Introduction
Acute lymphoblastic leukemia (ALL) is a
heterogeneous hematological malignancy characterized by the
abnormal proliferation of primitive and immature cells in the bone
marrow (1). ALL is the most common
hematologic malignancy in children, accounting for 29%, and it is
relatively rare in adults (2,3). Out of
all children with ALL, 85% have B-lymphoblastic leukemia (B-ALL)
(4). It is suggested that the
development of chemoresistance is a key regulator for relapse among
children (4,5). Therefore, further understanding of the
pathogenesis of B-ALL is of great significance to improve the
survival rate of patients (6).
French-American-British (FAB) (7) and
Morphology-Immunology-Cytogenetics-Molecular Biology (MICM)
(8) classification are the two main
diagnostic methods for acute leukemia (9). The former is easy to perform and does
not require expensive instruments and equipment, but it is strongly
subjective and has low classification accuracy, which makes it
impossible to achieve standardization and automation (10,11). The
MICM typing method, which was introduced by the World Health
Organization, is more comprehensive than the simple morphological
typing method (10,11). However, the performance of MICM is
cumbersome and expensive. The MICM and FAB morphological types
require lumbar puncture to obtain test samples, which increases the
pain and economic burden of the patient (10,11).
Therefore, searching for biomarkers for noninvasive diagnosis and
typing of acute leukemia has become a research focus (12).
The discovery of microRNAs (miRNAs/miRs) represents
a milestone in furthering the understanding of human cancer
(13,14). miRNAs have been demonstrated to be
influential factors in the pathogenesis of B-ALL (15,16).
Numerous studies have identified roles of miRNAs in the diagnosis,
treatment, classification, prognosis and risk assessment of tumors
(16–18). Studies have revealed that miRNAs are
involved in the occurrence and development of acute leukemia
(18,19). For example, miR-652-3p levels are
significantly increased in the bone marrow of patients with ALL
compared with in healthy controls, and miR-652-3p enhances the
progression of ALL by decreasing apoptosis and reducing sensitivity
to chemotherapeutic drugs (18). In
addition, miR-9 inhibits ALL cancer cell proliferation and cell
cycle progression by targeting neuropilin-1 (19). A previous study demonstrated that
miR-31 expression is decreased in chronic myeloid leukemia (CML)
cells compared with in controls (20). In adult T cell leukemia, polycomb
proteins lead to miR-31 downregulation in an epigenetic manner,
thereby activating the NF-κB signaling pathway and inducing
apoptosis resistance (21). At
present, to the best of our knowledge, no publication has described
miR-31 expression in patients with B-ALL. Therefore, the present
study aimed to explore whether miR-31 is involved in the
progression of B-ALL.
In the present study, miR-31 expression was
evaluated in children with B-ALL before and after treatment, and
the possible mechanism of miR-31 in children with B-ALL was
explored.
Materials and methods
General information
Children (n=38) with newly diagnosed B-ALL
(excluding mature B-ALL), according to the MICM classification, who
were admitted to Hongqi Hospital Affiliated to Mudanjiang Medical
University between April 2017 and December 2017, were designated as
the B-ALL group. Inclusion criteria were as follows: Infants
diagnosed as B-ALL by MICM classification and peripheral blood
collected prior to chemotherapy. Exclusion criteria were as
follows: History of malignant blood disease, immune diseases and
other tumors, and history of severe infection, trauma or surgery
prior to admission. All patients met the criteria set by the
National Conference on Pediatric Leukemia (9). There were 20 male patients and 18
female patients, with an average age of 5.6 years (range,
1.2–16.5), in the B-ALL group. Among the patients, 26 were <10
years old and 12 were ≥10 years old. A risk assessment (22) was carried out, in which the risk of
leukemia was stratified according to the age of pediatric patients
at initial diagnosis, peripheral leukocyte count, abnormalities in
cytogenetics and molecular biology, whether they were sensitive to
prednisone, bone marrow remission status at 33 days and the minimal
residual disease at 33 days. Based on the aforementioned
assessment, 17 cases were classified as low risk, 10 as medium risk
and 11 as high risk (including four cases of recurrence, 2 in
medium risk and 2 in high risk). The treatment regimen was based on
the Chinese Children's Leukemia Group (CCLG)-2008 protocol
(modified Berlin-Frankfurt-Münster ALL-95 protocol) (23). Patients were followed up until
December 31, 2018, and the median follow-up time was 14.7 months
(range, 3.2–19.8 months). The 14 month-survival rate for patients
with pediatric B-ALL was 84.3%. Healthy controls (n=18) treated at
the Physical Examination Center of Hongqi Hospital Affiliated to
Mudanjiang Medical University, due to suspicion of B-ALL that was
not further confirmed, were designated as the control group. The
control group were recruited between April 2017 and December 2017,
including 10 male patients and 8 female patients, with an average
age of 6.2 years (range, 1.5–16.4).
