Introduction
Colorectal cancer (CRC) has high morbidity and
mortality rates worldwide, at 10.2 and 9.2%, respectively (1). Despite the therapeutic advances and
earlier detection, the 5-year survival rate of patients with CRC
remains unsatisfactory (2). One of
the main reasons for this is that the occurrence of CRC is a
complex multi-stage process, and involves further investigation
into the proliferation, differentiation, apoptosis and survival
mechanism of intestinal epithelial cells (3). Therefore, biomarkers for early
detection and targeted therapy are urgently required.
Biological rhythms are produced by conserved
transcription and translation feedback loops of circadian clock
genes within the cells (4). A
circadian disruption has been recognized as a potential independent
risk factor for cancer development (5). Circadian clock genes appear to have
multifaceted functions during cancer development and can act to
both suppress tumors and promote carcinogenesis (6). Research by the International Agency for
Research on Cancer has also demonstrated that this disruption
increases the risk of CRC (7).
Several previous studies have also demonstrated that large
variations in expression levels, both up- and downregulated, and
the circadian clock genes are associated with tumor progression and
mammalian tumorigenesis for several malignancies, such as breast
cancer (8), liver cancer (9) and colorectal carcinoma (10). In addition, the association between
single nucleotide polymorphisms (SNPs) in circadian clock genes and
disease has also been analyzed (11,12).
These studies indicate that mutations or deregulated expression of
circadian clock genes are frequently detected in different tumors.
Copy number variation (CNV) is a kind of structural variation at
the submicroscopic level, which refers to the complex chromosomal
structural variation forms derived from the deletion and/or
duplication of DNA fragments longer than 1 kb (13). Increasing research has shown that CNV
is closely associated with the risk of tumor occurrence (14,15). The
mechanism of action of CNV and circadian clock genes in many cancer
types has been intensively investigated, such as liver cancer
(16) and lung cancer (14); however, the study of the mechanism of
action of CNV-driven circadian clock genes in cancer (including
CRC) has not yet been reported.
The aryl hydrocarbon receptor nuclear
translocator-like 2 (ARNTL2) gene is also known as brain and muscle
ARNT-like 2 (BMAL2), which is mapped to human chromosome
12p11.22–11.23 and shares 52% amino acid identity with zebrafish
Bmal2 and 49% identity with human BMAL1 (17). Schoenhard et al (18) hypothesized that the different ARNTL2
spatiotemporal distributions allow intrinsic circadian clocks to
modulate the amplitudes of their oscillators while maintaining
circadian periodicity. Research on ARNTL2 in various complex
diseases (19,20), particularly cancer, has gradually
become accepted. Studies have shown that ARNTL2 is a potential
biomarker for tumor invasion in colorectal cancer (21), and it is significantly associated
with lung cancer risk (22). In
addition, a previous study has analyzed SNPs associated with ARNTL2
expression in patients with breast cancer (23).
The aim of the present study, using somatic CNV,
legacy clinical information and gene expression data from the
Cancer Genome Atlas (TCGA; http://tcga-data.nci.nih.gov/tcga/), was to
investigate the systematic association between somatic cell CNV and
circadian clock gene expression in patients with CRC, and to
identify ARNTL2 as a contributing gene in CRC development that may
serve as a promising therapeutic strategy.
Materials and methods
Data source and preprocessing
The CNV data were downloaded from TCGA data portal
on October 23, 2018. The data contained 979 files and 460 cases for
CNV analysis by setting specific parameters: Data Type was Masked
Copy Number Segment. In addition, mRNA expression profile data and
the corresponding legacy clinical information of patients with CRC
from TCGA were also downloaded and contained 480 CRC tumor
specimens and 41 tumor-adjacent tissue specimens. Firstly, 18
samples without adequate clinical information were removed, which
left 462 patients with CRC with complete survival information.
Subsequently, low-abundance mRNA expression data were removed;
mRNAs with expression value >1 in 90% samples were retained. For
the duplication data in one sample, the average values of the mRNA
expression were adopted. The 2,083 differentially expressed mRNAs
were analyzed using R/Bioconductor package edgeR (version 3.26)
(24), with the criteria of
|log2fold-change (FC)| >1.5 and q-value <0.01. No
patients were involved in clinical trials in this study.
Identification and functional analysis
of CNV-driven circadian clock genes
Gene Ontology (GO) analysis was performed to explore
the functional roles of the target genes using DAVID (http://www.david.abcc.ncifcrf.gov/) (25). Finally, the enriched GO terms with
gene count >5 and P<0.05 were selected for further analyses.
Cytoscape software (version 3.7.1) (26) (with ClueGO and CluePedia plugins) was
used for the Kyoto Encyclopedia of Genes and Genomes (KEGG)
analyses, showing only pathways with P<0.05.
Circadian clocks exist endogenously in almost every
organism (6). The Circadian Gene
Database (CGDB; version 1.0; http://cgdb.biocuckoo.org/index.php) (27) was used to identify the circadian
clock genes. Circadian genes were selected that had been identified
experimentally. A literature search using PubMed database was
performed to identify the latest candidate circadian clock
genes.
To verify the expression profile of ARNTL2 in CRC
tissues and their non-tumoral counterparts, a meta-analysis was
performed using the Oncomine database (version 4.5; www.oncomine.org) by setting specific parameters:
‘ARNTL2’, ‘Cancer vs. Normal Analysis’, ‘Colorectal Cancer’ and
‘mRNA’.
The java software Gene Set Enrichment Analysis
(GSEA; version 3.0) was employed to perform the statistical
significance test between two phenotypes (http://software.broadinstitute.org/gsea/index.jsp),
with gene expression data and phenotype data (high/low group of
expression values of ARNTL2) to be prepared according to the GSEA
guidelines (28). The parameters
were set as follows: Using KEGG pathway as a reference, permutation
type to be the phenotype, and at least 15 genes in a single
pathway. The mean expression levels (905.75) of ARNTL2 in all
cancer samples were obtained. In the GSEA analysis, the expression
level higher than this value is considered to be high expression,
and below this value is considered to be low expression.
