Introduction
Thyroid cancer is the most common malignancy of the
endocrine system and accounted for ~1% of all human malignancies in
2006 worldwide (1). In numerous
countries, the incidence of thyroid cancer has been increasing
faster than that of any other malignancy (2). According to a 2015 report by the
American Cancer Society, the probability of being diagnosed with
thyroid cancer has tripled over the last three decades, making it
the most increasingly prevalent cancer in the USA (3). Thyroid cancer consists of the following
four histological types: Papillary, follicular, medullary and
anaplastic thyroid carcinoma. Different surgical techniques
according to the tumor stage and carcinoma type are the primary
modes of therapy for thyroid cancer. Papillary thyroid carcinoma
(PTC) is the most common pathological type of this cancer,
accounting for ~80% of all cases between 1980 and 2005 in the USA
(4). Although thyroidectomy combined
with radioactive iodine treatment and chemotherapy has achieved
good clinical outcomes, recurrence and metastasis are the main
causes of mortality in patients with thyroid cancer (5,6). It is
common for PTC to metastasize to the lymph nodes (LNs) of the neck,
occurring in up to 50% of cases (7,8).
Therefore, identifying protein markers that are associated with the
recurrence, metastasis and prognosis of thyroid carcinoma is
particularly important for clinical practice. Such newly discovered
markers may also promote the continuous development of efficient
treatments for thyroid carcinoma.
Sphingosine kinase (SPHK), an ATP-dependent protein,
is expressed in various organisms such as mammals, insects,
invertebrates, yeast strains and plants. In mammals, SPHK has been
identified to consist of two isozymes, SPHK1 and SPHK2. SPHK1
catalyzes the phosphorylation of sphingosine to produce
sphingosine-1-phosphate (S1P), which is a potent lipid mediator
involved in a variety of physiological processes, including
angiogenesis, cell proliferation, apoptosis, motility and migration
(9). Ceramides and sphingosine, the
metabolic precursors of S1P, are known to possess regulatory
functions in cell apoptosis (10).
Thus, the balance between these precursors and S1P within the cell
has been suggested to function as a switch that drives the decision
between cell proliferation and death (11). However, the key regulator of this
switch is SPHK1, which converts sphingosine into prosurvival S1P.
Previous studies have demonstrated that the activity of SPHK1 is
associated with anti-phagocytosis, cell transformation,
proliferation and the survival of tumor cells (12,13). In
addition, SPHK1 mRNA is overexpressed in a number of types of solid
tumor, including those of the stomach, rectum, kidney, breast,
colon, ovary, uterus and lung (14).
Other studies have demonstrated that high expression levels of
SPHK1 in breast cancer and glioblastoma are associated with poor
patient prognosis, suggesting that SPHK1 functions as a promoter of
cancer in human tumors (15,16). Furthermore, it has been reported that
high levels of SPHK1 expression were associated with clinical
stages, locoregional recurrence and distant metastasis of
nasopharyngeal carcinoma (17).
Therefore, SPHK1 may serve distinct roles in different tumor
types.
However, to the best of our knowledge, few
comprehensive studies exist that have investigated the expression
and significance of SPHK1 with respect to the long-term prognosis
of patients with thyroid carcinoma. To date, whether SPHK1 is
involved in the recurrence of thyroid carcinoma remains unknown.
Therefore, the aim of the present study was to investigate the
expression of SPHK1 in human PTC tissue samples using
immunohistochemistry (IHC), and its association with clinical
characteristics and pathological parameters. In addition, the
effect of SPHK1 positivity on the recurrence and prognosis of
patients with PTC was investigated, assessing its effectiveness as
a potential novel prognostic molecular marker for the management of
PTC in the clinical setting.
Materials and methods
Tissue specimens
The protocol and tissue specimen collection from
human participants for the present study was approved by the
Medical Institutional and Clinical Research Ethics Committee of
Cangzhou Central Hospital (Cangzhou, China). Formalin-fixed
paraffin-embedded tissue microarrays consisting of 92 samples of
PTC were used in this study. The tissue specimens were collected
during thyroidectomies from Cangzhou Central Hospital, and the
tissue microarrays with these specimens were produced by the
Alenabio and Shrbio Biological Technology companies. The patients
included in the study met the following criteria: i)
Histopathologic diagnosis of PTC; ii) complete clinical data; and
iii) patients underwent a total or near-total thyroidectomy for
PTC. Written informed consent was previously obtained from the 92
patients diagnosed with PTC who received surgical treatment.
Patients who had received radiotherapy or neoadjuvant therapy prior
to the surgery were excluded from the study. All patients were
followed up, and their clinicopathological parameters and survival
times were obtained. The follow-up was conducted primarily via
outpatient reviews, telephone, mail and home visit for at least 120
months.
The PTC tumors were staged based on the current
Tumor-Node-Metastasis (TNM) classification system (18), and the patients underwent
post-operative radioactive iodine ablation, according to the
guidelines of the American Thyroid Association (19). Clinical data, including sex, age,
tumor size, thyroid capsular invasion (TCI), extrathyroidal
extension (ETE), histological type, LN status, distant metastasis,
vascular invasion and recurrence state, were retrieved for all
patients, as were complete follow-up details.