Therapeutic regimen
All pediatric patients were treated according to the
CCLG-2008-ALL protocol; chemotherapy regimens with different
intensities were administered according to the different risk
degrees, as follows (23): i)
Remission induction: Pretreatment with prednisone for 7 days,
vincristine, daunorubicin, L-asparaginase or pegaspargase,
dexamethasone (VDLD) regimen; ii) early intensive treatment:
Cyclophosphamide, cytarabine, 6-mercaptopurine (6-MP) or
thioguanine (CAM) program (low risk group, one round; medium and
high risk groups, two rounds); iii) consolidation therapy: Low risk
and medium risk groups, HD-methotrexate (MTX) + 6-MP program (2.0
g/m2 MTX in low risk group; 5.0 g/m2 MTX in
medium risk group); high risk group, two rounds of treatment of the
aforementioned regimen; iv) delayed enhancement: The same VDLD and
CAM programs as aforementioned [two rounds of delayed enhancement
for medium risk group (one round of maintenance chemotherapy
between the two rounds of delayed enhancement)]; and v) maintenance
treatment: 6-MP + MTX. The single or triple chemotherapy drugs were
regularly injected intrathecally to prevent central nervous system
leukemia. All patients responded well to the therapeutic
regimen.
Reverse transcription-quantitative PCR
(RT-qPCR)
A total of 5 ml peripheral blood samples and 3 ml
bone marrow were collected prior to treatment, and subsequently 30
days and 12 weeks after treatment completion. In the control group,
5 ml peripheral blood samples and 3 ml bone marrow were collected
at admission at −20°C. Heparin anticoagulant was added to the bone
marrow, and Ficoll-Hypaque lymphocyte separating liquid was added.
Subsequently, mononuclear cells from bone marrow were separated by
density-gradient centrifugation at 2,000 × g at 4°C for 20 min.
Total RNA was isolated from the whole blood samples
(5 ml; collected in tubes containing EDTA) or mononuclear cells
using RNAVzol LS (Vigorous Biotechnology Beijing Co., Ltd.),
according to the manufacturer's protocol. The concentration and
purity of RNA samples were determined by measuring the optical
density (OD) 260/OD280.
A total of 1 µg RNA was reverse transcribed using
Moloney murine leukemia virus RT enzyme (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with specific primers. The temperature
protocol used for RT was as follows: 72°C for 10 min, 42°C for 60
min, 72°C for 5 min and 95°C for 2 min. To quantify the relative
expression levels, qPCR was performed using SYBR Green Supermix
(Bio-Rad Laboratories, Inc.) in an iCycleriQ real-time PCR
detection system (Bio-Rad Laboratories, Inc.). PCR amplification
was performed in a 10 µl reaction mixture containing 5 µl SYBR
Green Supermix, 0.4 µl forward primer, 0.4 µl reverse primer, 2.2
µl double-distilled H2O and 2 µl template cDNA.
Thermocycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles at 95°C for 15 sec, 60°C for 1 min, and 72°C for 15
sec. Dissolution curve with only one peak for miR-31 and U6
confirmed that there was no contamination in the experiment, which
ensured the test efficiency. Relative expression was normalized to
that of U6 using the 2−∆∆Cq method (24). Primer sequences were as follows:
miR-31-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCTATG-3′; U6-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATG-3′; miR-31,
forward 5′-GCGCAGGCAAGAUGCUGGC-3′; U6, forward
5′-GCGCGTCGTGAAGCGTTC-3′; and universal reverse primer,
5′-GTGCAGGGTCCGAGGT-3′.
Statistical analysis
Data are presented as the means ± SD. Each
experiment was performed in triplicate. SPSS version 13.0 (SPSS,
Inc.) was used to perform statistical analysis. A two-tailed
unpaired Student's t-test was used for comparisons of two groups.
One-way ANOVA followed by the Tukey post hoc test was used for
comparisons of more than two groups. A receiver operating
characteristic (ROC) curve for diagnostic B-ALL was generated, and
the area under the curve (AUC) was calculated. The AUC range is
0.5–1; it is generally considered that the AUC has low diagnostic
value when it is 0.5–0.7, medium diagnostic value when it is
0.7–0.9 and high diagnostic value when it is >0.9. P<0.05 was
considered to indicate a statistically significant difference.
Results
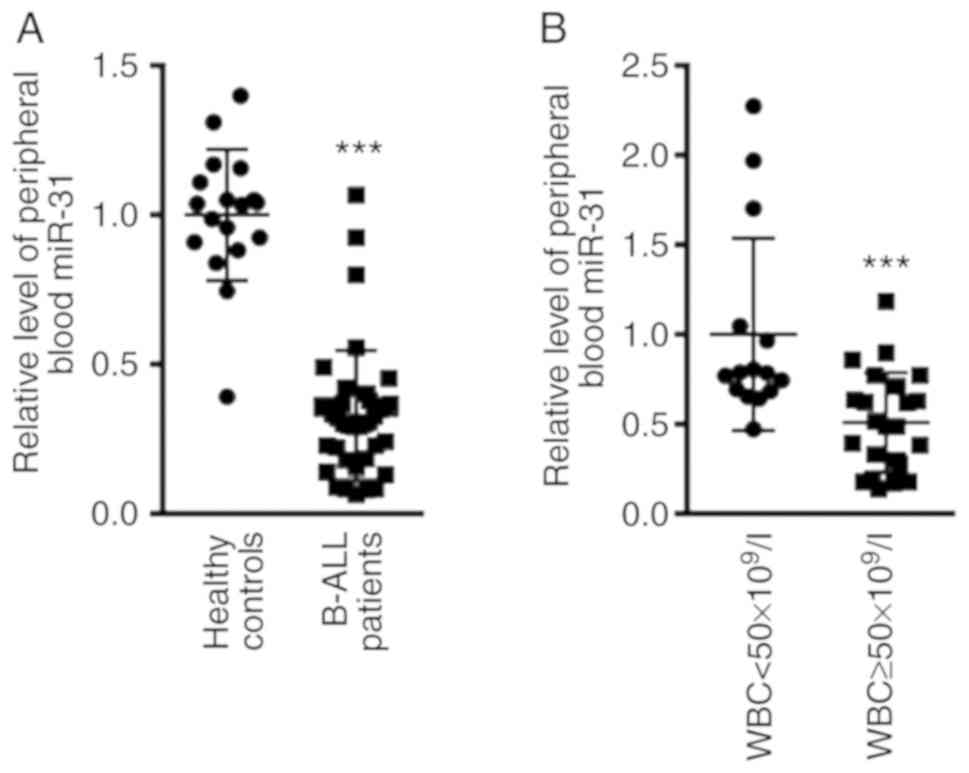
miR-31 expression is decreased in the
peripheral blood of patients with B-ALL
The expression levels of miR-31 in patients with
B-ALL before treatment were compared with those of patients in the
healthy control group. The results revealed that miR-31 expression
in the peripheral blood of patients with B-ALL was 0.33±0.21, and
that in the healthy control group was 1.00±0.36 (Fig. 1A; P<0.001). The present study
further evaluated the expression levels of miR-31 according to
white blood cell (WBC) count at initial diagnosis, by dividing
patients into WBC <50×109/l and WBC
≥50×109/l groups. As shown in Fig. 1B, the expression levels of miR-31
were significantly reduced in patients with B-ALL with WBC
≥50×109/l (0.51±0.28; n=23) compared with in patients
with WBC <50×109/l (1±0.54; n=15; P<0.001).
