Introduction
Gastric cancer is one of the most common cancer
types worldwide and remains the third leading cause of
cancer-associated mortality, accounting for >783,000 deaths
worldwide in 2018 (1). The
diagnostic rate of early gastric cancer is only 10% in China, and
most patients are at an advanced stage when clinically diagnosed,
which confers a poor prognosis (2).
Systematic chemotherapy is the major treatment for
patients with advanced gastric cancer (AGC).
Platinum-fluoropyrimidine- and paclitaxel-fluoropyrimidine-based
chemotherapy regimens are recommended as the first-line treatments
in line with the Chinese Society of Clinical Oncology guidelines
(3).
However, patients with the same tumor stage and
receiving similar treatment can exhibit different clinical
outcomes, and gastric cancer is a complex disease and its prognosis
and progression are significantly affected by genetic and
environmental factors (4).
Identifying predictive genetic biomarkers could therefore
contribute to the development of individualized therapy and
follow-up strategies (5).
Neuropilin-1 (NRP-1) is a type I
transmembrane glycoprotein distributed at the surface of cells that
has been reported to affect neuronal axon guidance and embryonic
angiogenesis (6), and to serve as a
co-receptor regulating tumorigenesis in the vascular endothelial
growth factor (VEGF)-VEGF receptor 2 (VEGFR2)
[kinase insert domain receptor (KDR)] or platelet-derived
growth factor (PDGF)-PDGF receptor (PDGFR) signaling
pathways (7,8). VEGFRs are a type of tyrosine
kinase receptor, and include VEGFR1 and VEGFR2 (KDR),
which can be activated by binding with VEGF ligands
(9). The VEGF-VEGFR2
signaling pathway is the leading pathway that activates the
proliferation and migration of endothelial cells, therefore
promoting angiogenesis and stimulating tumor growth and invasion
(10,11). Furthermore, PDGF isoforms can
transduce signals via binding to structurally similar α- and
β-tyrosine kinase receptors, known as PDGFRα and
PDGFRβ, respectively. The PDGF-PDGFR signaling
pathway serves critical roles in regulating proliferation and
survival of certain cell types (e.g. hematopoietic stem cell,
vascular endothelial cell and vascular smooth muscle cell) during
embryogenesis, and overexpression or mutation of the
PDGF-PDGFR pathway can stimulate tumor cell proliferation
(12,13). Previous studies have reported that
polymorphisms within VEGF and KDR impacted their
expression at the gene level (14,15).
Thus, polymorphisms of these two signaling pathways may affect AGC
prognosis by regulating the expression of the aforementioned genes
and therefore affect the survival of patients with AGC. The present
study investigated the association between polymorphisms of the
NRP-1, KDR, PDGFβ, PDGFRβ and PDGFRα genes and
the prognosis of patients with AGC.
Materials and methods
Study population
A total of 100 patients with AGC from the Second
Affiliated Hospital of Dalian Medical University (Dalian, Liaoning,
China) were recruited between January 2011 and June 2016. The
inclusion criteria were as follows: i) Patients were
histopathologically diagnosed with gastric adenocarcinoma; ii)
patients had inoperable locally advanced, metastatic or recurrent
gastric cancer (AGC); iii) patients had an Eastern Cooperative
Oncology Group performance status (ECOG-PS) ≤2 (16); and iv) patients underwent at least 2
cycles of chemotherapy at the Second Affiliated Hospital when
diagnosed with postoperative recurrence or inoperable advanced
gastric cancer. The exclusion criteria were as follows: i) Patients
who received chemotherapy, radiotherapy and/or biological treatment
previously; ii) patients with an ECOG-PS >2; and iii) patients
with multiple primary malignant neoplasms. The 100 patients were
followed up by clinic visits and phone calls every 2 months, and
clinical outcomes were recorded until October 2018. Genotype
information was not available for 8 patients, 5 cases were lost to
follow-up and 6 patients failed to receive the protocol treatment.
Therefore, 81 patients were analyzed in the present study.
Unresectable patients were staged according to imaging and
gastroscopy when histopathologically diagnosed by biospy, and the
postoperative recurrence patients were staged according to
postoperative pathology. Tumors were staged using the 7th edition
of the Tumor-Node-Metastasis (TNM) staging system of the
International Union Against Cancer/American Joint Committee on
Cancer (17). Chemotherapy was given
prior to the present study, and regimens included platinum and
fluoropyrimidine [cisplatin (D) 80 mg/m2 on day 1 and
fluorouracil (F) 750 mg/m2 from day 1–4; D 80
mg/m2 on day 1 and capecitabine (X) 1,000
mg/m2 from day 1–14], and paclitaxel (P) and
fluoropyrimidine [P, 150 mg/m2 on day 1 and F 750
mg/m2 from day 1–5; P 150 mg/m2 on day 1 and
X 1,000 mg/m2 from day 1–14]. The regimens were repeated
every 21 days. Chemotherapy was stopped in case of disease
progression, patient refusal or grade 3–4 toxicity according to
National Cancer Institute Common Terminology Criteria for Adverse
Events version 4.03 (18).
SNP selection
The SNP loci of the target genes were selected from
the public SNP database of the 1,000 Genome Project in the National
Center for Biotechnology Information (NCBI) using minor allele
frequency (MAF) >0.1 in the Chinese Han population and the
Hardy-Weinberg equilibrium with a P-value of >0.1, then tagSNPs
with a cut-off value of R2>0.8, and covering the gene
and flanking 3 kb either side of the gene regions were chosen by
the Genome Variation Server (https://gvs.gs.washington.edu). In total, 66 tagSNPs
(27 from KDR gene, 32 from NRP-1 gene and 7 from PDGFβ) were
selected, however due to financial constraints, 10 SNPs (rs7692791,
rs6838752, rs2034965, rs1531290, rs13109660 from KDR, rs2070296,
rs2804495, rs2065364 from NRP-1, and rs4821877, and rs9622978 from
PDGFβ) were randomly selected from the tagSNPs. In addition, five
disease-associated SNPs (rs1870377 and rs2305948 from KDR,
rs6554162 and rs1800812 from PDGFRα, and rs2302273 from PDGFRβ)
were selected according to their use in previous literature
(19–24). Finally, the 15 SNPs (Table SI) of KDR rs7692791, rs2305948,
rs6838752, rs2034965, rs1531290, rs13109660 and rs1870377, of NRP-1
rs2070296, rs2804495 and rs2065364, of PDGFβ rs4821877 and
rs9622978, of PDGFRα rs6554162 and rs1800812, and of PDGFRβ
rs2302273, were obtained from the SNP database of the NCBI
(http://www.ncbi.nlm.nih.gov/SNP).