IHC
PTC tissue microarray slides were deparaffinized in
xylene and rehydrated with 70, 80, 90 and 100% graded ethanol
solutions, and then, the endogenous peroxidase activity was
inhibited by a 0.3% hydrogen peroxide solution. The sections were
immersed in sodium citrate buffer (10 mM sodium citrate; 0.05%
Tween-20; pH 6.0) at 105°C for the antigen retrieval. The sections
were then incubated with 5% goat serum (Solarbio Biotechnology,
Inc.) to block the non-specific background peroxidase activity at
room temperature for 10 min. Subsequently, the slides were
incubated with a rabbit polyclonal anti-human SPHK1 primary
antibody (1:100; cat. no. ab71700; Abcam) at 4°C overnight. The
sections were then incubated with goat anti-rabbit secondary
antibody (1:1,000; cat. no. ab7090; Abcam) at 37°C for 15 min, and
the results were visualized using a diaminobenzidine
tetrahydrochloride detection kit (Santa Cruz Biotechnology, Inc.)
according to the manufacturer's protocol. Finally, the sections
were rinsed for 5 min in PBS, counterstained with 2.5% hematoxylin
for 5 min at room temperature and rinsed again with tap water. For
negative controls, the antibody was replaced by PBS. Images were
captured with an Olympus BX42 light microscope (Olympus
Corporation; magnification, ×40).
Evaluation of staining
IHC was performed as aforementioned (20). A total of five high-power fields of
view of the tumor sections were randomly selected, and 500 cells in
each field were counted to determine the labeling index, which
represents the estimated percentage of positive cells relative to
the total cells. Sections with <5% labeled cells were scored as
0; sections with 5–30% labeled cells were scored as 1; 31–70%
labeled cells were scored as 2; and sections with ≥71% labeled
cells were scored as 3. Staining intensity was evaluated similarly,
with a score of 0 for negative staining; 1 for weak positive; 2 for
moderate positive; and 3 for strong positive staining. The scores
of the percentage and intensity were added, and final scores of 0–1
indicated negative expression (−), 2–3 indicated weak expression
(+), 4–5 indicated moderate expression (++) and 6 indicated strong
expression (+++). The sections were evaluated by two pathologists
from Cangzhou Central Hospital blinded to the study. For the
statistical analysis, staining was classified into high-(score,
4–6) and low-(score, 0–3) expression groups.
Cell culture and transfection
The human PTC TPC-1 cell line was purchased from the
Cell Bank of the Chinese Academy of Science. The TPC-1 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE
Healthcare Life Sciences) supplemented with 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.), and were maintained
in an incubator at 37°C with a humidified atmosphere containing 5%
CO2.
For overexpression of the SPHK1 gene, pcDNA3.1
vector was purchased from Thermo Fisher Scientific (cat. no.
V79020) and pcDNA3.1-SphK1 was purchased from Shanghai GenePharma
Co., Ltd. The pcDNA3.1-SPHK1 plasmid was constructed as previously
described (21). The empty vector
pcDNA3.1 plasmid was transfected into the cells as a negative
control. The PTC TPC-1 cells were seeded in 6-well plates at
density of 5×104 cells per well. The cells were
transfected with the pcDNA3.1-SPHK1 plasmid once they had reached
~80% confluence. To transfect the cells, 2 µg plasmid and 6 µl
Lipofectamine® 3000 solution (Invitrogen; Thermo Fisher
Scientific, Inc.) were briefly mixed and incubated with the cells
for 5 min at room temperature. The overexpression experiment was
conducted in OptiMEM (Invitrogen) for 48 h at 37°C and 2 ml DMEM
was then added to the cell culture. The transfection efficiency was
confirmed by western blotting.
For SPHK1 knockdown, small interfering RNA (siRNA)
targeting SPHK1 and negative control scrambled siRNA were designed
and synthesized by Guangzhou RiboBio Co., Ltd. (SPHK1-siRNA,
forward, 5′-GGGCAAGGCCUUGCAGCUCd(TT)-3′ and reverse,
5′-GAGCUGCAAGGCCUUGCCCd(TT)-3′; and scrambled siRNA, forward,
5′-UUCUCCGAACGUGUCACGUd(TT)-3′ and reverse,
5′-ACGUGACACGUUCGGAGAAd(TT)-3′). The TPC-1 cells were seeded on
6-well plates at an initial density of 5×104 cells per
well and were then transfected using 50 nM
Lipofectamine® RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.) reagent for 5 min at room temperature, according
to the manufacturer's protocol. The concentration of siRNA duplexes
was 100 nmol/l. The cells were cultured in OptiMEM (Invitrogen) for
24 h at 37°C and 2 ml DMEM was then added to the cell culture. The
transfection efficiency was confirmed with western blotting.
Western blotting
To detect protein expression, TPC-1 cells that had
been transfected for 24 h were washed in PBS and lysed with
radioimmunoprecipitation assay buffer (50 mM TrisHCl pH 7.4; 150 mM
NaCl; 2 mM EDTA; 1% NP-40; 0.1% SDS). The protein concentrations
were determined using a bicinchoninic acid protein quantitation
assay kit (Beyotime Institute of Biotechnology). Protein (20 µg)
were separated by 10% SDS-PAGE and transferred onto a PVDF nylon
membrane (Merck KGaA) at 4°C. Membranes were blocked for 30 min in
5% non-fat powdered milk at room temperature. After washing with
TBS/Tween-20 (TBST) three times for 5 min each, the membranes were
incubated with the anti-SPHK1 primary antibody (1:100; cat. no.
ab71700; Abcam) for 8 h at 4°C. The membranes were then washed
three times with TBST for 10 min each to remove free primary
antibody. Mouse anti-GAPDH antibody (1:1,000 dilution; cat. no.
ab8245; Abcam) was used as the loading control. After further
washing, the membranes were incubated in a horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody (1:1,000;
cat. no. sc2004; Santa Cruz Biotechnology, Inc.) solution for 1 h
at room temperature. All blots were visualized using an enhanced
chemiluminescence solution (Merck KGaA) and analyzed using Quantity
One Protein Analysis software version 4.6.7 (Bio-Rad Laboratories,
Inc.).