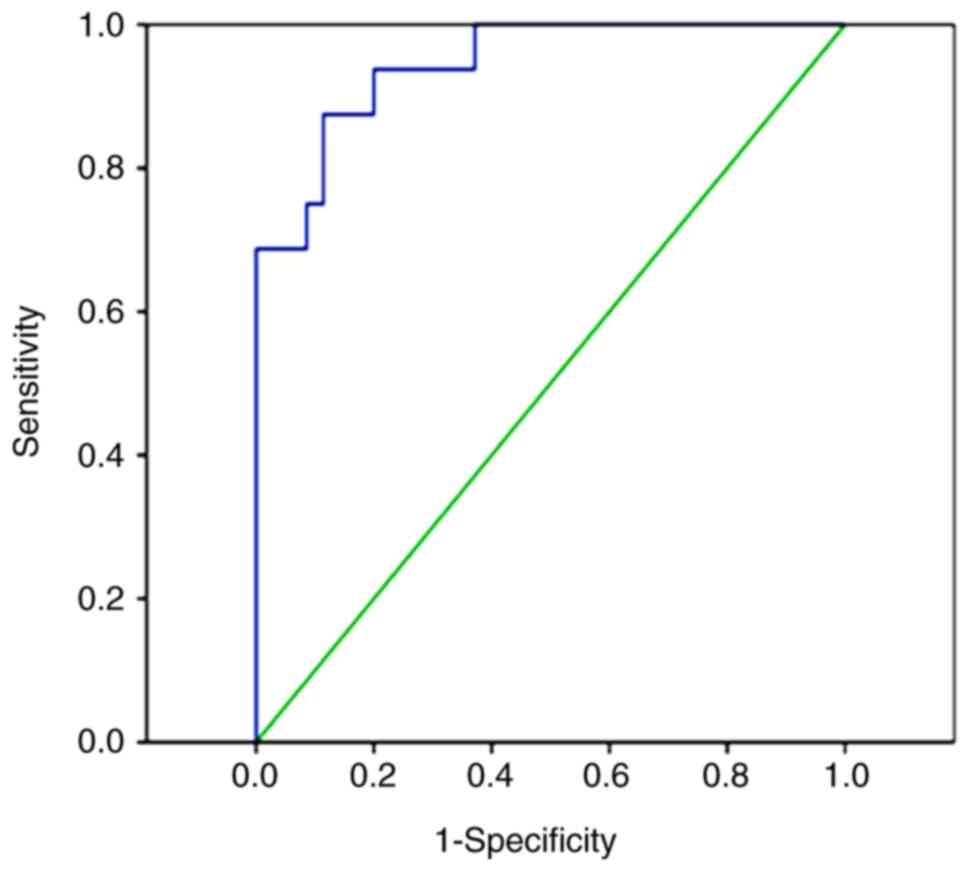
miR-31 may be used to differentiate
patients with B-ALL from healthy controls
Subsequently, a ROC curve was plotted for miR-31
expression and the diagnosis of B-ALL, and the AUC was calculated.
The present study revealed that when the cutoff value was 0.18, the
AUC of miR-31 for the diagnosis of B-ALL was 0.915 (95% CI,
0.828–1.000; P<0.0001), with a sensitivity and specificity of
80.8 and 100%, respectively (Fig.