SNP genotyping
The tissues from patients with AGC were obtained via
biopsy or surgery, fixed with 10% neutral buffer formalin for 24 h
at room temperature, immersed in 60°C paraffin, embedded in a
paraffin block and stored at 4°C. Genomic DNA was extracted from
paraffin-embedded tissues of patients with AGC using the QIAamp DNA
FFPE Tissue kit (Qiagen GmbH) according to the manufacturer's
instructions. The polymerase chain reaction (PCR) primers for SNPs
were designed using Sequenom Assay Design 3.1 software (Sequenom)
and are listed in Table SII. A
thermocycler (PTC-100PCR; MJ Research) and KAPA Taq HotStart DNA
polymerase (Kapa Biosystems; Roche Diagnostics) were used for PCR
amplification, the thermal cycling program employed was as follows:
94°C for 5 min, followed by 35 cycles of 30 sec at 94°C, then 30
sec of annealing at 60°C, 30 sec of extension at 72°C, and a final
elongation step at 72°C for 10 min. The PCR products were sequenced
using a 3730XL DNA Analyzer (Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Statistical analysis
In the present study, the genetic model was divided
into 3 types, namely, general, dominant and recessive models, as
follows: Dominant model, MW+MM vs. WW; recessive model, WW+WM vs.
MM; and general model, MM vs. WM vs. WW, where W indicates the
wild-type allele and M the mutant allele). Before analysis, the
Hardy-Weinberg equation for the equilibrium of allele distributions
was tested by the χ2 test (Table I) and the SNPs with a P-value of
<0.05 were excluded. The progression-free survival (PFS) and
overall survival (OS) probabilities were estimated using the
Kaplan-Meier method. The association between SNPs and PFS and OS
were analyzed by log-rank tests and Cox regression analyses. Hazard
ratios (HR) and 95% confidence intervals (CIs) were estimated for
the uivariate and multivariate analyses usingy Cox regression
analyses. Bonferroni's correction was applied for multiple
comparisons (with the significance level set at P<0.025).
Statistical analyses were conducted using SPSS v.21.0 (IBM Corp.).
All tests were two-sided, and P<0.05 was considered to indicate
a statistically significant difference.
 | Table I.Hardy-Weinberg equilibrium test
results of selected SNPs. |
Table I.
Hardy-Weinberg equilibrium test
results of selected SNPs.
| Gene | SNP | χ2 | P-value |
|---|
| KDR | rs7692791 | 0.11 | 0.739 |
|
| rs2305948 | 0.02 | 0.902 |
|
| rs6838752 | 0.65 | 0.418 |
|
| rs2034965 | 1.66 | 0.197 |
|
| rs13109660 | 0.03 | 0.860 |
|
| rs1870377 | 0.58 | 0.455 |
|
| rs1531290 | 3.37 | 0.067 |
| NRP-1 | rs2070296 | 3.46 | 0.062 |
|
| rs2804495 | 0.08 | 0.775 |
|
| rs2065364 | 1.00 | 0.317 |
| PDGFβ | rs9622978 | 11.54 | 0.007a |
|
| rs4821877 | 0.00 | 0.998 |
| PDGFRα | rs6554162 | 0.00 | 0.951 |
|
| rs1800812 | 30.3 |
<0.001a |
| PDGFRβ | rs2302273 | 7.04 | 0.007a |
Results
Patient clinical characteristics
A total of 81 patients were recruited in the present
study, including 56 men (69.1%) and 25 women (30.9%). The age of
the patients ranged from 30 to 83 years, and the mean age was
60.7±10.1 years. By October 2018, 79 patients were deceased, 2 had
been lost during follow-up, and the median PFS and OS times were
5.5 and 11.0 months, respectively. The association between clinical
pathological features and survival time are listed in Table II. The results demonstrated that TNM
stage analyzed by Kaplan-Meier analysis was significantly
associated with longer OS time (log-rank, P=0.047), and the
platinum-based chemotherapy regimen was significantly associated
with longer PFS time (log-rank, P=0.025). Associations between
survival time and other clinical characteristics were not
identified.
 | Table II.Association between characteristics
and prognosis of patients with advanced gastric cancer. |
Table II.
Association between characteristics
and prognosis of patients with advanced gastric cancer.