Proliferation and invasion assay
Cell proliferation was assessed using a Cell
Counting Kit-8 (CCK-8; MedChemExpress) according to the
manufacturer's protocol. Briefly, the TPC-1 cells were seeded into
96-well plates at a density of 1×103 cells per well (200
µl per well). Transfected TPC-1 cells were cultured for 24 h and
used for proliferation assay. In order to detect the relative cell
proliferation rate, CCK-8 reagent was added to the medium and
incubated for 3 h at 37°C in 5% CO2. Then, a microplate
reader was utilized to measure the absorbance at 450 nm. Each
experiment was performed at least three times.
Transwell inserts were used to assess the cell
invasion ability. The extent of cell invasion was assessed using a
BD BioCoat™ Matrigel™ invasion chamber with 8-µm pores (BD
Biosciences), according to the manufacturer's protocol. In brief,
for the invasion assay, 3×104 transfected cells in 200
µl serum-free DMEM were seeded in the upper chambers of
Matrigel-coated Transwell plates. Complete DMEM containing 10% FBS
was added to the lower chambers. The Transwell chambers were then
incubated at 37°C for 48 h. The upper chamber was washed with PBS
and cells were fixed with pure methanol for 15 min at room
temperature. Subsequently, the cells were stained with 0.1% crystal
violet for 30 min at room temperature. The stained cells were
viewed under a light microscope (magnification, ×40), images were
captured and the cell number was counted in five random sights.
Colony-formation assay
Cells in the logarithmic growth phase
(1×103 cells/well) were plated into 6-well plates.
Transfected TPC-1 cells were culture for 24 h before
colony-formation assay. Following incubation in DMEM with 10% FBS
at 37°C for 2 weeks, the cells were washed with PBS and fixed with
pure methanol for 30 min at room temperature, and colonies were
stained with 0.1% crystal violet at room temperature for 10 min.
Images were captured with an Olympus BX42 light microscope
(magnification, ×40). The number of clones (≥50 cells were
considered to be a colony) was recorded using Image J software
version 1.4 (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using the SPSS
statistical software package (version 20.0; IBM Corp.). The SPHK1
protein relative expression levels, PTC cell proliferation rate,
invasion cell numbers and colony numbers were analyzed using a
one-way ANOVA followed by a Fisher's least significant difference
post-hoc test. A χ2 test was performed to analyze the
association between SPHK1 expression and the clinicopathological
characteristics of patients. Univariate and multivariate regression
analysis was performed using the Cox proportional hazards
regression model to determine the effects of potential risk factors
on survival. Survival analysis was carried out using the
Kaplan-Meier method and tested with the log-rank test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The present study included samples from 92 patients
with PTC. A total of 66 patients (71.7%) were female, and the mean
age of the cohort was 49.2 years (range, 19–77 years). The
characteristics of the patients are presented in Table I. A total of 53 patients (57.6%) had
tumors ≤2 cm in diameter, and the mean tumor size was 1.9 cm
(range, 0.1–4.4 cm). More patients presented without TCI than with
it (69.6% vs. 30.4%) and nearly half presented with ETE (48.9%).
Regional LN metastasis was reported as absent in the majority of
patients (73.9%; 68/92). A total of 65 individuals were diagnosed
with stage I–II (70.7%), and 27 (29.3%) with more advanced stages
of the disease. The proportions of patients positive for distant
metastasis, vascular invasion and recurrence were 13.0, 28.3 and
14.1%, respectively.
 | Table I.Characteristics of the patients with
papillary thyroid carcinoma and associations with high (n=35) and
low (n=57) SPHK1 expression level. |
Table I.
Characteristics of the patients with
papillary thyroid carcinoma and associations with high (n=35) and
low (n=57) SPHK1 expression level.
|
|
| SPHK1
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases, n (%) | Low, n (%) | High, n (%) | P-value |
|---|
| Age, years |
|
|
| 0.262 |
|
≤46 | 41 (44.6) | 28 (49.1) | 13 (37.1) |
|
|
>46 | 51 (55.4) | 29 (50.9) | 22 (62.9) |
|
| Sex |
|
|
| 0.138 |
|
Male | 26 (28.3) | 13 (22.8) | 13 (37.1) |
|
|
Female | 66 (71.7) | 44 (77.2) | 22 (62.9) |
|
| Tumor size, cm |
|
|
| 0.001 |
| ≤2 | 53 (57.6) | 47 (82.5) | 6 (17.1) |
|
|
>2 | 38 (41.3) | 10 (17.5) | 29 (82.9) |
|
| TCI |
|
|
| 0.118 |
|
Negative | 64 (69.6) | 43 (75.4) | 21 (60.0) |
|
|
Positive | 28 (30.4) | 14 (24.6) | 14 (40.0) |
|
| ETE |
|
|
| 0.096 |
|
Negative | 47 (51.1) | 33 (57.9) | 14 (40.0) |
|
|
Positive | 45 (48.9) | 24 (42.1) | 21 (60.0) |
|
| LN metastasis |
|
|
| 0.004 |
|
Negative | 68 (73.9) | 48 (84.2) | 20 (57.1) |
|
|
Positive | 24 (26.1) | 9 (15.8) | 15 (42.9) |
|
| Distant
metastasis |
|
|
| 0.005 |
|
Negative | 80 (87.0) | 54 (94.7) | 26 (74.3) |
|
|
Positive | 12 (13.0) | 3 (5.3) | 9 (25.7) |
|
| TNM stage |
|
|
| 0.001 |
|
I–II | 65 (70.7) | 48 (84.2) | 17 (48.6) |
|
|
III–IV | 27 (29.3) | 9 (15.8) | 18 (51.4) |
|
| Vascular
invasion |
|
|
| 0.001 |
|
Negative | 66 (71.7) | 49 (86.0) | 17 (48.6) |
|
|
Positive | 26 (28.3) | 8 (14.0) | 18 (51.4) |
|
| Recurrence |
|
|
| 0.012 |
|
None | 79 (85.9) | 53 (93.0) | 26 (74.3) |
|
| Local
and distant | 13 (14.1) | 4 (7.0) | 9 (25.7) |
|
Association between SPHK1 expression
and clinicopathological characteristics
The present study aimed to determine the expression
patterns of this protein in PTC tumors. First, the protein
expression levels of SPHK1 in PTC tissues were assessed using IHC
on tissue microarrays that comprised of a total of 92 clinical
samples. Representative IHC images exhibiting weak or strong
staining for SPHK1 are presented in Fig.