Statistical analysis
The segment mean at (−0.2, 0.2) was generated by the
error of the instrument measurement, so the copy number of such
genes was confirmed as unchanged. A χ2 test was used to
compare the number of CNVs in cancer tissue and paracancer tissue,
with a criterion of false discovery rate (FDR) <0.01. A
Kolmogorov-Smirnov test was used to identify genes with CNV and
expression consistency, with the criterion of P<0.005. A
Kolmogorov-Smirnov test is based on cumulative distribution
functions to test whether a distribution conforms to a theoretical
distribution or whether there is a significant difference between
two empirical distributions. To assess the result set of genes, a
hypergeometric test was used to verify whether known CRC-related
genes were enriched on the set. To identify the associations
between clinicopathological parameters and the presence of copy
number loss or gain in the regions containing selected genes, a
Pearson's χ2 test was performed. A Kaplan-Meier curve
analysis was performed to analyze the association between the gene
and survival time, and statistical significance was assessed using
the R package ‘survival’ (29).
P<0.05 (two-sided) was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The detailed clinical and pathological
characteristics of the study population, including age, sex,
pathological stage, pathological tumor (pathological T),
pathological node (pathological N) and pathological metastasis
(pathologic M), were summarized in Table
I. All the 462 patients were pathologically diagnosed with
colorectal cancer. The median age for all patients was 60 years
(interquartile range, 31–90 years).
 | Table I.Clinicopathological features of the
462 patients with colorectal cancer. |
Table I.
Clinicopathological features of the
462 patients with colorectal cancer.
| Feature | Primary, n (%) | Metastatic, n
(%) | NA, n (%) |
|---|
| Age, years |
|
|
|
|
<60 | 81 (24.0) | 42 (36.5) | 4 (40.0) |
|
>60 | 256 (76.0) | 73 (63.5) | 6 (60.0) |
| Sex |
|
|
|
|
Male | 177 (52.5) | 53 (46.1) | 6 (60.0) |
|
Female | 160 (47.5) | 62 (53.9) | 4 (40.0) |
| Pathological T |
|
|
|
|
T1-T2 | 77 (22.8) | 10 (8.7) | 3 (30.0) |
|
T3-T4 | 260 (77.2) | 105 (91.3) | 7 (70.0) |
| Pathological n
stage |
|
|
|
| N0 | 231 (68.5) | 35 (30.4) | 5 (50.0) |
|
N1-N2 | 106 (31.5) | 80 (69.6) | 5 (50.0) |
| Pathological
stage |
|
|
|
|
I–II | 228 (67.7) | 23 (20.0) | 2 (20.0) |
|
III–IV | 106 (31.5) | 87 (75.7) | 5 (50.0) |
| NA | 3 (0.9) | 5 (4.3) | 3 (30.0) |
| Vital status |
|
|
|
|
Alive | 288 (85.5) | 73 (63.5) | 7 (70.0) |
|
Death | 49 (14.5) | 42 (36.5) | 3 (30.0) |
Screening of potential CRC-related
gene CNVs
To identify potential candidate genes within the
regions exhibiting CNVs in the TCGA dataset, the frequency of copy
number loss and gain in the regions was obtained. First, the
instrument measurement error was filtered, and the area where the
CNV number was significantly different located, and finally the
genes in these areas were identified. Finally, the χ2
test was conducted on CNV, and a total of 10,256 genes with
significant differences in CNV expression were obtained. KEGG and
GO enrichment analyses was then performed with a smaller set of
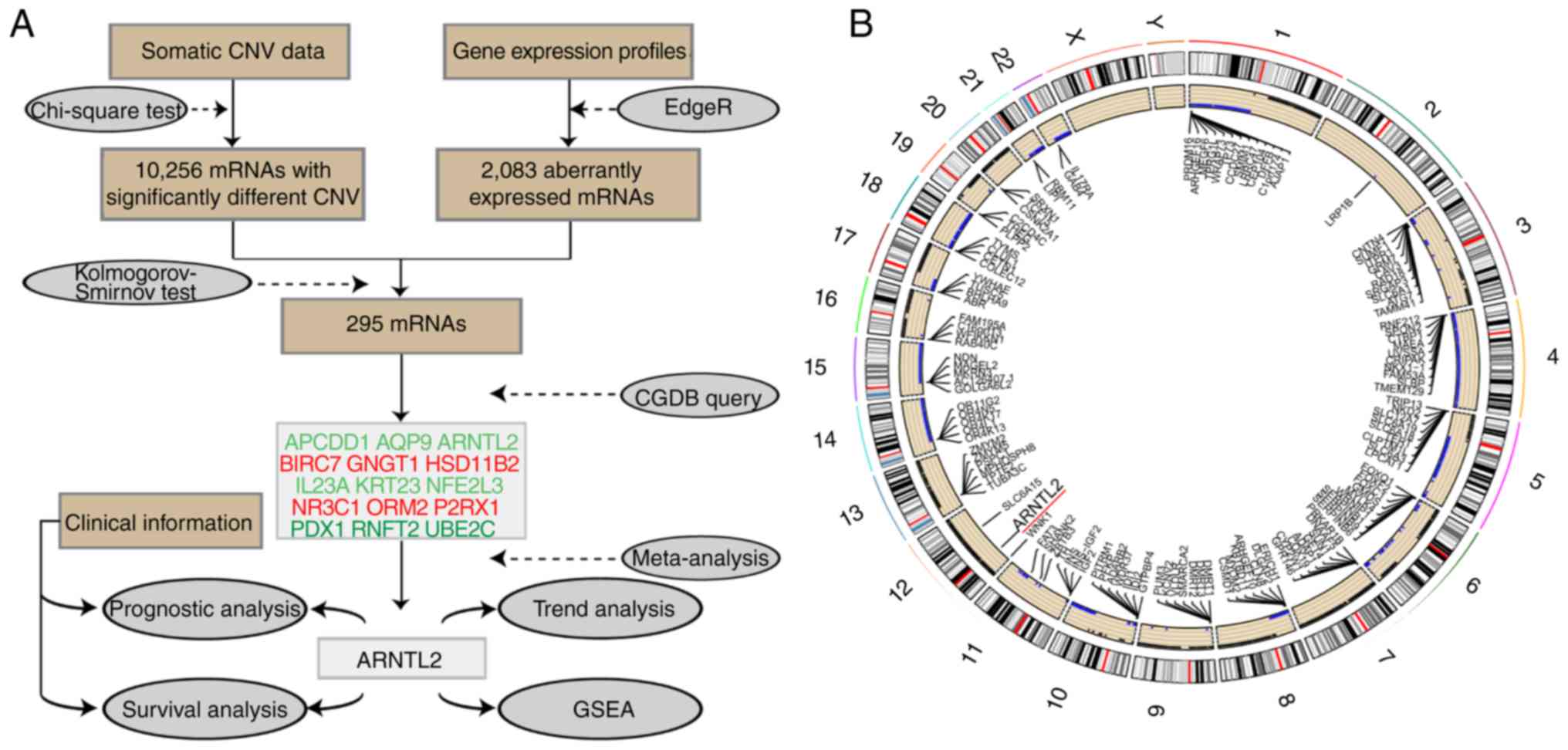
genes (n=295). A detailed workflow chart of the methodology is
illustrated in Fig. 1A. CNV occurred
differently on each chromosome in patients with CRC. Large-scale
losses of copy numbers occurred only on certain chromosomal
regions, such as chromosomes 4, 11, 14, 15, 18, 21 and 22. However,
on other chromosomes, such as chromosomes 7, 12 and 13, only gains
occurred (Fig. 1B).