|
|
|
|
| Log-rank
P-value |
|---|
|
|
|
|
|
|
|---|
| Variables | n | mPFS (95% CI) | mOS (95% CI) | PFS | OS |
|---|
| Sex |
|
|
| 0.433 | 0.703 |
|
Male | 56 | 5.0 (3.3–6.7) | 11 (9.6–12.4) |
|
|
|
Female | 25 | 6.0 (3.6–8.4) | 12 (9.4–14.5) |
|
|
| Age, years |
|
|
| 0.773 | 0.898 |
|
>60 | 33 | 5.5 (2.8–8.2) | 10.2
(6.0–14.4) |
|
|
|
≥60 | 48 | 6.0 (4.8–7.2) | 11.0
(10.0–12.0) |
|
|
| N stage |
|
|
| 0.590 | 0.081 |
|
N1+N2 | 49 | 5.0 (3.6–6.4) | 11.6
(10.4–12.8) |
|
|
| N3 | 32 | 5.0 (2.8–7.2) | 10.2
(8.6–11.8) |
|
|
| TNM stage |
|
|
| 0.080 | 0.047a |
| I, II
and III | 26 | 6.8 (5.9–7.7) | 12.0
(8.5–15.5) |
|
|
| IV | 55 | 4.5 (2.8–6.2) | 10.5
(8.5–12.5) |
|
|
| Tumor size, cm |
|
|
| 0.803 | 0.916 |
|
>5 | 31 | 5.0 (2.9–7.1) | 11.0
(9.0–13.0) |
|
|
| ≥5 | 50 | 6.0 (4.4–7.6) | 11.0
(9.5–12.5) |
|
|
|
Differentiation |
|
|
| 0.415 | 0.079 |
| Well to
moderate | 27 | 6.0 (4.5–7.5) | 14.8
(8.0–21.6) |
|
|
|
Poor | 54 | 4.5 (3.5–5.5) | 10.2
(8.2–12.2) |
|
|
| Platinum
chemotherapy regimen |
|
|
| 0.025a | 0.359 |
|
Platinum included | 38 | 6 (4.6–7.4) | 11.6
(9.3–13.9) |
|
|
|
Non-platinum included | 43 | 4.5 (3.3–5.7) | 10.5
(9.0–12.0) |
|
|
| Paclitaxel
chemotherapy regimen |
|
|
| 0.393 | 0.484 |
|
Paclitaxel included | 39 | 4.4 (2.9–5.9) | 11.0
(7.1–14.9) |
|
|
|
Non-paclitaxel included | 42 | 6 (4.6–7.4) | 11.0
(10.2–11.8) |
|
|
Associations between genotype and
survival time
Associations between genotype and prognosis were
estimated by Kaplan-Meier analysis, statistical significance was
determined by the log-rank test and the genotype information are
listed in Table SIII. The
associations between the three types of genetic models (general,
dominant and recessive) and survival time were analyzed (Table III). The results demonstrated that
of all the selected SNPs, five SNPs (KDR rs7692791, KDR rs1870377,
KDR rs2034965, NRP-1 rs2065364 and NRP-1 rs2804495) were
significantly associated with PFS or OS; however, SNPs from PDGF
and PDGFR genes were not associated with clinical outcomes.
 | Table III.Effect of SNPs in selected genes on
the prognosis in patients with advanced gastric cancer. |
Table III.
Effect of SNPs in selected genes on
the prognosis in patients with advanced gastric cancer.
|
|
|
| Log-rank P-value
for PFS | Log-rank P-value
for OS |
|---|
|
|
|
|
|
|
