–817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonnin P, Villemain A, Vincent F, Debbabi
H, Silvestre JS, Contreres JO, Levy BI, Tobelem G and Dupuy E:
Ultrasonic assessment of hepatic blood flow as a marker of mouse
hepatocarcinoma. Ultrasound Med Biol. 33:561–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rappaport AM: Hepatic blood flow:
Morphologic aspects and physiologic regulation. Int Rev Physiol.
21:1–63. 1980.PubMed/NCBI
|
|
34
|
Kamiyama T, Kakisaka T, Orimo T and
Wakayama K: Hepatectomy for hepatocellular carcinoma with portal
vein tumor thrombus. World J Hepatol. 9:1296–1304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong YH and Lin G: Experimental studies of
portal venous embolization with iodized oil in rats with
experimentally induced liver cancer. J Vasc Int Radiol. 4:621–624.
1993. View Article : Google Scholar
|
|
36
|
Han K, Kim JH, Ko GY, Gwon DI and Sung KB:
Treatment of hepatocellular carcinoma with portal venous tumor
thrombosis: A comprehensive review. World J Gastroenterol.
22:407–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giorgio A, Calisti G, Montesarchio L,
Scognamiglio U, Matteucci P, Coppola C, Scarano F, Amendola F and
Giorgio V: Hepatocellular carcinoma invading portal venous system
in cirrhosis: Long-term results of percutaneous radiofrequency
ablation of both the nodule and portal vein tumor thrombus. A case
control study. Anticancer Res. 34:6785–6790. 2014.PubMed/NCBI
|
|
38
|
Shimada S, Kamiyama T, Yokoo H, Orimo T,
Wakayama K, Einama T, Kakisaka T, Kamachi H and Taketomi A:
Clinicopathological characteristics of hepatocellular carcinoma
with microscopic portal venous invasion and the role of anatomical
liver resection in these cases. World J Surg. 41:2087–2094. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanaka S, Kitamura T, Fujita M, Nakanishi
K and Okuda S: Color Doppler flow imaging of liver tumors. AJR Am J
Roentgenol. 154:509–514. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kamalov IR, Sandrikov VA, Gautier SV,
Tsirulnikova OM and Skipenko OG: The significance of colour
velocity and spectral Doppler ultrasound in the differentiation of
liver tumours. Eur J Ultrasound. 7:101–108. 1998. View Article : Google Scholar : PubMed/NCBI
|