1. SPHK1 protein was predominantly detected in the cytoplasm of
tumor cells.
According to the percentage of positively stained
cells and the staining intensity of the PTC sections, the samples
were classified into low- and high-expression groups (Table I). The χ2 test was used to
analyze the association between the characteristics of the patients
with PTC and SPHK1 expression. Increased SPHK1 expression in PTC
tissue was revealed to be significantly associated with tumor size,
LN metastasis, TNM stage, distant metastasis, vascular invasion and
recurrence (all P<0.05). However, no association was observed
between SPHK1 expression and age, sex, TCI or ETE occurrence (all
P>0.05).
SPHK1 expression is associated with
the prognosis of patients with PTC
Since high expression levels of SPHK1 were
associated with larger tumor size and advanced TNM stage, it was
hypothesized that this may play an important role in predicting the
clinical outcome of patients with PTC. Therefore, the association
between disease-free survival (DFS) time of patients and SPHK1
expression was analyzed using Kaplan-Meier analysis and the
log-rank test. The results revealed that the patients that
exhibited high SPHK1 expression levels had significantly shorter
DFS times than those with low expression (mean DFS time, 80.3±6.9
months vs. 103.9±3.7; P=0.003; Fig.
2H). The prognostic value of the other clinical characteristics
were also analyzed. A tumor size of ≥2 cm (P=0.009; Fig. 2E), LN metastasis (P=0.017; Fig. 2F), advanced TNM stage (P=0.006;
Fig. 2G), distant metastasis
(P=0.044; Fig. 2I), vascular
invasion (P=0.013; Fig. 2J) and
recurrence (P=0.003; Fig. 2K) were
all revealed to be unfavorable prognostic factors. However, no
significant association was observed between prognosis and age,
sex, TCI or ETE status (all P>0.05; Fig. 2A-D).
Subsequently, the independent hazard effect of each
factor was identified using a univariate and multivariate Cox
regression analysis model (Table
II). Univariate Cox regression analysis indicated that tumor
size, TNM stage, distant metastasis, vascular invasion, recurrence
state and SPHK1 expression were significantly associated with
disease-free survival time of patients with PTC (P<0.05;
Table II). Furthermore, the
multivariate Cox regression analysis demonstrated that tumor size,
TNM stage, distant metastasis, vascular invasion, recurrence state
and SPHK1 expression were all independent prognostic factors for
these patients (all P<0.05; Table
II).
 | Table II.Univariate and multivariate Cox
regression analyses of disease-free survival in patients with
papillary thyroid carcinoma. |
Table II.
Univariate and multivariate Cox
regression analyses of disease-free survival in patients with
papillary thyroid carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≤46 years vs.
>46 years) | 0.868 | 0.487–1.302 | 0.441 | – | – | – |
| Sex (male vs.
female) | 0.770 | 0.369–1.227 | 0.335 | – | – | – |
| TCI (positive vs.
negative) | 1.562 | 0.937–2.210 | 0.148 | – | – | – |
| ETE (positive vs.
negative) | 1.173 | 0.775–1.650 | 0.292 | – | – | – |
| LN metastasis
(positive vs. negative) | 1.631 | 0.880–2.936 | 0.351 | – | – | – |
| Tumor size (>2
cm vs. ≤2 cm) | 4.148 | 2.134–6.279 | 0.002 | 2.568 | 1.224–5.387 | 0.013 |
| TNM stage (I–II vs.
III–IV) | 3.539 | 1.763–5.195 | 0.003 | 2.493 | 1.382–4.330 | 0.010 |
| Distant metastasis
(positive vs. negative) | 2.448 | 1.639–3.760 | 0.013 | 1.636 | 1.275–2.994 | 0.037 |
| Vascular invasion
(positive vs. negative) | 2.918 | 1.720–4.889 | 0.001 | 2.330 | 1.378–4.195 | 0.008 |
| Recurrence (local
and distant vs. none) | 2.483 | 1.704–3.955 | 0.005 | 1.878 | 1.135–3.014 | 0.012 |
| SPHK1 (high vs.