2).
miR-31 expression is lower in patients
<10 years old
The present study further divided the patients with
B-ALL according to age, including 26 patients who were younger than
10 years and 12 who were older than 10 years. As shown in Fig. 3, the expression levels of miR-31
before treatment in the peripheral blood (0.44±0.12; n=26) and
mononuclear cells (0.38±0.15; n=26) were markedly lower in the
group of children <10 years old compared with in the group of
children >10 years old (1±0.42; n=12; and 1±0.38; n=12,
respectively).
miR-31 expression is increased in
patients with B-ALL after treatment
Furthermore, the present study compared the
expression levels of miR-31 before treatment (n=38) and after
treatment for 30 days (n=38) or 12 weeks (n=36). A total of 36
patients were analyzed in the 12 weeks group as two patients
succumbed to the disease. qPCR analysis demonstrated that miR-31
expression in peripheral blood was 0.33±0.21 before treatment,
whereas miR-31 expression was 0.68±0.17 after treatment for 30 days
and 0.92±0.28 after treatment for 12 weeks (Fig. 4A). Additionally, the data
demonstrated that miR-31 expression was lowest in mononuclear cells
in the group before treatment (0.28±0.15). Conversely, miR-31
expression gradually increased in the mononuclear cells after
treatment for 30 days (0.62±0.16) and 12 weeks (0.87±0.23; Fig. 4B).
Lowest expression levels of miR-31 are
observed in the high-risk group
Additionally, the present study evaluated the
expression levels of miR-31 before treatment according to a risk
assessment. The present study identified 17, 10 and 11 cases with
low, medium and high risk, respectively. As shown in Fig. 5, the expression levels of miR-31 were
higher in the peripheral blood (1±0.23) and mononuclear cells
(1±0.28) of the low-risk group compared with in the medium-risk
(0.61±0.18 and 0.58±0.13) and high-risk (0.35±0.16 and 0.28±0.10)
groups. No differences were identified between male and female
patients (data not shown).
Discussion
The present study first demonstrated that the
expression levels of circulating miR-31 in patients with B-ALL were
significantly decreased compared with those in the healthy control
group, suggesting that miR-31 could be used as a diagnostic marker
in B-ALL. Subsequently, a ROC curve was plotted and the AUC was
calculated. The AUC was 0.915, suggesting that miR-355 has a high
diagnostic value for B-ALL. When an optimal cutoff value of 0.18
was selected, the probability of the correct diagnosis of patients
with B-ALL based on miR-31 was 80.8%, while the probability of
excluding B-ALL was 100%. Therefore, decreased circulating miR-31
levels in patients with B-ALL may have a high diagnostic efficiency
for B-ALL.
The expression levels of miR-31 were also compared
at the different treatment stages for patients with B-ALL. Notably,
miR-31 expression gradually increased with the progression of
treatment stages, suggesting that miR-31 may improve the
progression of B-ALL in children. The present study also revealed
that the expression levels of miR-31 were higher in children with
B-ALL who were >10 years old than those in children with B-ALL
who were <10 years old, indicating that the expression levels of
miR-31 were associated with age, and suggesting that detecting the
expression levels of miR-31 may be helpful in evaluating the
prognosis of children with B-ALL. The expression levels of miR-31
prior to treatment in the different risk groups were further
compared. The data revealed that miR-31 expression in the high-risk
group was lower than that in the medium- and low-risk groups, and
the expression levels of miR-31 also differed between the medium-
and high-risk groups, indicating that when miR-31 expression was
lower, the prognosis of the children was worse.
However, there were some limitations of the present
study. Due to limitations of time and number of specimens, it
remains to be investigated as to whether miR-31 acts as an
independent factor or in combination with other pathogenic factors
in the pathogenesis of B-ALL. Additionally, miR-31 expression in T
cell acute lymphoblastic leukemia, acute myeloid leukemia or other
hematological disease samples was not analyzed. Therefore, whether
miR-31 is a specific diagnostic marker for B-ALL requires further
study.
It is well known that miRNAs can modulate the
expression of multiple target genes, thus exerting roles in
numerous signaling pathways (20).
In a previous study, Rokah et al (20) determined potential target genes and
signaling pathways that may be involved in the progression of CML.