Screening of differentially expressed
mRNAs
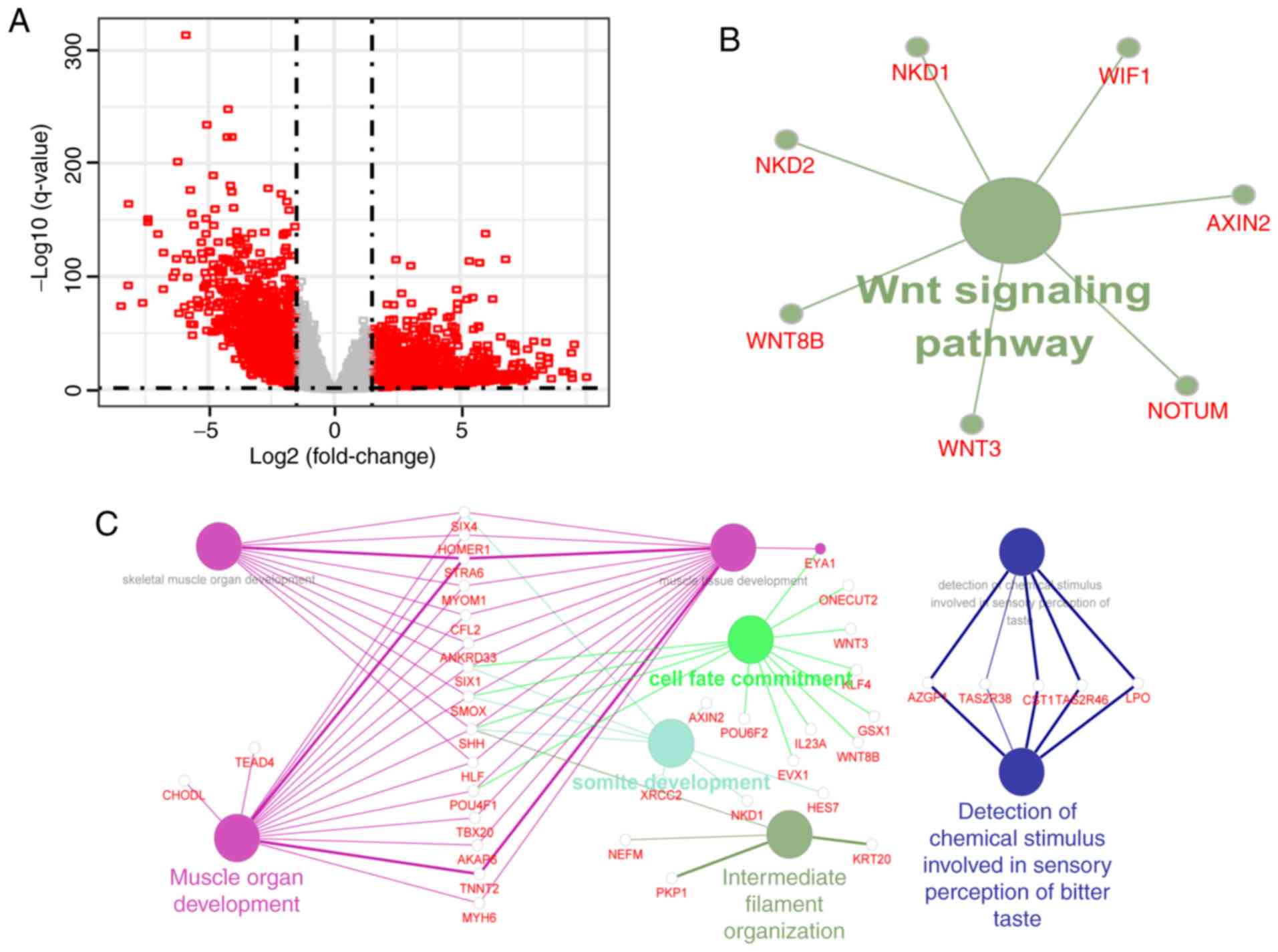
Based on the threshold criteria of
|log2FC| >1.5 and q-value <0.01, 2,083 mRNAs were
identified as aberrantly expressed mRNAs in the CRC tissues
compared with that in the adjacent non-tumorous tissues. It was
found that a number of mRNAs were upregulated or downregulated
>100-fold (Fig. 2A). To further
investigate the mechanism of CNV in the development and progression
of CRC, the intersection of genes involved in significant abnormal
CNV and differentially expressed genes was obtained. Subsequently
the association analysis of the expression profiles and copy number
profiles for the aforementioned small gene set was performed, with
a result that 295 mRNAs had statistically significant differential
expression and a difference in CNV. Finally, GO enrichment analysis
and a KEGG pathway analysis of these mRNAs were performed,
suggesting that the mRNAs were primarily enriched in only one KEGG
pathway (P<0.05; Fig. 2B) and
eight GO terms (Benjamin P<0.01; Fig.
2C). Recent evidence suggests that the circadian system can
influence the Wnt/β-catenin signaling pathway (30), which is a critical pathway for the
development and progression of CRC (31). Known CRC-related genes were mapped to
the set of 295 mRNAs, and the 73 CRC-related genes were
significantly enriched in this gene set (hypergeometric test,
P=4.725561×10−9).
PubMed and CGDB databases were searched, and 15 of
the 295 mRNAs were found to be circadian clock genes (Table II). Among the 15 circadian clock
genes, NR3C2 and P2RX1 were downregulated, and the remaining 13
genes were upregulated in patients with CRC. Gain was the
predominant type of alteration for BIRC7, GNGT1, NFE2L3, PDX1 and
UBE2C, while loss of APCDD1 and P2RX1 was found in >30% of
cases. No significant changes in the expression levels of other
important genes, such as PER and ARNTL1, in the circadian clock
signaling pathway, were found.
 | Table II.Information of the 15 circadian clock
genes. |
Table II.