|---|
| Gene | SNP | Allelic change | General | Dominant | Recessive | General | Dominant | Recessive |
|---|
| KDR | rs7692791 | T/C | 0.032a | 0.009a | 0.281 | 0.227 | 0.093 | 0.364 |
|
| rs2305948 | C/T | 0.619 | 0.329 | 0.871 | 0.277 | 0.109 | 0.748 |
|
| rs6838752 | T/C | 0.097 | 0.137 | 0.053 | 0.203 | 0.254 | 0.095 |
|
| rs2034965 | G/A | 0.155 | 0.065 | 0.240 | 0.065 | 0.031 | 0.883 |
|
| rs13109660 | G/A | 0.795 | 0.522 | 0.687 | 0.365 | 0.376 | 0.481 |
|
| rs1870377 | T/A | 0.030a | 0.008a | 0.256 | 0.091 | 0.032a | 0.250 |
|
| rs1531290 | A/G | 0.236 | 0.128 | 0.313 | 0.451 | 0.845 | 0.207 |
| NRP-1 | rs2070296 | G/A | 0.498 | 0.417 | 0.486 | 0.993 | 0.964 | 0.909 |
|
| rs2804495 | G/T | 0.064 | 0.150 | 0.028a | 0.085 | 0.308 | 0.029 |
|
| rs2065364 | G/A | 0.052 | 0.300 | 0.015a | 0.113 | 0.587 | 0.037a |
| PDGFβ | rs4821877 | C/T | 0.712 | 0.490 | 0.862 | 0.949 | 0.933 | 0.747 |
| PDGFRα | rs6554162 | G/A | 0.513 | 0.322 | 0.751 | 0.501 | 0.413 | 0.561 |
Following univariate analysis (Tables IV and V), the dominant model of KDR
rs1870377 indicated that AA+AT carriers were associated with
shorter PFS and OS times compared with TT carriers [PFS: HR, 2.618;
95% CI, 1.235–5.550; P=0.012; OS: HR, 2.041; 95% CI, 1.042–3.999;
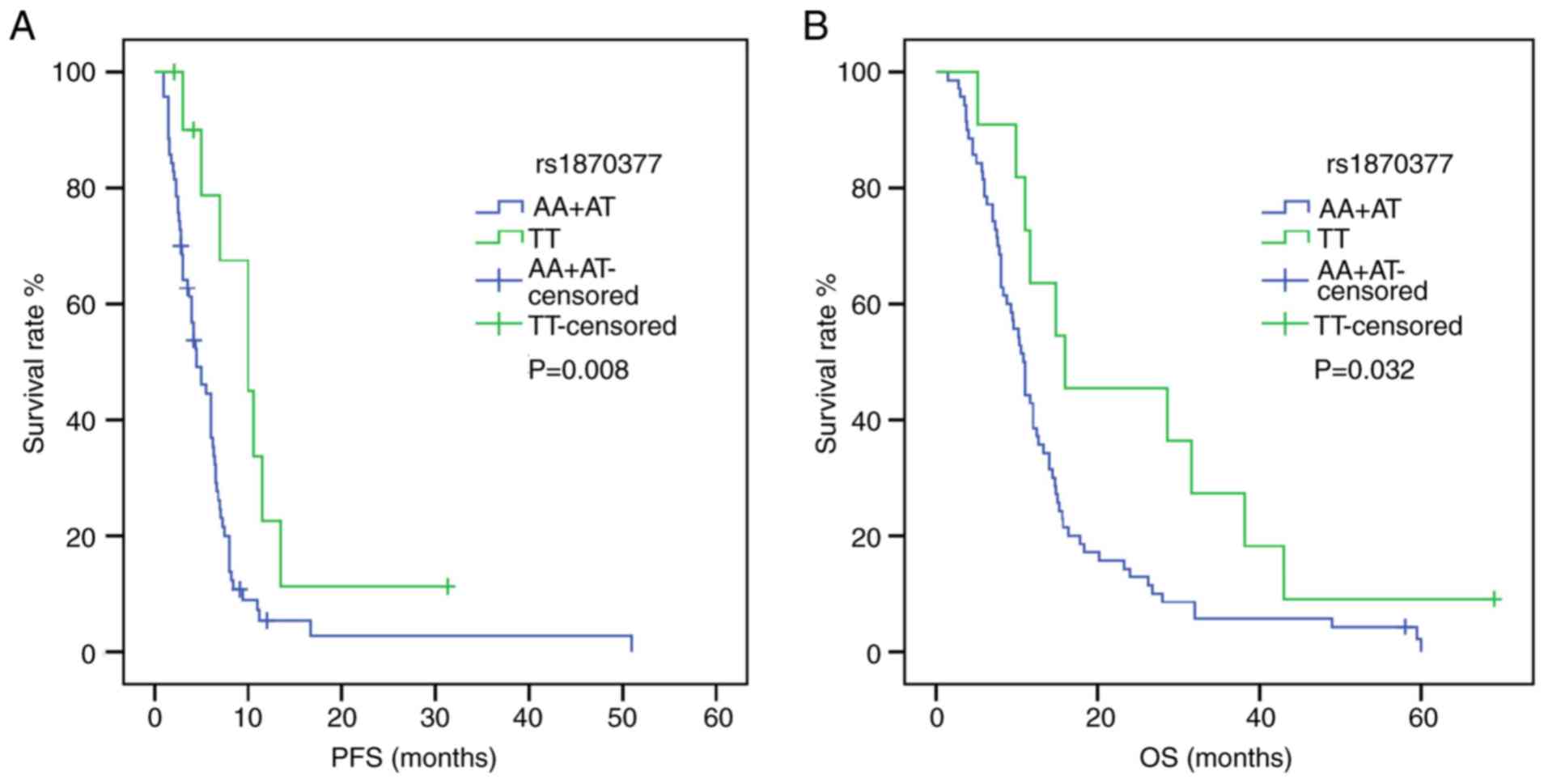
P=0.038) (Fig. 1). Furthermore, in a
recessive model of NRP-1 rs2065364, AA genotype carriers
exhibited more favorable PFS and OS times compared with the GG+AG
genotypes (PFS: HR, 2.896; 95% CI, 1.159–7.237; P=0.023; OS: HR,
2.367; 95% CI, 1.019–5.496; P=0.045) (Fig. 2). However, KDR rs1870377
variant AA and AT genotype were significantly associated with poor
PFS times compared with wild-type TT (AA vs. TT: HR, 3.221; 95% CI,
1.356–7.651; P=0.008; AT vs. TT: HR, 2.545, 95% CI, 1.159–5.589;
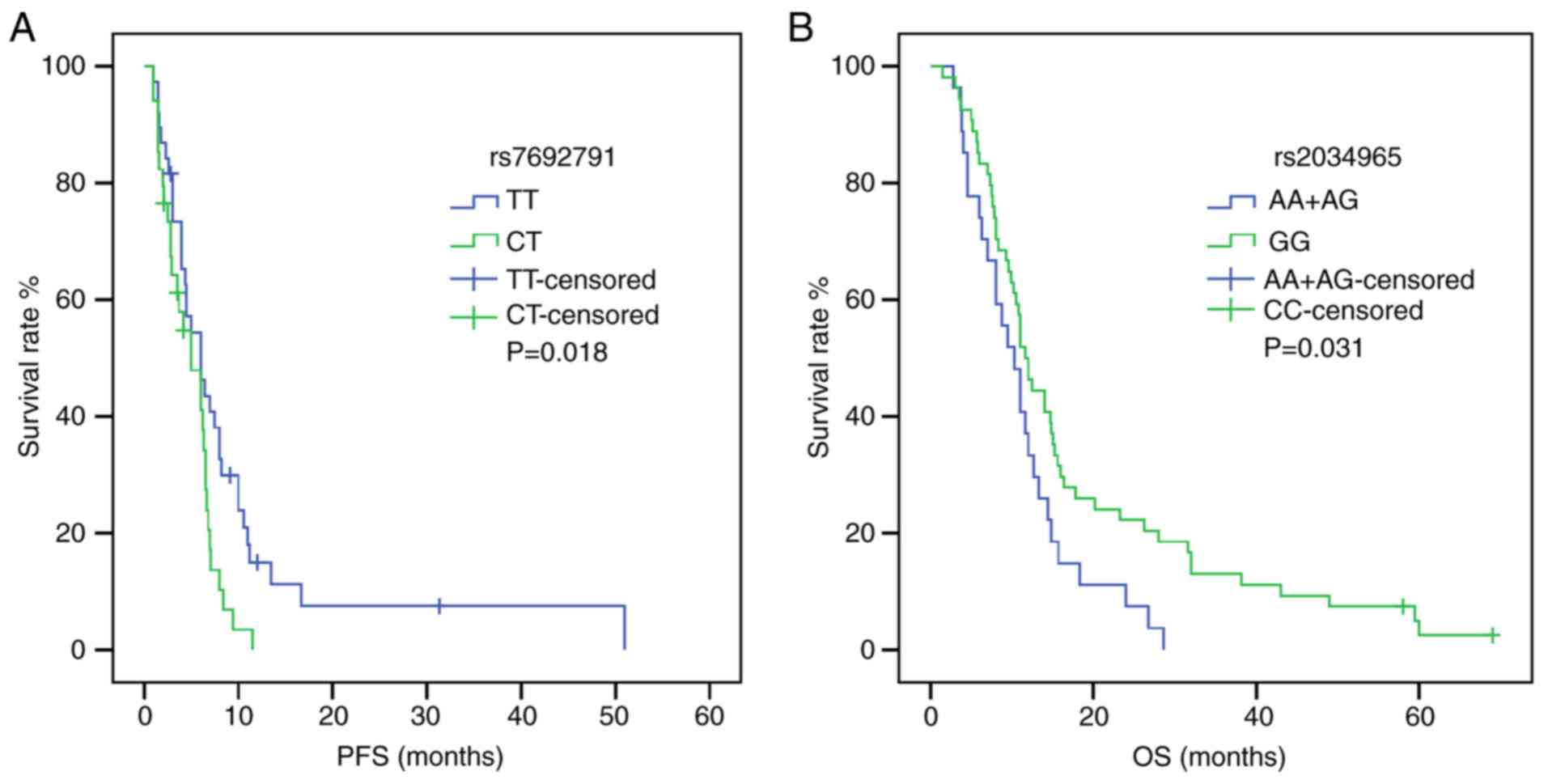
P=0.020; Table IV) (Fig. S1). Furthermore, the KDR
rs7692791 CT genotype was associated with lower PFS times compared
with the wild-type TT genotype (HR, 1.829, 95% CI, 1.091–3.066,
P=0.022; Table IV) (Fig. 3). In addition, in the dominant model
of KDR rs2034965, AA+GA genotypes were significantly
associated with reduced OS times (HR, 1.687; 95% CI, 1.039–2.738;
P=0.034; Table IV) (Fig. 3). Statistical significance between
SNPs and survival time in other polymorphisms was not found.
