Introduction
Colorectal cancer (CRC) is the third most common
cancer in men (1,006,000 cases, 10.6% of the total) and the second
in women (795,000 cases, 9.2% of the total) worldwide (1). CRC is the second leading tumor
diagnosis in women and in men in the Czech Republic (2). Patient outcome strongly depends on the
stage of tumor at the time of diagnosis and, in metastatic disease,
the prognosis depends on localization and extent of distant
metastases (3). Early detection and
diagnosis still present the best chance for successful treatment
and improved outcome. Early detection of metastatic liver disease
is important for indication of liver surgery. Therefore, novel
biomarkers for early cancer detection and early detection of
metastatic disease are strongly needed.
Heat shock protein 60 (HSP60) belongs to a
functional superfamily which is highly conserved during evolution
and play important roles in protein folding and translocation
(4,5). Hsp60 can be considered as a protein
with moonlighting functions (6).
Abnormalities in expression level have been detected from different
diseased tissues including inflammatory diseases and various
cancers. The HSP60 acts as an autoantigen in the development of a
range of autoimmune diseases including Hashimoto's thyroiditis
(7), inflammatory bowel diseases
(8) and chronic obstructive
pulmonary diseases (9). HSP60 is
also implicated in the cell survival and apoptosis signaling
pathways (10). An increased level
of HSP60 has been detected in colon cancer (11,12),
breast cancer (13), prostate
(14) and others. In patients with
CRC HSP60 levels have been correlated with tumor grade and stage
and with occurrence of lymph node metastases (15).
A few studies have suggested that circulating Hsp60
protein level has potential value in early detection of CRC
(12,16) Serum levels in patients with CRC were
compared to standard serum tumor markers by Hamelin et al
(16), but the majority of studied
patients had localized CRC (only 36 patients with distant
metastasis).
Chitinase-3-like protein 1 (CHI3L1) also known as
YKL-40, is a highly conserved glycoprotein produced by cancer cells
(including CRC cells), macrophages and neutrophils and by fetal and
embryonic stem cells (17–19). CHI3L1 regulates VEGF and plays an
important role in angiogenesis (19–21) and
inflammation (17,22), including inflammation-associated
carcinogenic changes of colonic epithelial cells (23,24).
Furthermore, YKL-40 participates in the activation of Akt signaling
pathways in these cells (25), in
cell proliferation and differentiation (18,19), and
in apoptosis (26). Serum
concentration of CHI3L1 is emerging as a new biomarker in patients
with CRC (17,27) High serum CHI3L1 levels from the
general population are associated with an increased risk of
development (27,28) and death from gastrointestinal cancer
(29). In addition, high serum
CHI3L1 levels before and after operation for CRC are independent
prognostic biomarkers of short overall survival (30–32).
IGFBP-2 is an extracellular protein that binds IGF-2
and, with a smaller affinity, IGF-1 (33). Tumor growth is assisted by various
growth factors, and insulin-like growth factors (IGF-1 and IGF-2)
are among the most important (34,35).
They stimulate cell proliferation, regulate differentiation, and
prevent apoptosis. A majority of the IGFs are bound to IGFBPs.
Their release is dependent on the rate of IGFBP proteolysis and may
also induce IGF-independent effects after interaction with specific
cell membrane structures (36,37).
IGFBP-3 is the principal binding protein in healthy persons, but in
patients with CRC the expression of IGFBP-2 may become dominant
(38). Activation of the type 1
receptor (IGF-1R) by binding of IGFs is the key point in triggering
intracellular metabolic and mitogenic mechanisms. IGFs are
recognized as mitogens for colon mucosa (39). IGFBP-2 plays an important role in
heat shock protein 27-mediated cancer progression and metastasis
(40). IGFBP-2 serum levels are
significantly elevated in patients with prostate cancer (41), colon cancer (28,42,43),
lung cancer (44) and others.
In the present study, we investigated the serum
levels of HSP60, CHI3L1 and IGFBP-2 in patients with metastatic CRC
compared to healthy controls. This is the first study that compares
the levels of these markers to standard biomarkers used in the
monitoring of CRC (CEA and cancer antigen 19-9 (CA19-9)) and that
also studies the correlation of tumor characteristics and treatment
outcomes to the serum levels of HSP60, CHI3L1 and IGFBP-2.