Information of the 15 circadian clock
genes.
| Gene | Location |
Log2FC | FDR | Loss | Gain | Normal | FDR |
P-valuea |
|---|
| APCDD1 | Chr18:
10,454,628-10,489,948 | 2.483785 |
2.96×10−12 | 154 | 9 | 289 |
1.14×10−87 |
1.93×10−5 |
| AQP9 | Chr15:
58,138,169-58,185,911 | 2.470799 |
6.41×10−10 | 59 | 2 | 391 |
6.65×10−12 |
2.16×10−3 |
| ARNTL2 | Chr12:
27,332,854-27,425,289 | 2.484382 |
1.58×10−41 | 4 | 44 | 404 |
2.46×10−8 |
1.28×10−7 |
| BIRC7 | Chr20:
63,235,883-63,240,507 | 2.466525 |
1.09×10−11 | 0 | 272 | 180 |
3.08×10−87 |
1.37×10−6 |
| GNGT1 | Chr7:
93,591,573-93,911,265 | 3.164943 |
4.55×10−10 | 1 | 109 | 342 |
2.94×10−25 |
5.17×10−6 |
| HSD11B2 | Chr16:
67,430,652-67,437,553 | −2.35877 |
5.09×10−65 | 2 | 27 | 423 |
1.08×10−4 |
1.44×10−3 |
| IL23A | Chr12:
56,334,174-56,340,410 | 3.021143 |
4.60×10−23 | 0 | 35 | 417 |
6.42×10−5 |
4.20×10−3 |
| KRT23 | Chr17:
40,922,696-40,937,634 | 7.179667 |
2.02×10−34 | 9 | 38 | 405 |
8.50×10−9 |
7.79×10−7 |
| NFE2L3 | Chr7:
26,152,240-26,187,125 | 2.676112 |
1.01×10−85 | 0 | 161 | 291 |
1.53×10−41 |
3.89×10−17 |
| NR3C2 | Chr4:
148,078,762-148,444,698 | −2.63761 |
4.00×10−84 | 29 | 2 | 421 |
1.74×10−3 |
4.86×10−5 |
| ORM2 | Chr9:
114,329,869-114,333,252 | 2.989211 |
1.39×10−11 | 9 | 19 | 424 |
1.42×10−3 |
3.11×10−3 |
| P2RX1 | Chr17:
3,896,592-3,916,500 | −2.29178 |
2.75×10−55 | 138 | 3 | 311 |
1.42×10−36 |
5.26×10−7 |
| PDX1 | Chr13:
27,920,020-27,926,231 | 4.797965 |
1.54×10−55 | 0 | 200 | 252 |
1.37×10−58 |
8.04×10−18 |
| RNFT2 | Chr12:
116,738,178-116,853,631 | 2.006432 |
4.31×10−30 | 3 | 31 | 418 |
1.08×10−4 |
4.09×10−3 |
| UBE2C | Chr20:
45,812,576-45,816,957 | 2.15803 |
5.49×10−43 | 1 | 276 | 175 |
5.72×10−89 |
6.51×10−36 |
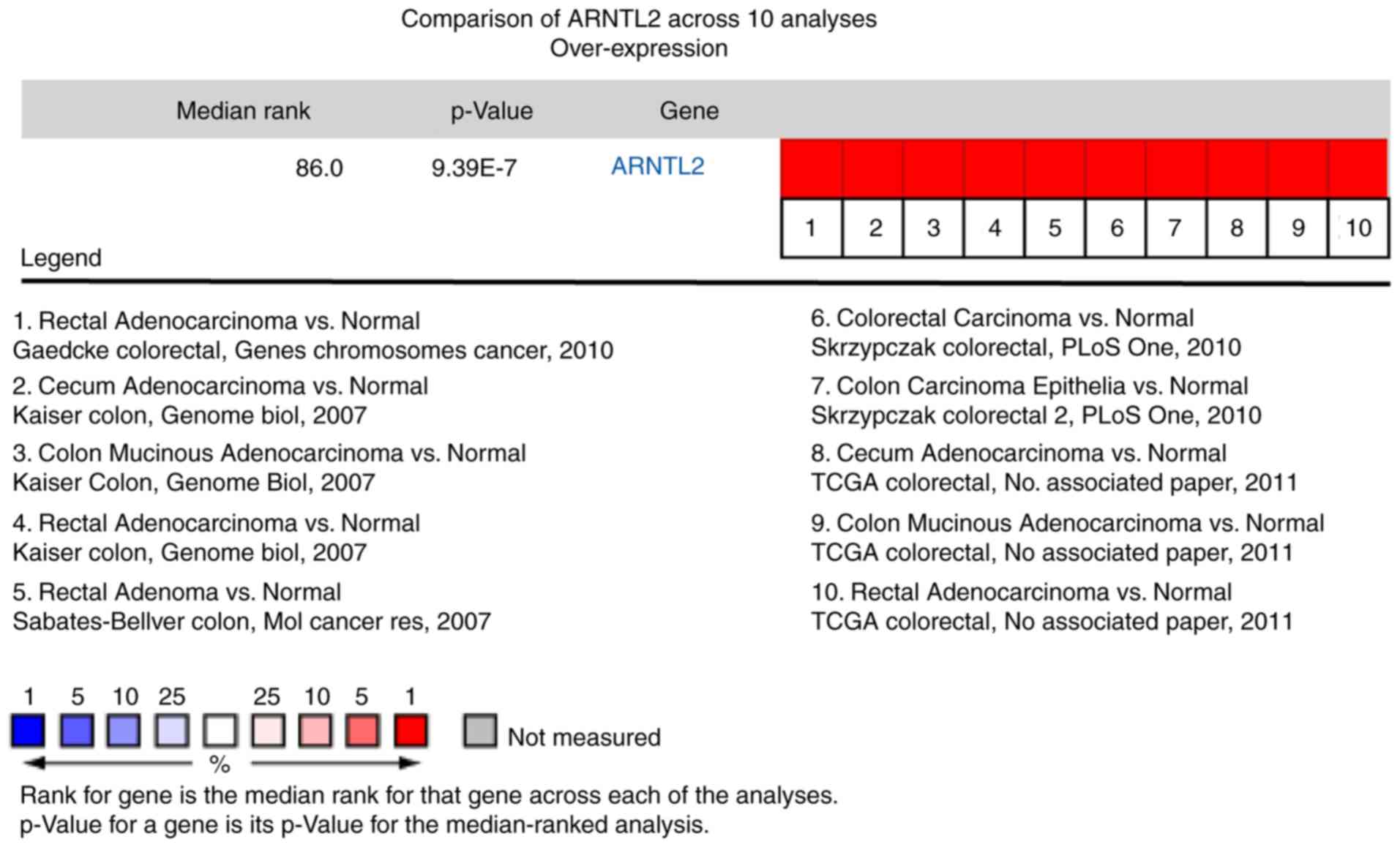
Subsequently, a meta-analysis on the expression of
the 15 clock genes in CRC using public microarray datasets from the
Oncomine database was performed. As presented in Fig. 3, the expression patterns of the clock
gene ARNTL2 in 10 independent microarray datasets and TCGA datasets
were consistent with previous analyses (32,33).