low) | 4.980 | 2.156–9.562 | 0.001 | 3.649 | 1.584–7.371 | 0.004 |
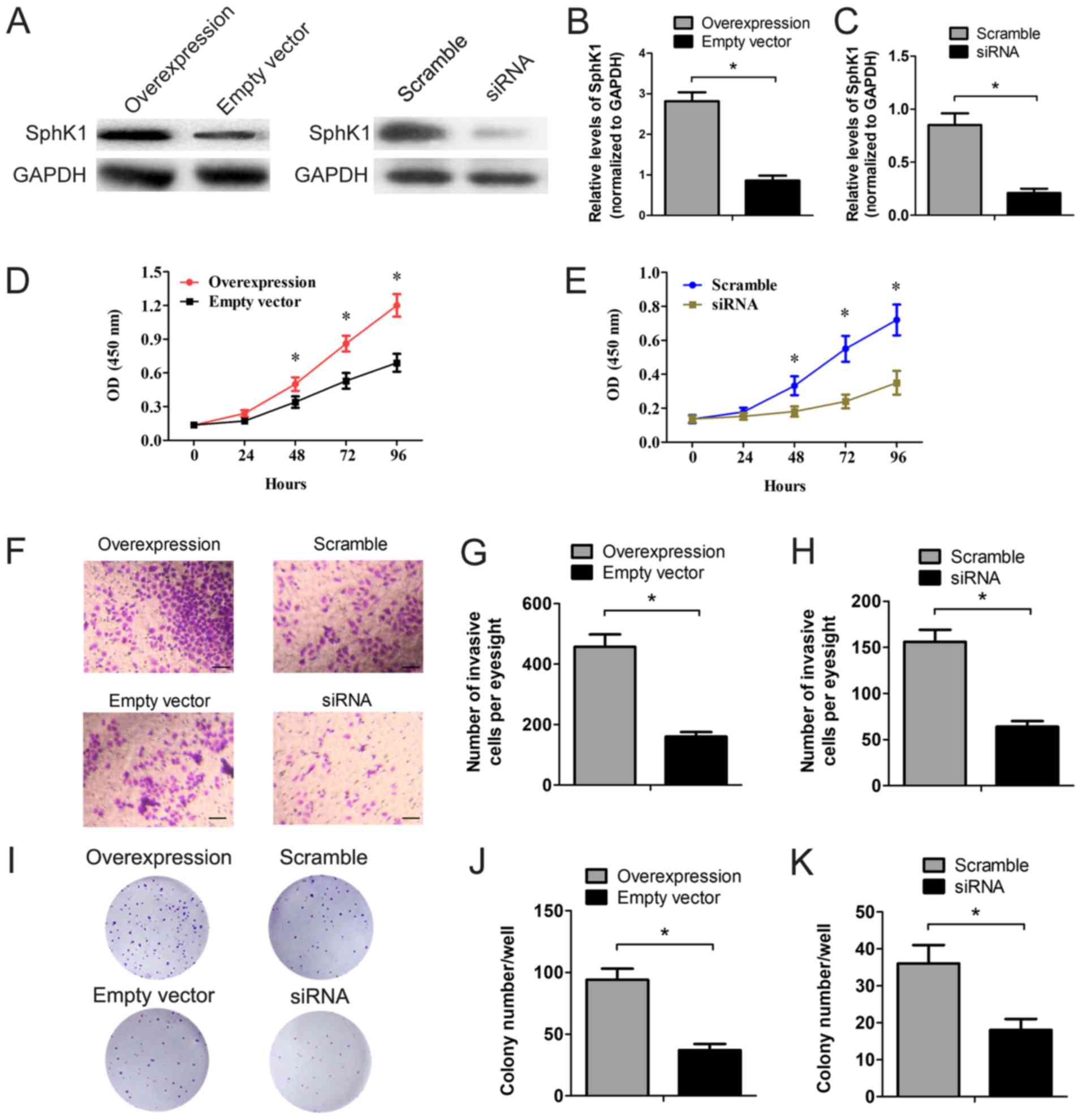
SPHK1 directly promotes PTC cell
proliferation and invasion
In order to investigate the role of SPHK1 in PTC
cell proliferation and invasion, SPHK1 was overexpressed and
downregulated in TPC-1 cells using the pcDNA3.1-SPHK1 plasmid and
SPHK1-siRNA transfection. As presented in Fig. 3A-C, the protein level of SPHK1 was
significantly higher in TPC-1 cells transfected with the
overexpression plasmid compared with the corresponding empty
vector-transfected TPC-1 cells (control), and significantly lower
in the cells transfected with the siRNA compared with those
transfected with the scrambled siRNA control. A CCK-8 assay was
performed to test cell proliferation, which demonstrated a
significantly enhanced proliferation pattern in SPHK1
overexpressing cells (Fig. 3D). In
contrast, TPC-1 cells transfected with SPHK1 siRNA exhibited a
strong decrease in cell viability (Fig.
3E). In addition, cell invasion was evaluated using the
Transwell Matrigel invasion assay in vitro. Overexpression
of SPHK1 significantly increased the invasiveness of TPC-1 cells by
~2.9 times more than the control group. Cells with SPHK1 inhibition
exhibited a 53% decrease in invasion capacity (Fig. 3F-H). Finally, colony-formation assays
were also used to investigate the role of SPHK1 in TPC-1 cell
survival. Fig. 3I-K demonstrate that
the ability of TPC-1 cells to form colonies was significantly
altered in cells with overexpressed or silenced SPHK1.
Colony-formation number increased significantly in TPC-1 cells
overexpressing SPHK1 compared with the empty vector control group,
and decreased significantly on the knockdown of SPHK1.