Materials and methods
Patients and healthy control
characteristics
Between November 2011 and May 2013, 97 patients with
metastatic CRC and 79 relatively age- and gender matched healthy
individuals were enrolled in this study. Serum samples were
collected before beginning treatment for mCRC (n=52) or at the time
of progression during palliative treatment for mCRC (n=45). The
majority of patients were after resection of primary tumour
(79.4%). In the control group 79 healthy controls after negative
colonoscopy were included (45.6% women, median age 62 years).
Table I shows the characteristics of
the patients and healthy controls.
 | Table I.Basic characteristics of patients and
healthy controls. |
Table I.
Basic characteristics of patients and
healthy controls.
|
Characteristics | Cancer group
(N=97) | Control group
(N=79) |
|---|
| Age |
|
|
|
Median | 64.4 | 61.5 |
|
<50 | 13 (13.4%) | 24 (30.1%) |
|
>50 | 84 (86.6%) | 55 (69.6%) |
| Sex |
|
|
|
Male | 60 (61.9%) | 43 (54.4%) |
|
Female | 37 (38.1%) | 36 (45.6%) |
| Histological
type |
|
|
|
Adenocarcinoma | 91 (93.8%) | – |
|
Mucinous carcinoma | 6 (6.2%) | – |
| Primary site |
|
|
| Right
colon (caecum, ascendens, transversum) | 20 (20.6%) | – |
| Left
colon (descendens, sigmoideum, rectosigma) | 50 (51.5%) | – |
|
Rectum | 27 (27.9%) | – |
| Side of
metastasis |
|
|
|
Liver | 64 (66.0%) | – |
|
Lung | 31 (32.0%) | – |
|
Peritoneal | 14 (14.4%) | – |
|
Lymphatic nodules | 22 (22.7%) | – |
| Number of
metastatic sides |
|
|
| 1 | 57 (58.7) | – |
| 2 | 28 (28.9) |
|
| 3 or
more | 12 (12.4) | – |
| Number of previous
treatment line |
| – |
| 0 | 52 (53.6) | – |
| 1 | 26 (26.8) | – |
| 2 | 19 (19.6) | – |
All patients and controls had adequate liver and
renal function (transaminases <2× and creatinine clearance
<1,5× upper normal limit) and signed informed consent.
In the cancer group the serum levels of CEA and
CA19-9 (median (25–75% percentile); N=97) were 26.1 (4.3–82.9) ug/l
and 25.2 (9.95–372.8) kIU/l, respectively. Both markers were
significantly elevated compared to the control group (N=79),
difference was counted by Mann-Whitney U test (P<0.001; P=0.006,
respectively) (45).
Laboratory analyses
Blood for laboratory analyses was collected after
overnight fasting via puncture of the cubital vein simultaneously
with blood collection for routine examinations. Routine biochemical
parameters were measured in fresh samples. For special parameters,
blood was standardly centrifuged for 10 min at 3,000 rpm (rotations
per minute) and serum was stored at −80°C until analysis. Levels of
HSP60 (StressMarq Biosciences), CHI3L1 (R&D Systems) and
IGFBP-2 (Mediagnost) in serum samples were determined by
commercially available ELISA (enzyme linked immunoassay) kits
according to the manufacturer's protocols. The intraassay (or
interassay) coefficient of variation (CV) was always less than 10%.
Concentrations of CEA and CA19-9 in serum samples were determined
by chemiluminiscent immunoanalysis (Architect, Abbott, USA).
Statistical analysis
Statistical analysis was performed using SAS (SAS
Institute Inc., Cary, NC, USA). Basic statistics were calculated
for parameters measured in the whole group and in different groups
and subgroups. Selected statistical data were also graphically
processed via Box & Whisker plot diagrams. A non-parametric
analysis of variance Mann-Whitney U test was used for comparison of
the distribution of the individual parameters in the different
groups and subgroups [median (25–75% percentile)] and a
Kruskal-Wallis test followed by Mann Whitney U with Bonferroni's
correction was used in case of comparing 3 subgroups. Due to
non-Gaussian distribution of variables Spearman's correlation
coefficient was used to determine the dependency of characters.
Statistical significance was determined at the border of
alpha=0.05. Receiver operating characteristic (ROC) curves were
generated to assess the diagnostic accuracy of each parameter; the
sensitivity and specificity of optimum cut off point were found.
Overall survival was defined as the interval between serum
collection and death or the last follow-up.
Results
Baseline characteristic of patients
and controls
In the cancer group serum 97 patients with
generalized CRC were included (38.1% women, median age 64 years).