Overexpression was found in all CRC tissues compared with that in
the tumor-adjacent tissue (gene median rank, 86.0;
P=9.39×10−7).
Function analysis of the clock gene
ARNTL2 driven by CNV in CRC
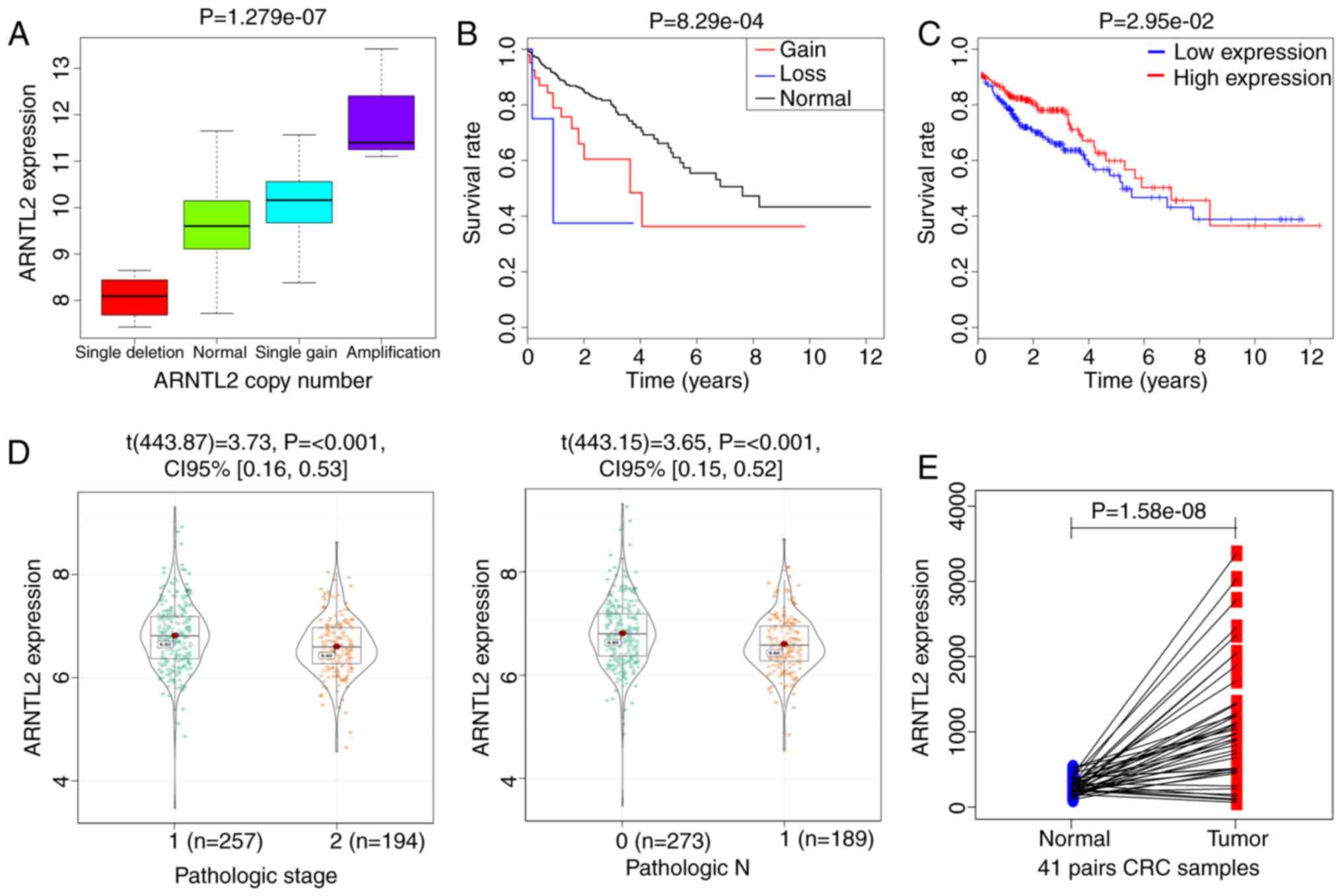
The expression of the gene ARNTL2 was found in the
452 patients with CRC, among which a total of 48 CNVs occurred,
with the presence of copy number gain in 44 patients and copy
number loss in 4 patients. ARNTL2 was null in 10 samples, which
were consequently removed from the study. The association of ARNTL2
mRNA expression levels with CNV type was identified. As shown in
Fig. 4A, single gain and
amplification of ARNTL2 were associated with increased mRNA
expression, and deletion of ARNTL2 was associated with decreased
mRNA expression. Therefore, ARNTL2 gene expression and CNV in CRC
tissues show the same trend.
A Kaplan-Meier curve analysis was performed to
investigate the overall survival time for ARNTL2 in patients with
CRC. Compared with that of the patients with normal copy number,
the survival rate of the patients with abnormal copy number (gain
or loss) of ARNTL2 was significantly decreased (Fig. 4B), whereas the overall survival of
patients with CRC with ARNTL2 CNV was significantly decreased. The
expression levels of ARNTL2 were also associated with the overall
patient survival; higher expression levels indicated greater
survival time (Fig. 4C).
To further investigate whether ARNTL2 is involved in
the development and progression of CRC, the tumor tissue samples
were divided into several subgroups based on pathological TNM
(T3+T4 vs. T1+T2, N2+N3 vs. N0+N1, M1 vs. M0) and pathological
stages (I–II vs. III–IV) (34). A
comparative analysis of ARNTL2 expression profiles was performed.