 | Table IV.Associations of SNPs in selected
genes and PFS in patients with advanced gastric cancer. |
Table IV.
Associations of SNPs in selected
genes and PFS in patients with advanced gastric cancer.
|
|
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|
|---|
| SNP | Outcome | mPFS, months | Model | Log-rank
P-value | HR (95% CI) | P-value | HR (95%
CI)a |
P-valuea |
|---|
| KDR
rs7692791 | PFS |
| General | 0.032 |
| 0.020 |
| 0.012 |
|
|
| 4.2 | CC | 0.099b | 1.926
(0.859–4.319) | 0.112 | 2.053
(0.855–4.929) | 0.107 |
|
|
| 5.0 | CT | 0.018b | 1.829
(1.091–3.066) | 0.022 | 1.969
(1.150–3.369) | 0.013 |
|
|
| 6.0 | TT |
| Reference |
| Reference |
|
|
|
|
| Dominant | 0.009 |
| 0.010 |
| 0.006 |
|
|
| 6.0 | TT |
| Reference |
| Reference |
|
|
|
| 5.0 | CC+CT |
| 1.892
(1.156–3.098) | 0.011 | 1.982
(1.196–3.284) | 0.008 |
| KDR
rs1870377 | PFS |
| General | 0.030 |
| 0.017 |
| 0.127 |
|
|
| 4.0 | AA | 0.005b | 3.221
(1.356–7.651) | 0.008 | 2.892
(0.987–8.474) | 0.053 |
|
|
| 5.5 | AT | 0.015b | 2.545
(1.159–5.589) | 0.020 | 1.778
(0.724–4.366) | 0.209 |
|
|
| 10.0 | TT |
| Reference |
| Reference |
|
|
|
|
| Dominant | 0.008 |
| 0.009 |
| 0.051 |
|
|
| 10.0 | TT |
| Reference |
| Reference |
|
|
|
| 4.5 | AA+AT |
| 2.618
(1.235–5.550) | 0.012 | 1.970
(0.861–4.503) | 0.108 |
| NRP-1
rs2065364 | PFS |
| Recessive | 0.015 |
| 0.017 |
| 0.004 |
|
|
| 8.0 | AA |
| Reference |
| Reference |
|
|
|
| 4.5 | AG+GG |
| 2.896
(1.159–7.237) | 0.023 | 3.905
(1.485–10.268) | 0.006 |
 | Table V.Associations of SNPs in selected
genes and OS in patients with advanced gastric cancer. |
Table V.
Associations of SNPs in selected
genes and OS in patients with advanced gastric cancer.
|
|
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|
|
|---|
| SNP | Outcome | mOS, months | Model | Log-rank
P-value | HR (95% CI) | P-value | HR (95%
CI)a | P-value |
|---|
| KDR
rs2034965 | OS |
| Dominant | 0.031 |
| 0.032 |
| 0.029 |
|
|
| 11.6 | GG |
| Reference |
| Reference |
|
|
|
| 10.3 | AA+GA |
| 1.687
(1.039–2.738) | 0.034 | 1.978
(1.193–3.280) | 0.008 |
| NRP1
rs2065364 | OS |
| Recessive | 0.037 |
| 0.039 |
| 0.105 |
|
|
| 17.8 | AA |
| Reference |
| Reference |
|
|
|
| 11.0 | AG+GG |
| 2.367
(1.019–5.496) | 0.045 | 2.048
(0.847–4.952) | 0.112 |
| KDR
rs1870377 | OS |
| Dominant | 0.032 |
| 0.034 |
| 0.035 |
|
|
| 16.0 | TT |
| Reference |
| Reference |
|
|
|
| 10.8 | AA+AT |
| 2.041
(1.042–3.999) | 0.038 | 2.264
(1.130–4.536) | 0.021 |
| NRP1
rs2804495 | OS |
| Recessive | 0.029 |
| 0.031 |
| 0.084 |
|
|
| 8.8 | TT |
| Reference |
| Reference |
|
|
|
| 12.0 | GT+GG |
| 1.710
(1.046–2.796) | 0.033 | 1.570
(0.924–2.667) | 0.095 |
For multivariate analysis (Tables IV and V), adjustments were performed for different
variables in PFS and OS. Variables that were considered clinically
relevant, such as age and TNM stage, or that presented an
association with survival time following univariate analysis as
listed in Table II were entered
into a multivariate Cox proportional-hazards regression model.
KDR rs7692791 remained significantly associated with PFS,
and the TT genotype was associated with better prognosis compared
with the CT genotype (HR, 1.969; 95% CI, 1.150–3.369; P=0.013).
Furthermore, the association between NRP-1 rs2065364 AG+GG
genotypes and shorter PFS remained significant following adjustment
(HR, 3.905; 95% CI, 1.485–10.268; P=0.006). Furthermore, KDR
rs2034965 AA+GA genotypes remained significantly associated with
worse OS following adjustment (HR, 1.978; 95% CI, 1.193–3.280,
P=0.008), and the KDR rs1870377 AA+AT genotypes were also
associated with shorter OS compared with the wild-type TT genotype
following adjustment (HR, 2.264; 95% CI, 1.130–4.536; P=0.021).