The most common site of metastases was liver in 66.0% and lung in
32.0% patients. In the control group 79 healthy controls after
negative colonoscopy were included (45.6 women, median age 62
years).
Serum levels of HSP60, CHI3L1 and
IGFBP-2
The serum level of HSP60 in the cancer group was
significantly increased compared to healthy controls [mean was 0.51
(25–75% percentile 0.34–0.86) ng/ml and 0.25 (0.18–0.34) ng/ml,
respectively, P<0.001, Fig. 1A].
In the control group there was no significant difference in HSP60
levels between men and women (P=0.844), but there was significant
difference in younger than or equal to 55 years [N=24, 0.20
(0.15–0.30) ng/ml] and older than 55 [N=55; 0.27 (0.22–0.35) ng/ml;
P=0.021]. Compared to the same aged group with CRC there was
significant difference, P<0.001 and <0.001, respectively. In
the cancer group there was no significant difference in serum level
of HSP60 between sex, age and part of colon with primary tumor
(Table II). Insinuate difference
(P=0.084) was found between good or moderately and poorly
differentiated tumors. No statistically significant differences
were found between good or moderately (N=75) and poorly
differentiated tumors (N=22, P=0.309) and patients with liver
metastases (N=64) and those without (N=33; P=0.217). Positive
association was found between patients with non-resected primary
tumor (N=20) and patients after resection (N=77; P=0.039) Negative
association was identified between patients with and without
pulmonary metastases [0.42 (0.25–0.63) ng/l, N=31; 0.52 (0.37–1.22)
ng/ml, N=66; P=0.010].
 | Table II.Serum HSP-60, CHI3L1 and IGFBP-2
levels in the different groups (difference was calculated by
Mann-Whitney U test). |
Table II.
Serum HSP-60, CHI3L1 and IGFBP-2
levels in the different groups (difference was calculated by
Mann-Whitney U test).
|
| Serum HSP60
(ng/ml) | Serum CHI3L1
(ng/ml) | Serum IGFBP-2
(ng/ml) |
|---|
|
|
|
|
|
|---|
| Variable | Median | 25–75%
percentile | P-value | Median | 25–75%
percentile | P-value | Median | 25–75%
percentile | p-value |
|---|
| Age (n=97),
years |
|
|
|
|
|
|
|
| 0.289 |
| ≤55
(n=24) | 0.51 | 0.36–1.02 | 0.878 | 125.0 | 93.0–227.5 | 0.025a | 551.3 | 373.8–843.6 |
|
| >55
(n=73) | 0.51 | 0.34–0.85 |
| 195.5 | 125.3–609.0 |
| 634.9 | 435.6–1026.0 |
|
| Sex, |
|
|
|
|
|
|
|
| 0.870 |
| Female
(n=37) | 0.52 | 0.35–1.19 | 0.329 | 153.0 | 78.0–384.0 | 0.114 | 622.9 | 377.5–1006.0 |
|
| Male
(n=60) | 0.45 | 0.34–0.82 |
| 194.5 | 112.8–593.5 |
| 613.4 | 448.5–955.3 |
|
| Primary tumor
localization |
|
|
|
|
|
|
|
|
|
| Right
colon (n=20) (caecum, ascendens, transversum) | 0.56 | 0.38–1.13 | 0.367a >0.99 vs. leftb | 211.0 | 113.3–573.3 | .179a >0.99 vs. leftb | 578.9 | 290.0–725.4 | 0.322a 0.401 vs. leftb |
| Left
colon (n=50) (descendens, sigmoideum, rectosigma) | 0.52 | 0.38–1.10 | 0. 571 vs.
rectumb | 190.5 | 106.5–593.5 | 0.227 vs.
rectumb | 639.3 | 447.0–1029.0 | >0.99 vs.
rectumb |
|
Sigmoideum, rectosigma, rectum
(n=27) | 0.46 | 0.31–0.87 | 0.754 vs.
rightb | 127.0 | 73.0–334.0 | 0.512 vs.
rightb | 677.2 | 404.1–975.0 | 0.817 vs.