It was suggested that Casitas B-lineage lymphoma and E2F
transcription factor 3 may be possible target genes of miR-31 in
CML initiation and progression (20). Additionally, in ALL, reduced miR-31
expression has been demonstrated to activate the NF-κB signaling
pathway and suppress cell apoptosis via inhibiting NF-κB inducing
kinase (21). The underlying
mechanism by which the occurrence of B-ALL was regulated via miR-31
remains to be further investigated by evaluating the role of these
target genes.
In conclusion, in children with B-ALL, the
expression levels of miR-31 were downregulated compared with those
in the healthy controls, and this downregulation was associated
with age of onset, risk and treatment stage. These findings
suggested that miR-31 expression may be an important prognostic
indicator in B-ALL.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
Hongqi Hospital Affiliated to Mudanjiang Medical University (grant
no. HAMDMU-20170615).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ performed the experiments and analyzed the data.
XL, LB and LL analyzed and interpreted the work. DL, XD and BW
performed the reverse transcription-quantitative PCR experiments.
CL designed the experiments, analyzed the data and gave the final
approval of the version of the manuscript to be published. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Hongqi Hospital Affiliated to Mudanjiang
Medical University (Mudanjiang, China), and all guardians of the
patients provided written informed consent for this study.
Patient consent for publication
All patients provided written informed consent for
the publication of data in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akiyama M, Yamada O, Agawa M, Yuza Y,
Yanagisawa T, Eto Y and Yamada H: Effects of prednisolone on
specifically expressed genes in pediatric acute B-lymphoblastic
leukemia. J Pediatr Hematol Oncol. 30:313–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luan C, Yang Z and Chen B: The functional
role of microRNA in acute lymphoblastic leukemia: Relevance for
diagnosis, differential diagnosis, prognosis, and therapy. Onco
Targets Ther. 8:2903–2914. 2015.PubMed/NCBI
|
|
3
|
Fang M, Becker PS, Linenberger M, Eaton
KD, Appelbaum FR, Dreyer Z, Airewele G, Redell M, Lopez-Terrada D,
Patel A, et al: Adult low-hypodiploid acute B-Lymphoblastic
leukemia with IKZF3 deletion and TP53 mutation: Comparison with
pediatric patients. Am J Clin Pathol. 144:263–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Severson EA, Vergilio JA, Gay LM, Daniel
S, Hemmerich AC, Elvin JA, Britt N, Nahas M, Cohen MB, Brown C, et
al: Genomic landscape of adult and pediatric BCR-ABL1-like
b-lymphoblastic leukemia using parallel DNA and RNA sequencing.
Oncologist. 24:372–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evensen NA, Madhusoodhan PP, Meyer J,
Saliba J, Chowdhury A, Araten DJ, Nersting J, Bhatla T, Vincent TL,
Teachey D, et al: MSH6 haploinsufficiency at relapse contributes to
the development of thiopurine resistance in pediatric
B-lymphoblastic leukemia. Haematologica. 103:830–839. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oberley MJ, Li S, Orgel E, Phei Wee C,
Hagiya A and O'Gorman MRG: Clinical significance of isolated
myeloperoxidase expression in pediatric B-Lymphoblastic leukemia.
Am J Clin Pathol. 147:374–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crist WM, Furman W and Strother D.and Pui
CH: Acute lymphocytic leukemia in childhood: Immunologic marker,
cytogenetic, and molecular studies. South Med J. 80:841–847. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Subspecialty Group of Hematology and The
Society of Pediatrics, Chinese Medical Association, . Summary of
the 16th national conference of pediatric hematology. Zhonghua Er
Ke Za Zhi (Chinese). 51:76–77. 2013.
|
|
10
|
Douet-Guilbert N, Chauveau A, Gueganic N,
Guillerm G, Tous C, Le Bris MJ, Basinko A, Morel F, Ugo V and De
Braekeleer M: Acute myeloid leukaemia (FAB AML-M4Eo) with cryptic
insertion of cbfb resulting in cbfb-Myh11 fusion. Hematol Oncol.