Effect of risk allele combinations on
PFS and OS
To study the combined effects of polymorphisms on
survival time, risk alleles were selected according to the
aforementioned results. The NRP-1 rs2065364G allele and the
KDR rs1870377 A allele were found to be unfavorable for PFS
and OS. Subsequently, the NRP-1 rs2065364/KDR
rs1870377 combination was tested for its association with survival
time and numbers of ‘risk alleles’ (Tables VI and VII). The results suggested that patients
carrying >2 risk alleles were more likely to have shorter PFS
and OS times compared with carriers with 1–2 risk alleles (PFS: HR,
0.427; 95% CI, 0.260–0.701; P=0.008; OS: HR, 0.523; 95% CI,
0.323–0.845; P=0.008; Tables VI and
VII) (Fig. 4). Following adjustment, this
association was also significant (PFS: HR, 0.427; 95% CI,
0.257–0.709; P=0.001; OS: HR, 0.511; 95% CI, 0.314–0.833;
P=0.007).
 | Table VI.Association between number of risk
alleles and overall survival in patients with advanced gastric
cancer. |
Table VI.
Association between number of risk
alleles and overall survival in patients with advanced gastric
cancer.
|
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|
|---|
| Alleles
combination | n | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
|
rs2065364/rs1870377 |
| 1-2 risk
alleles | 39 | 0.523
(0.323–0.845) | 0.008 | 0.511
(0.314–0.833) | 0.007 |
| 3-4 risk
alleles | 42 | Reference |
| Reference |
|
 | Table VII.Association between number of risk
alleles and progression-free survival in patients with advanced
gastric cancer. |
Table VII.
Association between number of risk
alleles and progression-free survival in patients with advanced
gastric cancer.
|
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|
|---|
| Alleles
combination | n | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
|
rs2065364/rs1870377 |
| 1-2 risk
alleles | 39 | 0.427
(0.260–0.701) | 0.008 | 0.427
(0.257–0.709) | 0.001 |
| 3-4 risk
alleles | 42 | Reference |
| Reference |
|
Discussion
The results from the present study demonstrated that
polymorphisms of NRP-1 and KDR genes were associated
with clinical outcome in patients with AGC. Following univariate
analysis, KDR rs1870377 AA+AT genotypes were found to be
associated with shorter PFS and OS times compared with the
wild-type TT genotype, and the KDR rs1870377 variant AA and
AT genotypes were significantly associated with poor PFS time
compared with wild-type TT genotype. Furthermore, the NRP-1
rs2065364 homozygous mutant AA genotype was significantly
associated with higher PFS and OS times compared with the GG+AG
genotypes. The genotypes of KDR rs7692971 and KDR
rs2034965 were also significantly associated with higher PFS and OS
times, respectively. Following adjustment, the KDR rs7692791
TT genotype was associated with increased PFS time compared with
the CT genotype, and the NRP-1 rs2065364 AG+GG genotypes
were associated with shorter PFS times compared with the AA
genotype. The KDR rs2034965 AA+GA genotypes were associated
with worse OS times compared with the GG genotype. The KDR
rs1870377 AA+AT genotypes were associated with shorter OS times
compared with the TT genotype. Additionally, increasing number of
risk alleles with the NRP-1 rs2065364/KDR rs1870377
combination was significantly associated with shorter OS and PFS
times. These results demonstrated that NRP-1 rs2065364,
KDR rs7692791, KDR rs2034965 and KDR rs1870377
may be considered as independent indicators of prognosis in
patients with AGC.
NRP-1 was originally found to be crucial for
neuronal axon guidance and embryonic angiogenesis, and was
identified as a novel receptor involved in angiogenesis (6–8).
Previous studies reported that the NRP-1 gene is associated
with tumorigenesis and progression. One study reported that
NRP-1 overexpression is associated with the promotion of
gastric cancer migration, invasion and growth (25). Lin et al (26) demonstrated that NRP-1 is a
novel TEA domain transcription factor target that serves a crucial
role in hepatocellular carcinoma tumorigenesis. A previous
demonstrated that NRP-1 is abnormally highly expressed in
non-small cell lung tumor tissue, and is associated with patient
prognosis (27). Another study
reported that NRP-1 affects the chemosensitivity of cancer
cells (28), Wey et al
(28) demonstrated that NRP-1
overexpression in pancreatic cancer cell lines is associated with
increased chemoresistance to gemcitabine in vitro. Yue et
al (29) reported that NRP-1
overexpression increases osteosarcoma cell survival following
exposure to doxorubicin. To the best of our knowledge, no study has
demonstrated the association between NRP-1 SNPs and cancer.
The present study confirmed that the NRP-1 rs2065364 AA
genotype was associated with increased PFS time compared with the
AG+GG genotypes. Further molecular investigation is required to
reveal the underlying mechanisms involved.
KDR (VEGFR-2) is a tyrosine kinase
receptor that can regulate signal transduction by binding to
VEGF via its extracellular domain (9). VEGF/VEGFR2 is an important
signaling pathway that can promote proliferation, survival and
migration of vascular endothelial cells and increase vascular
permeability (9,10). The cellular processes mediated by the
VEGF-VEGFR2 signaling cascade can lead to angiogenesis and
therefore regulate tumor growth and invasion, and therapeutic
resistance (10,11). Previous studies reported that
KDR gene polymorphisms are associated with clinical outcomes
in various types of cancer, including colorectal cancer, glioma,
hepatocellular carcinoma and gastric cancer. Torben et al
(30) reported that VEGFR2
1192C>T and −604T>C polymorphisms were associated with
increased microvessel density in colorectal cancer. A previous
study of glioma in the Chinese population demonstrated that three
SNPs of VEGFR2 (rs7667298, rs2305948 and rs1870377) are
correlated with an increased risk of a glioma when homozygous
(31). Another study described that
the VEGFR-2 rs2305948 T polymorphism frequency is higher in
patients with gastroenteropancreatic neuroendocrine neoplasms
compared with that in the healthy population (19). In the present study, among the
genetic variations of the VEGFR2 gene, the KDR
rs1870377 and KDR rs7692791 TT genotypes were found to be
associated with a better prognosis, and the KDR rs2034965 GG
genotype was associated with increased OS time. Zhu et al
(20) demonstrated that the
VEGFR2 rs1870377 TT genotype confers a favorable prognosis
in gastric cancer. Furthermore, Wang et al (21) investigated the correlation between
polymorphisms of four genes from the epidermal growth factor
receptor (EGFR) pathway and the clinical outcome of 363
patients with hepatocellular carcinoma, and reported that
EGFR rs2034965 with the AA genotype is negatively correlated
with disease-free survival. These results were consistent with the
results from the present study; however, inconsistent results were
reported in other types of cancer, and Kim et al (22) reported that the VEGFR2
rs1870377 TT genotype is associated with shorter OS time in
patients with diffuse large B cell lymphoma. Furthermore, it was
reported that rs7692791 C allele is significantly correlated with
increased OS and DFS in hepatocellular carcinoma (21). These discordances may be partly
attributed to the different types of cancer, the different clinical
characteristics of the patients and the study sizes. The rs1870377
mutation is located in the coding region of KDR and is a
missense mutation. The functional role of this gene polymorphism
remains unclear.