As a result, ARNTL2 expression demonstrated a statistically
significant association with pathological stages (P<0.001) and
pathological N (P<0.001) (Fig.
4D).
To gain a clearer understanding of the expression of
ARNTL2 in patients with cancer and adjacent tissues, a paired
difference analysis of ARNTL2 from 41 patients with cancer and
adjacent tissues was performed. The expression of ARNTL2 in cancer
tissues was significantly higher compared with that in adjacent
tissues (P=1.058×108; Fig.
4E). This is consistent with the results of our previous
analysis of the difference.
To investigate the biological characteristics shared
by the different ARNTL2 expression levels, a GSEA was performed.
The most significant pathways for the upregulated gene sets in the
significance order (nominal P<0.05) are shown in Fig. 5. The six pathways, including ‘natural
killer cell-mediated cytotoxicity’, ‘oocyte meiosis’, the ‘p53
signaling pathway’, ‘pancreatic cancer’, ‘prostate cancer’ and the
‘toll like receptor signaling pathway’ were significant in the
ARNTL2 high expression phenotype. Among these pathways, some were
directly linked to cancer pathogenesis, such as ‘pancreatic
cancer’, the ‘p53 signaling pathway’ (35) and ‘prostate cancer’. There were no
significant pathways for downregulated gene sets with nominal
P<0.05.
Discussion
CRC is the third most commonly occurring cancer
worldwide and the fourth most frequent cause of death having an
oncological origin (1); it is
considered to be a complex disease resulting from a combination of
environmental factors, genetic/epigenetic predisposing variants and
specific molecular mechanisms. Chromosomal instability (CIN) has
been defined as a major factor contributing to CRC carcinogenesis
(36). CNV exists as a genetic
polymorphism in the human genome that is a type of CIN (37). The form of CNV directly affecting the
expression of a gene is mainly the deletion or amplification of a
copy number of a gene, causing an increase or decrease in the
amount of gene expression and increasing the occurrence of the
disease (38). A previous study
found that tumor necrosis factor receptor superfamily member 10C
CNV is associated with metastatic colorectal cancer (39). In the present study, an integrated
analysis of CNV data and gene expression profile for CRC with a
large sample size (n=503, including 462 patient samples and 41
tumor-adjacent tissue samples) was performed. A total of 10,256
genes with significantly different CNV and 2,083 aberrantly
expressed mRNAs were obtained, of which 295 genes showed a
statistically significant association between the gene expression
and CNV; therefore, these 295 genes were regarded as CRC-related
CNV-driven genes. The present findings may provide a new
theoretical basis for the pathogenesis of CRC and also contribute
to the development of new therapeutic strategies.
In the present study, CNV-driven genes were only
enriched in the Wnt signaling pathway. The Wnt pathway is involved
in the regulation of important physiological processes such as
normal embryo development, and cell proliferation and
differentiation, and its abnormal activation plays an important
role in the process of tumor development, metastasis and
therapeutic resistance (40). A
previous study showed that >90% of colorectal cancer cases have
abnormal activation of the Wnt classical signaling pathway
(41). Meanwhile, some studies
suggested that the regulation of circadian clock genes, such as
CRY1 (42) and Rev-erbα (43), was mediated by the classical
Wnt/β-catenin signaling pathway. Further study of the Wnt signaling
pathway will help to develop new strategies for CRC treatment. The
present findings provide a new clue to study this signaling
pathway.
Studies in circadian clock genes may expand the
knowledge regarding the mechanism of occurrence and development of
tumors, and may provide a new approach for tumor therapy (44). Indeed, multiple epidemiological
studies have shown that impaired function of the circadian clock
promotes development of cancer (45). For circadian clock genes, including
Per1, Per2, and Per3, the expression levels of which are often
found to be decreased in pancreatic cancer (46) and gastric cancer (47), as well as the disruption of autonomic
rhythm. Additionally, in a previous study, CRC showed lower
expression of NPAS2 compared with that in healthy tissues, and this
was negatively associated with tumor size, stage and metastasis
(48). A previous study has also
shown that varying degrees of biorhythm destruction are found in
50% of metastatic cancer cases (49). In the present study, 15 CNV-driven
circadian clock genes in CRC tissues were identified, indicating
that these circadian clock genes may play a role in cancer.
However, this requires further validation at the protein level.
ARNTL2 has been described as a candidate biomarker in different
cancer types, including kidney cancer (50), colorectal cancer (21) and hepatocellular carcinoma (51), and similar results for ARNTL2 were
obtained in the present study. As research continues to deepen,
numerous studies have found that genomic alterations involving
circadian clock genes, such as point mutations or CNV, are
frequently found in different human cancer types. The rs1801260
SNP, in the 3′ untranslated region of the clock circadian regulator
gene, was found to be associated with the development of CRC
(52). The CNV form of the BMAL1
gene has also been found in multiple cancers, such as breast and
colorectal cancer (35 gains and 7 losses in CNV numbers) (53). Previous studies have observed a close
association between ARNTL2 expression and various types of cancer
(21,22); however, no studies have characterized
the association between CNV in ARNTL2 and cancer. In the present
study, upregulation of ARNTL2 in patients with CRC was found, and
ARNTL2 CNV has three forms: Single loss, single gain, and
amplification. Further analysis found that the expression of ARNTL2
has the same trend as CNV. Our study showed that the expression
level of ARNTL2 was abnormal due to the presence of CNV, which
promoted the occurrence and development of CRC.
Genetic drifts in ARNTL2 polymorphisms have been
described in the human population leading to variation in the
circadian rhythm regulation (54).
The expression of ARNTL2 was significantly associated with survival
time in patients with CRC. A previous study found that high ARNTL2
expression predicted poor survival in patients with lung
adenocarcinoma (55). However, in
the present study, low ARNTL2 expression predicted poor survival in
patients with CRC. This may be due to the heterogeneity between
different types of cancer. Moreover, a previous report indicated
that ARNTL2 high levels significantly influence mammary tumor
metastasis (23). ARNTL2 levels were
also significantly associated with pathological stage and N stage
in patients with CRC in the present study. ARNTL2 CNV was also
significantly associated with survival time in patients with CRC.