Discussion
Thyroid cancer is one of the most common endocrine
tumors diagnosed worldwide, with an estimated annual incidence of
12.2 cases per 100,000 individuals in the USA (22). PTC is the most common pathological
type of thyroid carcinoma, and is generally characterized by an
improved prognosis compared with other subtypes of thyroid
carcinoma; however, the recurrence rate of PTC following the
initial surgical treatment has been reported to vary by 8–23%
(23,24). Therefore, investigating the
underlying molecular mechanisms and various clinical factors
associated with recurrence is essential in order to develop more
effective diagnosis and treatment strategies for PTC. Cumulative
data have revealed that SPHK1 is involved in cell proliferation,
cell survival and tumorigenesis, and that it acts as a predictor of
unfavorable prognosis in breast and colorectal cancers (16,25).
However, little is known about its role in the pathophysiology and
clinical practice of PTC. In the present study, SPHK1 expression
levels in human PTC tissue were investigated, in addition to its
association with clinico- and histopathological features. The role
of SPHK1 in the proliferation and invasion of PTC cells was also
identified.
SPHK1 has previously been demonstrated to be
upregulated in prostate and kidney cancers (12,26).
Consistent with these previous reports, the data from the present
study demonstrated that SPHK1 was upregulated in 38% of the
participating patients with PTC, according to tissue microarrays,
and that it was significantly associated with tumor size, LN
metastasis and TNM stage. Furthermore, multivariate analyses of DFS
revealed that high SPHK1 expression levels, a large tumor size and
an advanced TNM stage were independent predictive risk factors for
DFS, indicating a higher disease recurrence risk. These results
suggest that SPHK1 plays tumor-promoting roles in PTC. A previous
study demonstrated that SPHK1 is capable of promoting cell invasion
and proliferation in human hepatocellular carcinoma (27). Other in vivo studies have also
confirmed that SPHK1 is involved in increasing sacral chordoma and
esophageal carcinoma rapid cell growth in addition to spontaneous
metastasis (28,29). Consistent with these results, the
present study demonstrated that heterogenous overexpression of
SPHK1 could enhance cell proliferation and invasion processes,
while SPHK1 silencing impaired cell viability and invasion. These
results provide new direction for drug discovery and tumor clinical
therapy.
SPHK1 can be activated by a variety of growth
factors, cytokines and mitogens, such as platelet-derived growth
factor (PDGF), tumor necrosis factor-α, and vascular endothelial
growth factor (VEGF) (30,31). Pitson et al (32) reported that the phosphorylation of
SPHK1 and its translocation from the cytoplasm to the cell membrane
may be the key process responsible for inducing the malignant
phenotype of cells. This process not only promotes the
proliferation of malignant cells, but also protects the apoptotic
pathway from being destroyed, thereby producing carcinogenic
effects. Altering the subcellular location of SPHK1 exerts marked
effects on cell function, with cell membrane-translocated SPHK1
exhibiting a potent inhibitory effect on the G1-S phase
transition in 3T3-L1 fibroblasts, suggesting that the localization
of SPHK1 in cells may have an effect on tumor cell apoptosis.
Ogretmen (33) determined the
metabolism of ceramide for S1P biosynthesis, which is mediated by
SPHK1 and −2, and its role in influencing cancer cell growth, drug
resistance and tumor metastasis through S1PR-dependent or
receptor-independent signaling. In the present study, it was
observed that overexpression of SPHK1 promotes the malignant
biological behavior of TPC-1 cells. Recently, increasing evidence
has indicated that SPHK1 acts as an enzyme involved in
carcinogenesis (34,35). The potential underlying molecular
mechanisms of SPHK1 in the pathogenesis of PTC may include its
enzymatic activity of upregulating S1P. S1P, a bioactive lipid,
potentially contributes to tumorigenesis. S1P can bind to a family
of G protein-coupled receptors (S1PRs) to induce cellular responses
such as the survival, proliferation, apoptosis and migration of
cancer cells (35).
SPHK1 overexpression functions as a prognostic
marker for judging the survival time of patients with different
types of cancer (36). However, the
roles of SPHK1 have not been extensively investigated, particularly
in the long-term DFS time of patients with PTC. To this effect, the
present study investigated the associations between SPHK1
expression levels and the clinical characteristics of patients with
PTC, and the results revealed a significant association between
SPHK1 expression and tumor size, clinical stage and LN metastasis.
Notably, there was a significant association between a shorter DFS
time of patients with PTC and high SPHK1 expression, suggesting
that SPHK1 may be a useful prognostic marker for patients with PTC.
The prognostic role of SPHK1 has also been revealed in numerous
types of cancer, including astrocytoma, breast cancer and gastric
cancer, suggesting that patients with low levels of SPHK1
expression have longer overall survival time, while those with
higher SPHK1 expression levels survived for shorter durations
(16,37,38). In
addition, Rosa et al (39)
reported that SPHK1 overexpression contributes to cetuximab
resistance in human colorectal cancer models. Furthermore,
multivariate analysis of the results from the present study
confirmed that SPHK1 expression was an independent factor for
predicting DFS of patients with PTC. Consistent with the
observations in the present study, additional studies demonstrated
that SPHK1 upregulation was an independent prognostic factor for
nasopharyngeal carcinoma, salivary gland carcinoma, breast cancer
and hepatocellular carcinoma (16,17,40).
However, the present study had certain limitations, including a
lack of an intensive investigation of the molecular mechanisms
underlying the associations between SPHK1 expression and tumor
occurrence and metastasis in patients with PTC. Another limitation
of the present study is the lack of investigation of S1P, which is
released by SPHK1 activity and binds to S1P receptors, which has
been demonstrated to participate in the epithelial-to-mesenchymal
transition of a number of different types of tumor (41). Future studies must elucidate the
potential molecular mechanisms underlying SPHK1 in PTC, using a
much larger sample size and longer follow-up times.