rightb |
| Degree of
differentiation |
|
|
|
|
|
|
|
|
|
| Well or
moderate (n=75) | 0.50 | 0.35–0.78 | 0.307 | 188.0 166.0 | 105.0–592.0 | 0.410 | 613.4 | 435.5–954.0 | 0.743 |
| Poorly
(n=21) | 0.63 | 0.33–1.21 |
|
| 92.5–568.5 |
| 714.0 | 374.9–1204.0 |
|
| Primary tumor |
|
|
|
|
|
|
|
|
|
|
Presence (n=20) | 0.64 | 0.45–1.28 | 0.039a | 181.5 | 119.8–898.8 | 0.214 | 869.1 | 432.0–1063.0 | 0.190 |
| Absence
(n=77) | 0.46 | 0.34–0.77 |
| 177.0 | 99.0–402.0 |
| 565.7 | 424.0–913.2 |
|
| Liver
metastases |
|
|
|
|
|
|
|
|
|
|
Presence (n=64) | 0.52 | 0.36–1.20 | 0.217 | 180.0 | 102.0–628.5 | 0.568 | 613.4 | 435.6–1035.0 | 0.328 |
| Absence
(n=33) | 0.43 | 0.34–0.62 |
| 177.0 | 100.0–278.0 |
| 608.3 | 417.2–851.1 |
|
| Lung
metastases |
|
|
|
|
|
|
|
|
|
|
Presence (n=31) | 0.42 |
| 0.010a | 165.0 | 96.0–221.0 | 0.132 | 551.3 | 436.0–743.1 | 0.300 |
| Absence
(n=66) | 0.52 |
|
| 188.0 | 110.3–604.5 |
| 677.2 | 413.5–1031.0 |
|
The serum level of CHI3L1 in the cancer group was
177.0 (102.0–582.0) ng/ml and was significantly elevated compared
to 76.0 (50.0–109.0) ng/ml in the healthy control group (Fig. 1B, P<0.001). In the control group,
there was no significant difference in CHI3L1 levels between men
and women (P=0.927), between younger than or equal to 55 years
(N=24) and older than 55 (N=55; P=0.108). In the cancer group there
was no significant difference in CHI3L1 levels between men and
women (P=0.114), but there was significant difference in younger
than or equal to 55 years (P=0.025, Table I).
In the cancer group, there was no significant
difference between part of colon with primary tumor, tumor grade
and presence or absence of the primary tumor. No statistically
significant differences between patients with liver metastases were
found (P=0.568). There was indicated difference in presence or
absence of pulmonary metastases (P=0.132). The group of patients
with liver metastases without pulmonary metastases was divided into
patients with the sum of the longest dimension of liver metastases
smaller than 100 millimeters (N=20), larger than or equal to 100
millimeters (N=29) and control group (N=79). The difference in
CHI3L1 levels was statistically significant (P<0.001 by
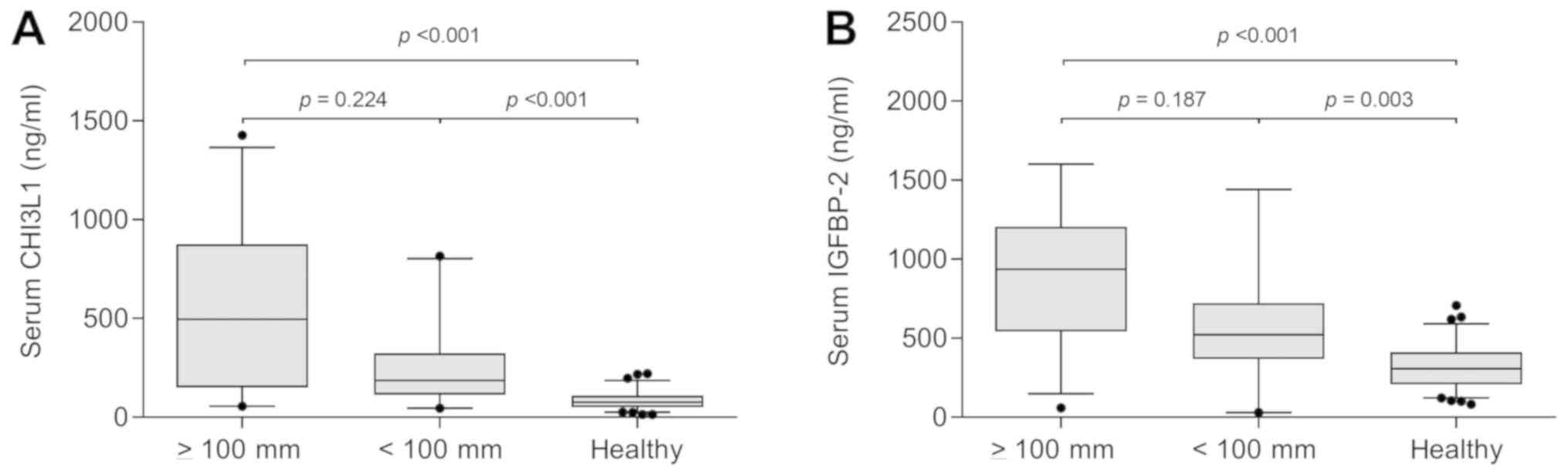
Kruskal-Wallis test), 496.0 (136.5–876.0) mg/ml, 188.0
(111.0–293.0) mg/ml and 76.0 (51.0–109.0), respectively (Fig. 2A).