Proteins from the PDGF family are crucial to
stimulate the proliferation, survival and migration of mesenchymal
cells (32), This family consists of
5 different isoforms, named disulphide-bonded homodimers of A-, B-,
C- and D-polypeptide chains and the heterodimer PDGF-AB.
PDGFR is classified as a receptor tyrosine kinase, and the 5
PDGF isoforms can activate cellular responses via
PDGFRα and PDGFRβ (32,33).
Overactivation of the PDGF-PDGFR signaling pathway has been
reported to be associated with tumorigenesis (34). PDGFR gene mutations have been
found in malignancies. Point mutations in PDGFRα were found
in ~5% of gastrointestinal stroma tumors, which led to amino acid
residue changes, therefore activating PDGFR kinase activity
(35). In addition, a study reported
that rs1800812 T allele and rs6554162 G allele in PDGFRα
were related to decreased frequency in patients with papillary
thyroid cancer compared with that in the healthy population
(23). A previous study demonstrated
that two SNPs in PDGFβ (rs5757573 T>C and rs6001516
C>T) were associated with an increased risk of pancreatic cancer
(36). Furthermore, Volz et
al (24) found that the SNP
(rs2302273 C>T) in PDGFRβ gene was associated with a
significantly longer PFS time in patients with metastatic
colorectal cancer. However, in the present study, no relevance was
found between SNPs and prognosis.
The present study had some limitations. Firstly,
only 81 patients with AGC were eligible for statistical analysis.
Since the sample size was relatively small, the results from this
study should be considered as preliminary data and for generation
of a hypothesis for subsequent investigation. Secondly, since the
patients studied had AGC, it is not known whether the results could
be applicable to patients with other types of gastric cancer.
Further investigation should therefore be conducted to validate the
results.
In conclusion, the results from the present study
demonstrated that KDR rs7692791 and NRP-1 rs2065364
were positively associated with PFS. Furthermore, KDR
rs2034965 and KDR rs1870377 significantly negatively
correlated with OS time following multivariate analysis in patients
with AGC. In addition, the numbers of ‘risk alleles’ of
NRP-1 rs2065364/KDR rs1870377 combination were
significantly associated with survival time. These results
suggested that genetic variants in NRP-1 and KDR
genes may affect the biological features and prognosis of patients
with AGC. Due to limited funding, the underlying mechanisms were
not explored, and further investigation is required to verify these
results.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Dan Lv
(Department of Medical Oncology, The Second Hospital Affiliated to
Dalian Medical University) and Professor Na Gao (Department of
Obstetrics and Gynecology, First Affiliated Hospital of Dalian
Medical University) for their technical assistance.
Funding
No funding was recieved.
Availability of data and materials
All data and materials generated and/or used during
the study are available from the corresponding author upon
reasonable request.
Authors' contributions
TW and YS designed the study. YS collected the
patients' clinical data. YJZ and YS performed the experiments. YJZ
and YS analyzed the data and wrote the manuscript. TW contributed
to the revision of the manuscript. All the authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Research Ethics Committee of The Second Hospital Affiliated to
Dalian Medical University and was conducted in accordance with The
Declaration of Helsinki. Participants were fully informed of the
procedures and provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Li Z, Shan F, Miao R, Xue K, Li Z,
Gao C, Chen N, Gao X, Li S and Ji J: Current status of diagnosis
and treatment of early gastric cancer in China-Data from China
gastrointestinal cancer surgery union. Zhonghua Wei Chang Wai Ke Za
Zhi. 21:168–174. 2018.(In Chinese). PubMed/NCBI
|
|
3
|
Wang FH, Shen L, Li J, Zhou ZW, Liang H,
Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, et al: The Chinese
Society of Clinical Oncology (CSCO): Clinical Guidelines for the
Diagnosis and Treatment of Gastric Cancer. Cancer Commun (Lond).
39:102019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen K, Yang D, Li X, Sun B, Song F, Cao
W, Brat DJ, Gao Z, Li H, Liang H, et al: Mutational landscape of
gastric adenocarcinoma in Chinese: Implications for prognosis and
therapy. Proc Natl Acad Sci USA. 112:1107–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujisawa H, Takagi S and Hirata T:
Growth-associated expression of a membrane protein, neuropilin, in
Xenopus optic nerve fibers. Dev Neurosci. 17:343–349. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muhl L, Folestad EB, Gladh H, Wang Y,
Moessinger C, Jakobsson L and Eriksson U: Neuropilin 1 binds PDGF-D
and is a co-receptor in PDGF-D-PDGFRβ signaling. J Cell Sci.
130:1365–1378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raimondi C: Neuropilin-1 enforces
extracellular matrix signalling via ABL1 to promote angiogenesis.