In summary, the present study demonstrated that
increased SPHK1 expression levels were significantly associated
with the progression and poor prognosis of patients with PTC,
indicating that SPHK1 may represent a novel and valuable predictor
for the prognosis of patients with PTC. In addition, the present
study demonstrated that overexpression of SPHK1 with the
pcDNA3.1-SPHK1 plasmid results in the enhanced malignant behavior
of PTC cells in vitro. Further studies on the possible
molecular mechanisms of SPHK1 participation in PTC tumor
progression may ultimately lead to the development of a new potent
anti-PTC strategy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hebei
Cangzhou Science and Technology Plan Project of China (grant no.
131302136).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL designed the study. JL, BZ, YB, YL, BZ and JJ
performed the experiments and analyzed the data. JL and BZ wrote
the manuscript. BZ and JJ helped to revise the manuscript. All
authors read and approved the final version of the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The protocol for the present study was approved by
the Medical Institutional and Clinical Research Ethics Committee of
Cangzhou Central Hospital (Cangzhou, China). All patients included
in the present study previously provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Al-Brahim N and Asa SL: Papillary thyroid
carcinoma: An overview. Arch Pathol Lab Med. 130:1057–1062.
2006.PubMed/NCBI
|
|
2
|
Nikiforova MN and Nikiforov YE: Molecular
genetics of thyroid cancer: Implications for diagnosis, treatment
and prognosis. Expert Rev Mol Diagn. 8:83–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enewold L, Zhu K, Ron E, Marrogi AJ,
Stojadinovic A, Peoples GE and Devesa SS: Rising thyroid cancer
incidence in the United States by demographic and tumor
characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev.
18:784–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang LY and Ganly I: Nodal metastases in
thyroid cancer: Prognostic implications and management. Future
Oncol. 12:981–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asimakopoulos P, Nixon IJ and Shaha AR:
Differentiated and medullary thyroid cancer: Surgical management of
cervical lymph nodes. Clin Oncol (R Coll Radiol). 29:283–289. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grant CS: Recurrence of papillary thyroid
cancer after optimized surgery. Gland Surg. 4:52–62.
2015.PubMed/NCBI
|
|
8
|
Giusca SE, Amalinei C, Lozneanu L, Ciobanu
Apostol D, Andriescu EC, Scripcariu A, Balan R, Avadanei ER and
Căruntu ID: Heterogeneous periostin expression in different
histological variants of papillary thyroid carcinoma. Biomed Res
Int. 2017:87013862017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spiegel S and Milstien S: Functions of the
multifaceted family of sphingosine kinases and some close
relatives. J Biol Chem. 282:2125–2129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat Rev Mol
Cell Biol. 4:397–407. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Green JA, Suzuki K, Cho B, Willison LD,
Palmer D, Allen CD, Schmidt TH, Xu Y, Proia RL, Coughlin SR and
Cyster JG: The sphingosine 1-phosphate receptor S1P2
maintains the homeostasis of germinal center B cells and promotes
niche confinement. Nat Immunol. 12:672–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malavaud B, Pchejetski D, Mazerolles C, de
Paiva GR, Calvet C, Doumerc N, Pitson S, Rischmann P and Cuvillier
O: Sphingosine kinase-1 activity and expression in human prostate
cancer resection specimens. Eur J Cancer. 46:3417–3424. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bayerl MG, Bruggeman RD, Conroy EJ, Hengst
JA, King TS, Jimenez M, Claxton DF and Yun JK: Sphingosine kinase 1
protein and mRNA are overexpressed in non-Hodgkin lymphomas and are
attractive targets for novel pharmacological interventions. Leuk
Lymphoma. 49:948–954. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
French KJ, Schrecengost RS, Lee BD, Zhuang
Y, Smith SN, Eberly JL, Yun JK and Smith CD: Discovery and
evaluation of inhibitors of human sphingosine kinase. Cancer Res.
63:5962–5969. 2003.PubMed/NCBI
|
|
15
|
Van Brocklyn JR, Jackson CA, Pearl DK,
Kotur MS, Snyder PJ and Prior TW: Sphingosine kinase-1 expression
correlates with poor survival of patients with glioblastoma
multiforme: Roles of sphingosine kinase isoforms in growth of
glioblastoma cell lines. J Neuropathol Exp Neurol. 64:695–705.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu YJ, You H, Tan JX, Li F, Qiu Z, Li HZ,
Huang HY, Zheng K and Ren GS: Overexpression of sphingosine kinase
1 is predictive of poor prognosis in human breast cancer. Oncol
Lett. 14:63–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Tian Z, Qin H, Li N, Zhou X, Li J,
Ni B and Ruan Z: High expression of sphingosine kinase 1 is
associated with poor prognosis in nasopharyngeal carcinoma. Biochem
Biophys Res Commun. 460:341–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tran B, Roshan D, Abraham E, Wang L,
Garibotto N, Wykes J, Campbell P and Ebrahimi A: An analysis of the
American Joint Committee on Cancer 8th edition T staging system for
papillary thyroid carcinoma. J Clin Endocrinol Metab.