These data suggest that ARNTL2 can be used as a prognostic factor
for CRC, which may bring more personalized treatment to patients
with CRC. GSEA analysis showed that ARNTL2 is enriched for gene
sets associated with CRC pathogenesis, such as the ‘p53 signaling
pathway’. These findings suggest that the CNV-driven clock gene
ARNTL2 plays a crucial role in the development and progression of
CRC. However, this study has some limitations as it was an in
silico study. Further in vivo investigations would be
beneficial to fully understand the roles of ARNTL1 in CRC
initiation and development.
In summary, to the best of our knowledge, the
present study demonstrates for the first time that circadian clock
genes play an important role in CRC in the form of CNV, and that 15
CNV-driven clock genes are associated with the etiology and
pathogenesis of CRC. Finally, it was concluded that CNV in the
circadian clock gene ARNTL2 may be a useful genetic biomarker for
the treatment of individualized CRC patients and may identify
patients who may benefit from more aggressive systemic treatment
strategies.
Acknowledgements
Not applicable.
Funding
This study was partly supported by the National
Natural Science Foundation of China (grant no. 61775139 and grant
no. 61373057), the Shanghai Natural Science Foundation (grant no.
15ZR1420800) and the Key Projects of Humanities and Social Sciences
Research in Colleges and Universities in Anhui Province (grant no.
SK2018A0630).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WLY, SHP and LHJ conceived the study. WLY collected
the data and performed the bioinformatics analyses. LL, XBL, CW,
DS, CXD, and SCL performed quality control of the raw data and
performed data analyses. WLY and SHP wrote the manuscript. SHP and
LHJ supervised the study and agreed to be responsible for ensuring
that all aspects of the study are accurate and have been
appropriately investigated. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calvert PM and Frucht H: The genetics of
colorectal cancer. Ann Intern Med. 137:603–612. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu F and Chang HC: Physiological links of
circadian clock and biological clock of aging. Protein Cell.
8:477–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kondratov R: Circadian clock and cancer
therapy: An unexpected journey. Ann Med. 46:189–190. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sahar S and Sassone-Corsi P: Metabolism
and cancer: The circadian clock connection. Nat Rev Cancer.
9:886–896. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kelleher FC, Rao A and Maguire A:
Circadian molecular clocks and cancer. Cancer Lett. 342:9–18. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stevens RG, Brainard GC, Blask DE, Lockley
SW and Motta ME: Breast cancer and circadian disruption from
electric lighting in the modern world. CA Cancer J Clin.
64:207–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polo A, Singh S, Crispo A, Russo M,
Giudice A, Montella M, Colonna G and Costantini S: Evaluating the
associations between human circadian rhythms and dysregulated genes
in liver cancer cells. Oncol Lett. 14:7353–7359. 2017.PubMed/NCBI
|
|
10
|
Momma T, Okayama H, Saitou M, Sugeno H,
Yoshimoto N, Takebayashi Y, Ohki S and Takenoshita S: Expression of
circadian clock genes in human colorectal adenoma and carcinoma.
Oncol Lett. 14:5319–5325. 2017.PubMed/NCBI
|
|
11
|
Wendeu-Foyet MG, Koudou Y, Cénée S,
Trétarre B, Rébillard X, Cancel-Tassin G, Cussenot O, Boland A,
Bacq D, Deleuze JF, et al: Circadian genes and risk of prostate
cancer: Findings from the EPICAP study. Int J Cancer.
145:1745–1753. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bragantini D, Sivertsen B, Gehrman P,
Lydersen S and Güzey IC: Variations in circadian genes and
individual nocturnal symptoms of insomnia. The HUNT study.
Chronobiol Int. 36:681–688. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cooper GM, Nickerson DA and Eichler EE:
Mutational and selective effects on copy-number variants in the
human genome. Nat Genet. 39:S22–S29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng ZM, Liu L, Qiu WH, Zhang YQ, Zhong
HY, Liao P and Wu YH: Analysis of genomic variation in lung
adenocarcinoma patients revealed the critical role of PI3K complex.
PeerJ. 5:e32162017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diskin SJ, Hou C, Glessner JT, Attiyeh EF,
Laudenslager M, Bosse K, Cole K, Mossé YP, Wood A, Lynch JE, et al:
Copy number variation at 1q21.1 associated with neuroblastoma.
Nature. 459:987–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui M, Zheng M, Sun B, Wang Y, Ye L and
Zhang X: A long noncoding RNA perturbs the circadian rhythm of
hepatoma cells to facilitate hepatocarcinogenesis. Neoplasia.
17:79–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda M, Yu W, Hirai M, Ebisawa T, Honma
S, Yoshimura K, Honma KI and Nomura M: cDNA cloning of a novel
bHLH-PAS transcription factor superfamily gene, BMAL2: Its mRNA
expression, subcellular distribution, and chromosomal localization.
Biochem Biophys Res Commun. 275:493–502. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schoenhard JA, Smith LH, Painter CA, Eren
M, Johnson CH and Vaughan DE: Regulation of the PAI-1 promoter by
circadian clock components: Differential activation by BMAL1 and
BMAL2. J Mol Cell Cardiol. 35:473–481. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He CX, Avner P, Boitard C and Rogner UC:
Downregulation of the circadian rhythm related gene Arntl2
suppresses diabetes protection in Idd6 NOD.C3H congenic mice. Clin
Exp Pharmacol Physiol. 37:1154–1158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olkkonen J, Kouri VP, Kuusela E, Ainola M,
Nordström D, Eklund KK and Mandelin J: DEC2 blocks the effect of
the ARNTL2/NPAS2 dimer on the expression of PER3 and DBP. J
Circadian Rhythms. 15:62017. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mazzoccoli G, Pazienza V, Panza A, Valvano
MR, Benegiamo G, Vinciguerra M, Andriulli A and Piepoli A: ARNTL2
and SERPINE1: Potential biomarkers for tumor aggressiveness in
colorectal cancer. J Cancer Res Clin Oncol. 138:501–511. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mocellin S, Tropea S, Benna C and Rossi
CR: Circadian pathway genetic variation and cancer risk: Evidence
from genome-wide association studies. BMC Med. 16:202018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ha NH, Long J, Cai Q, Shu XO and Hunter
KW: The circadian rhythm gene Arntl2 is a metastasis susceptibility
gene for estrogen receptor-negative breast cancer. PLoS Genet.
12:e10062672016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S, Shui K, Zhang Y, Lv Y, Deng W, Ullah
S, Zhang L and Xue Y: CGDB: A database of circadian genes in
eukaryotes. Nucleic Acids Res. 45:D397–D403. 2017.PubMed/NCBI
|
|
28
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin H and Zelterman D: Modeling survival
data: Extending the Cox model. Taylor & Francis. 85–86.