35:385–389. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canaani J, Beohou E, Labopin M, Socié G,
Huynh A, Volin L, Cornelissen J, Milpied N, Gedde-Dahl T, Deconinck
E, et al: Impact of FAB classification on predicting outcome in
acute myeloid leukemia, not otherwise specified, patients
undergoing allogeneic stem cell transplantation in CR1: An analysis
of 1690 patients from the acute leukemia working party of EBMT. Am
J Hematol. 92:344–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balatti V, Tomasello L, Rassenti LZ,
Veneziano D, Nigita G, Wang HY, Thorson JA, Kipps TJ, Pekarsky Y,
Croce CM, et al: miR-125a and miR-34a expression predicts Richter
syndrome in chronic lymphocytic leukemia patients. Blood.
132:2179–2182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El-Khazragy N, Elshimy AA, Hassan SS,
Matbouly S, Safwat G, Zannoun M and Riad RA: Dysregulation of
miR-125b predicts poor response to therapy in pediatric acute
lymphoblastic leukemia. J Cell Biochem. 2–Nov;2018.(Epub ahead of
print).
|
|
14
|
Gutierrez-Camino A, Umerez M,
Martin-Guerrero I, García de Andoin N, Santos B, Sastre A,
Echebarria-Barona A, Astigarraga I, Navajas A and Garcia-Orad A:
Mir-pharmacogenetics of Vincristine and peripheral neurotoxicity in
childhood B-cell acute lymphoblastic leukemia. Pharmacogenomics J.
18:704–712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Z, Liao Z, Chen S, Li B, Yu Z, Luo G,
Yang L, Zeng C and Li Y: Downregulated miR-17, miR-29c, miR-92a and
miR-214 may be related to BCL11B overexpression in T cell acute
lymphoblastic leukemia. Asia Pac J Clin Oncol. 14:e259–e265. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong Z, Zhang R and Qi H: Diagnostic and
prognostic relevance of serum miR-195 in pediatric acute myeloid
leukemia. Cancer Biomark. 21:269–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Y, Zou Y, Lin L, Ma X and Chen H:
Identification of serum miR-34a as a potential biomarker in acute
myeloid leukemia. Cancer Biomark. 22:799–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Q, Lu X, Huang P, Gao C, Zhao X,
Xing T, Li G, Bao S and Zheng H: Expression of miR-652-3p and
effect on apoptosis and drug sensitivity in pediatric acute
lymphoblastic leukemia. Biomed Res Int. 2018:57246862018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zang Y, Yu R, Bai Y and Chen X: MicroRNA-9
suppresses cancer proliferation and cell cycle progression in acute
lymphoblastic leukemia with inverse association of neuropilin-1. J
Cell Biochem. 119:6604–6613. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rokah OH, Granot G, Ovcharenko A, Modai S,
Pasmanik-Chor M, Toren A, Shomron N and Shpilberg O: Downregulation
of miR-31, miR-155, and miR-564 in chronic myeloid leukemia cells.
PLoS One. 7:e355012012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamagishi M, Nakano K, Miyake A, Yamochi
T, Kagami Y, Tsutsumi A, Matsuda Y, Sato-Otsubo A, Muto S,
Utsunomiya A, et al: Polycomb-mediated loss of miR-31 activates
NIK-dependent NF-κB pathway in adult T cell leukemia and other
cancers. Cancer Cell. 21:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wood B, Wu D, Crossley B, Dai Y,
Williamson D, Gawad C, Borowitz MJ, Devidas M, Maloney KW, Larsen
E, et al: Measurable residual disease detection by high-throughput
sequencing improves risk stratification for pediatric B-ALL. Blood.
131:1350–1359. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moricke A, Reiter A, Zimmermann M, Gadner
H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig WD, Ratei R,
et al: Risk-adjusted therapy of acute lymphoblastic leukemia can
decrease treatment burden and improve survival: Treatment results
of 2169 unselected pediatric and adolescent patients enrolled in
the trial ALL-BFM 95. Blood. 111:4477–4489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|