103:2199–2206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Schlumberger M,
Sawka AM, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Zhang W, Yan W, Han L, Zhang K,
Shi Z, Zhang J, Wang Y, Li Y, Yu S, et al: The putative tumor
suppressor miR-524-5p directly targets Jagged-1 and Hes-1 in
glioma. Carcinogenesis. 33:2276–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HG, Cao B, Zhang LX, Song N, Li H,
Zhao WZ, Li YS, Ma SM and Yin DJ: KLF2 inhibits cell growth via
regulating HIF-1a/Notch-1 signal pathway in human colorectal cancer
HCT116 cells. Oncol Rep. 38:584–590. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen QT, Lee EJ, Huang MG, Park YI,
Khullar A and Plodkowski RA: Diagnosis and treatment of patients
with thyroid cancer. Am Health Drug Benefits. 8:30–40.
2015.PubMed/NCBI
|
|
23
|
Popadich A, Levin O, Lee JC, Smooke-Praw
S, Ro K, Fazel M, Arora A, Tolley NS, Palazzo F, Learoyd DL, et al:
A multicenter cohort study of total thyroidectomy and routine
central lymph node dissection for cN0 papillary thyroid cancer.
Surgery. 150:1048–1057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartl DM, Mamelle E, Borget I, Leboulleux
S, Mirghani H and Schlumberger M: Influence of prophylactic neck
dissection on rate of retreatment for papillary thyroid carcinoma.
World J Surg. 37:1951–1958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bae GE, DO SI, Kim K, Park JH, Cho S and
Kim HS: Increased sphingosine kinase 1 expression predicts distant
metastasis and poor outcome in patients with colorectal cancer.
Anticancer Res. 39:663–670. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bouquerel P, Gstalder C, Müller D, Laurent
J, Brizuela L, Sabbadini RA, Malavaud B, Pyronnet S, Martineau Y,
Ader I and Cuvillier O: Essential role for SphK1/S1P signaling to
regulate hypoxia-inducible factor 2a expression and activity in
cancer. Oncogenesis. 5:e2092016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao M, Chen Z, Xu Y, Zhao Y, Zha R, Huang
S, Liu L, Chen T, Li J, Tu H and He X: Sphingosine kinase 1
promotes tumour cell migration and invasion via the S1P/EDG1 axis
in hepatocellular carcinoma. Liver Int. 32:331–338. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan J, Tao YF, Zhou Z, Cao BR, Wu SY,
Zhang YL, Hu SY, Zhao WL, Wang J, Lou GL, et al: An novel role of
sphingosine kinase-1 (SPHK1) in the invasion and metastasis of
esophageal carcinoma. J Transl Med. 9:1572011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang K, Chen H, Wu G, Chen K and Yang H:
High expression of SPHK1 in sacral chordoma and association with
patients' poor prognosis. Med Oncol. 31:2472014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia P, Wang L, Moretti PA, Albanese N,
Chai F, Pitson SM, D'Andrea RJ, Gamble JR and Vadas MA: Sphingosine
kinase interacts with TRAF2 and dissects tumor necrosis
factor-alpha signaling. J Biol Chem. 277:7996–8003. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wattenberg BW, Pitson SM and Raben DM: The
sphingosine and diacylglycerol kinase superfamily of signaling
kinases: Localization as a key to signaling function. J Lipid Res.
47:1128–1139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pitson SM, Xia P, Leclercq TM, Moretti PA,
Zebol JR, Lynn HE, Wattenberg BW and Vadas MA:
Phosphorylation-dependent translocation of sphingosine kinase to
the plasma membrane drives its oncogenic signalling. J Exp Med.
201:49–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ogretmen B: Sphingolipid metabolism in
cancer signalling and therapy. Nat Rev Cancer. 18:33–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nema R, Vishwakarma S, Agarwal R, Panday
RK and Kumar A: Emerging role of sphingosine-1-phosphate signaling
in head and neck squamous cell carcinoma. Onco Targets Ther.
9:3269–3280. 2016.PubMed/NCBI
|
|
35
|
Pyne S and Pyne NJ: Sphingosine
1-phosphate signalling in mammalian cells. Biochem J. 349:385–402.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Safadi-Chamberlain F, Wang LP, Payne SG,
Lim CU, Stratford S, Chavez JA, Fox MH, Spiegel S and Summers SA:
Effect of a membrane-targeted sphingosine kinase 1 on cell
proliferation and survival. Biochem J. 388:827–834. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li W, Yu CP, Xia JT, Zhang L, Weng GX,
Zheng HQ, Kong QL, Hu LJ, Zeng MS, Zeng YX, et al: Sphingosine
kinase 1 is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 15:1393–1399. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Guan HY, Gong LY, Song LB, Zhang N,
Wu J, Yuan J, Zheng YJ, Huang ZS and Li M: Clinical significance of
sphingosine kinase-1 expression in human astrocytomas progression
and overall patient survival. Clin Cancer Res. 14:6996–7003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosa R, Marciano R, Malapelle U, Formisano
L, Nappi L, D'Amato C, D'Amato V, Damiano V, Marfè G, Del Vecchio
S, et al: Sphingosine kinase 1 overexpression contributes to
cetuximab resistance in human colorectal cancer models. Clin Cancer
Res. 19:138–147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang F and Wu Z: Sphingosine kinase 1
overexpression is associated with poor prognosis and oxaliplatin
resistance in hepatocellular carcinoma. Exp Ther Med. 15:5371–5376.
2018.PubMed/NCBI
|
|
41
|
Brizuela L, Ader I, Mazerolles C, Bocquet
M, Malavaud B and Cuvillier O: First evidence of sphingosine
1-phosphate lyase protein expression and activity downregulation in
human neoplasm: Implication for resistance to therapeutics in
prostate cancer. Mol Cancer Ther. 11:1841–1851. 2012. View Article : Google Scholar : PubMed/NCBI
|