2002.
|
|
30
|
Karantanos T, Theodoropoulos G, Pektasides
D and Gazouli M: Clock genes: Their role in colorectal cancer.
World J Gastroenterol. 20:1986–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Emons G, Spitzner M, Reineke S, Möller J,
Auslander N, Kramer F, Hu Y, Beissbarth T, Wolff HA, Rave-Fränk M,
et al: Chemoradiotherapy resistance in colorectal cancer cells is
mediated by Wnt/β-catenin signaling. Mol Cancer Res. 15:1481–1490.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Skrzypczak M, Goryca K, Rubel T, Paziewska
A, Mikula M, Jarosz D, Pachlewski J, Oledzki J and Ostrowski J:
Modeling oncogenic signaling in colon tumors by multidirectional
analyses of microarray data directed for maximization of analytical
reliability. PLoS One. 5:e130912010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaiser S, Park YK, Franklin JL, Halberg
RB, Yu M, Jessen WJ, Freudenberg J, Chen X, Haigis K, Jegga AG, et
al: Transcriptional recapitulation and subversion of embryonic
colon development by mouse colon tumor models and human colon
cancer. Genome Biol. 8:R1312007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manualSpringer
International Publishing; 2017
|
|
35
|
Slattery ML, Mullany LE, Wolff RK, Sakoda
LC, Samowitz WS and Herrick JS: The p53-signaling pathway and
colorectal cancer: Interactions between downstream p53 target genes
and miRNAs. Genomics. 111:762–771. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laissue P: The forkhead-box family of
transcription factors: Key molecular players in colorectal cancer
pathogenesis. Mol Cancer. 18:52019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang F, Gu W, Hurles ME and Lupski JR:
Copy number variation in human health, disease, and evolution. Annu
Rev Genomics Hum Genet. 10:451–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Falchi M, El-Sayed Moustafa JS, Takousis
P, Pesce F, Bonnefond A, Andersson-Assarsson JC, Sudmant PH,
Dorajoo R, Al-Shafai MN, Bottolo L, et al: Low copy number of the
salivary amylase gene predisposes to obesity. Nat Genet.
46:492–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanenbaum DG, Hall WA, Colbert LE, Bastien
AJ, Brat DJ, Kong J, Kim S, Dwivedi B, Kowalski J, Landry JC and Yu
DS: TNFRSF10C copy number variation is associated with metastatic
colorectal cancer. J Gastrointest Oncol. 7:306–314. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Silva AL, Dawson SN, Arends MJ, Guttula K,
Hall N, Cameron EA, Huang TH, Brenton JD, Tavaré S, Bienz M and
Ibrahim AE: Boosting Wnt activity during colorectal cancer
progression through selective hypermethylation of Wnt signaling
antagonists. BMC Cancer. 14:8912014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun S, Zhou L, Yu Y, Zhang T and Wang M:
Knocking down clock control gene CRY1 decreases adipogenesis via
canonical Wnt/β-catenin signaling pathway. Biochem Biophys Res
Commun. 506:746–753. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He Y, Lin F, Chen Y, Tan Z, Bai D and Zhao
Q: Overexpression of the circadian clock gene Rev-erbα affects
murine bone mesenchymal stem cell proliferation and osteogenesis.
Stem Cells Dev. 24:1194–1204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kettner NM, Katchy CA and Fu L: Circadian
gene variants in cancer. Ann Med. 46:208–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Angelousi A, Kassi E, Nasiri-Ansari N,
Weickert MO, Randeva H and Kaltsas G: Clock genes alterations and
endocrine disorders. Eur J Clin Invest. 48:e129272018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Relles D, Sendecki J, Chipitsyna G, Hyslop
T, Yeo CJ and Arafat HA: Circadian gene expression and
clinicopathologic correlates in pancreatic cancer. J Gastrointest
Surg. 17:443–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao H, Zeng ZL, Yang J, Jin Y, Qiu MZ, Hu
XY, Han J, Liu KY, Liao JW, Xu RH and Zou QF: Prognostic relevance
of Period1 (Per1) and Period2 (Per2) expression in human gastric
cancer. Int J Clin Exp Pathol. 7:619–630. 2014.PubMed/NCBI
|
|
48
|
Xue X, Liu F, Han Y, Li P, Yuan B, Wang X,
Chen Y, Kuang Y, Zhi Q and Zhao H: Silencing NPAS2 promotes cell
growth and invasion in DLD-1 cells and correlated with poor
prognosis of colorectal cancer. Biochem Biophys Res Commun.
450:1058–1062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Innominato PF, Roche VP, Palesh OG,
Ulusakarya A, Spiegel D and Lévi FA: The circadian timing system in
clinical oncology. Ann Med. 46:191–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mazzoccoli G, Piepoli A, Carella M, Panza
A, Pazienza V, Benegiamo G, Palumbo O and Ranieri E: Altered
expression of the clock gene machinery in kidney cancer patients.
Biomed Pharmacother. 66:175–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kovanen L, Saarikoski ST, Haukka J,
Pirkola S, Aromaa A, Lönnqvist J and Partonen T: Circadian clock
gene polymorphisms in alcohol use disorders and alcohol
consumption. Alcohol Alcohol. 45:303–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Karantanos T, Theodoropoulos G, Gazouli M,
Vaiopoulou A, Karantanou C, Stravopodis DJ, Bramis K, Lymperi M and
Pektasidis D: Association of the clock genes polymorphisms with
colorectal cancer susceptibility. J Surg Oncol. 108:563–567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Forbes SA, Beare D, Boutselakis H, Bamford
S, Bindal N, Tate J, Cole CG, Ward S, Dawson E, Ponting L, et al:
COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids
Res. 45:D777–D783. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ciarleglio CM, Ryckman KK, Servick SV,
Hida A, Robbins S, Wells N, Hicks J, Larson SA, Wiedermann JP,
Carver K, et al: Genetic differences in human circadian clock genes
among worldwide populations. J Biol Rhythms. 23:330–340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brady JJ, Chuang CH, Greenside PG, Rogers
ZN, Murray CW, Caswell DR, Hartmann U, Connolly AJ, Sweet-Cordero
EA, Kundaje A and Winslow MM: An Arntl2-driven secretome enables
lung adenocarcinoma metastatic self-sufficiency. Cancer Cell.
29:697–710. 2016. View Article : Google Scholar : PubMed/NCBI
|