Biochem Soc Trans. 42:1429–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karaman S, Leppänen VM and Alitalo K:
Vascular endothelial growth factor signaling in development and
disease. Development. 145(pii): dev1510192018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farooqi AA and Siddik ZH: Platelet-derived
growth factor (PDGF) signalling in cancer: Rapidly emerging
signalling landscape. Cell Biochem Funct. 33:257–265. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heldin CH, Lennartsson J and Westermark B:
Involvement of platelet-derived growth factor ligands and receptors
in tumorigenesis. J Intern Med. 283:16–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sa-Nguanraksa D, Kooptiwut S,
Chuangsuwanich T, Pongpruttipan T, Malasit P and O-Charoenrat P:
Vascular endothelial growth factor polymorphisms affect gene
expression and tumor aggressiveness in patients with breast cancer.
Mol Med Rep. 9:1044–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glubb DM, Cerri E, Giese A, Zhang W, Mirza
O, Thompson EE, Chen P, Das S, Jassem J, Rzyman W, et al: Novel
functional germline variants in the VEGF receptor 2 gene and their
effect on gene expression and microvessel density in lung cancer.
Clin Cancer Res. 17:5257–5267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken M, Creech R, Tormey D, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A III: AJCC Cancer Staging Manual. 7th.
Springer; New York, NY: 2010
|
|
18
|
National Cancer Institute (NCI), . Common
Terminology Criteria for Adverse Events CTCAE). Version 4.03.
2010.
|
|
19
|
Berardi R, Torniai M, Partelli S, Rubini
C, Pagliaretta S, Savini A, Polenta V, Santoni M, Giampieri R,
Onorati S, et al: Impact of vascular endothelial growth factor
(VEGF) and vascular endothelial growth factor receptor (VEGFR)
single nucleotide polymorphisms on outcome in
gastroenteropancreatic neuroendocrine neoplasms. PLoS One.
13:e01970352018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Wang Y, Xue W, Wang R, Wang L, Zhu
ML and Zheng L: The VEGFR-2 protein and the VEGFR-2 rs1870377
A>T genetic polymorphism are prognostic factors for gastric
cancer. Cancer Biol Ther. 20:497–504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Ma XP, Shi Z, Zhang P, Ding DL,
Huang HX, Saiyin HG, Chen TY, Lu PX, Wang NJ, et al: Epidermal
growth factor receptor pathway polymorphisms and the prognosis of
hepatocellular carcinoma. Am J Cancer Res. 5:396–410.
2015.PubMed/NCBI
|
|
22
|
Kim MK, Suh C, Chi HS, Cho HS, Bae YK, Lee
KH, Lee GW, Kim IS, Eom HS, Kong SY, et al: VEGFA and VEGFR2
genetic polymorphisms and survival in patients with diffuse large B
cell lymphoma. Cancer Sci. 103:497–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MJ, Kim SK, Park HJ, Chung DH, Park
HK, Lee JS, Kwon KH and Chung JH: PDGFRA promoter polymorphisms are
associated with the risk of papillary thyroid cancer. Mol Med Rep.
5:1267–1270. 2012.PubMed/NCBI
|
|
24
|
Volz NB, Stintzing S, Zhang W, Yang D,
Ning Y, Wakatsuki T, El-Khoueiry RE, Li JE, Kardosh A, Loupakis F,
et al: Genes involved in pericyte-driven tumor maturation predict
treatment benefit of first-line FOLFIRI plus bevacizumab in
patients with metastatic colorectal cancer. Pharmacogenomics J.
15:69–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
MicroRNA-338 inhibits growth, invasion and metastasis of gastric
cancer by targeting NRP1 expression. PLoS One. 9:e944222014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin J, Zhang Y, Wu J, Li L, Chen N, Ni P,
Song L and Liu X: Neuropilin 1 (NRP1) is a novel tumor marker in
hepatocellular carcinoma. Clin Chim Acta. 485:158–165. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding M, Liu L, Hu C, Liu Y, Qiao Y and
Jiang X: Expression of VEGFR2 and NRP-1 in non-small cell lung
cancer and their clinical significance. Chin J Cancer Res.
26:669–677. 2014.PubMed/NCBI
|
|
28
|
Wey JS, Gray MJ, Fan F, Belcheva A,
McCarty MF, Stoeltzing O, Somcio R, Liu W, Evans DB, Klagsbrun M,
et al: Overexpression of neuropilin-1 promotes constitutive MAPK
signalling and chemoresistance in pancreatic cancer cells. Br J
Cancer. 93:233–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yue B, Ma JF, Yao G, Yang MD, Cheng H and
Liu GY: Knockdown of neuropilin-1 suppresses invasion, angiogenesis
and increases the chemosensitivity to doxorubicin in osteosarcoma
cells-an in vitro study. Eur Rev Med Pharmacol Sci. 18:1735–1741.
2014.PubMed/NCBI
|
|
30
|
Hansen TF, Sørensen FB, Spindler KL, Olsen
DA, Andersen RF, Lindebjerg J, Brandslund I and Jakobsen A:
Microvessel density and the association with single nucleotide
polymorphisms of the vascular endothelial growth factor receptor 2
in patients with colorectal cancer. Virchows Arch. 456:251–260.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang J, Yang J, Chen Y, Mao Q, Li S,
Xiong W, Lin Y, Chen J and Ge J: Genetic variants of VEGF (rs201963
and rs3025039) and KDR (rs7667298, rs2305948, and rs1870377) are
associated with glioma risk in a Han Chinese population: A
case-control study. Mol Neurobiol. 53:2610–2618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Andrae J, Gallini R and Betsholtz C: Role
of platelet-derived growth factors in physiology and medicine.
Genes Dev. 22:1276–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor.
Physiol Rev. 79:1283–1316. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pietras K, Sjöblom T, Rubin K, Heldin CH
and Ostman A: PDGF receptors as cancer drug targets. Cancer Cell.
3:439–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heinrich MC, Corless CL, Duensing A,
McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A,
Town A, et al: PDGFRA activating mutations, in gastrointestinal
stromal tumors. Science. 299:708–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duan B, Hu J, Liu H, Wang Y, Li H, Liu S,
Xie J, Owzar K, Abbruzzese J, Hurwitz H, et al: Genetic variants in
the platelet-derived growth factor subunit B gene associated with
pancreatic cancer risk. Int J Cancer. 142:1322–1331. 2018.
View Article : Google Scholar : PubMed/NCBI
|