The serum level of IGFBP-2 in the cancer group was
613.4 (427.9–968.6) ng/ml and was significantly elevated compared
to 308.1 (219.6–417.8) ng/ml in the healthy control group (Fig. 1C, P<0.001). In the control group
there was no significant difference in IGFBP-2 levels between men
and women (P=0.486), between younger than or equal to 55 years
(N=24) and older than 55 (N=55; P=0.189). Compared to the same aged
group with CRC there were significant differences, P<0.001 and
<0.001, respectively.
In the cancer group there were no significant
differences between sex, age, part of colon with primary tumor and
tumor grade (Table I). There was a
slight but non-statistically significant difference between
presence or absence of primary tumor, 869.1 (432.0–1063.0) ng/ml
(N=28) and 565.7 (424.0–913.2) ng/l (N=69) respectively (P=0.190).
No statistically significant differences between patients with and
without liver metastases (P=0.328) and with and without pulmonary
metastases (P=0.300).
The group of patients with liver metastases without
pulmonary metastases was divided into patients with the sum of the
longest dimension of liver metastases smaller than 100 millimeters
(N=20) and larger than or equal to 100 millimeters (N=29). and
control group (N=79). The difference in CHI3L1 levels was
statistically significant (P<0.001 by Kruskal-Wallis test),
935.5 (542.1–1204.0) ng/ml, 522.5 (368.6–722.0) ng/ml and 307.0
(207.0–411.8), respectively (Fig.
2B).
Sensitivity and specificity of HSP60, CHI3L1 and
IGFBP-2. ROC curve analysis showed that serum HSP60 with an AUC of
0.856 (Fig. 3A) and serum level
cut-off values of 0.42 ng/ml has the sensitivity and specificity to
distinguish CRC from healthy controls at a rate of 62.89 and
94.74%, respectively (P<0.001). CHI3L1 with an AUC of 0.808
(Fig. 3B) and serum level cut-off
values of 195.0 ng/ml has a sensitivity and specificity rate of
47.42 and 93.67%, respectively (P<0.001). IGFBP-2 with an AUC of
0.798 (Fig. 3C) and serum level
cut-off values of 630.0 ng/ml has a sensitivity and specificity
rate of 48.96 and 94.52%, respectively (P<0.001).
The level of HSP60, CHI3L1, IGFBP-2
and CEA as prognostic factor
The serum level of HSP60 and IGFBP-2 appears to have
a prognostic value. The cut-off for serum HSP60 level was
determined as 0.42 ng/ml. Patients were divided into groups with
negative serum levels of HSP60 (<0.42 ng/ml, N=41), slight
increase (0.42 and ≤0.84 ng/ml, N=33) and large increase (≥0.84
ng/ml, N=23). From all 97 patients overall survival (OS, time from
first sample collection to death) was calculated. No significant
differences (P=0.610) between groups with negative HSP60 levels
(mOS 17.2 months) and groups with slight increase (mOS 13.7 months)
were observed. But there were statistically significant differences
between the groups with negative (mOS 17.2 months) and large
increase of HSP60 serum levels (mOS 5.0 months, P<0.001;
Fig. 4A).
The cut-off for serum CHI3L1 level was determined as
195.0 ng/ml. Patients were divided into groups with negative serum
levels of HSP60 (<195.0 ng/ml, N=37), slight increase (195.0 and
<390.0 ng/ml, N=27) and large increase (>390.0 ng/ml, N=33).
No significant differences (P=0.284) between the groups with
negative CHI3L1 levels (mOS 13.7 months) and with slight increase
(mOS 12.9 months) were observed nor between the groups with
negative (mOS 13.7 months) and large increase of CHI3L1 serum
levels (mOS 9.7 months, P=0.680; Fig.
4B).
The cut-off for serum IGFBP-2 level was determined
as 630.0 ng/ml. Patients were divided into groups with negative
serum levels of IGFBP-2 (<630.0 ng/ml, N=51), slight increase
(630.0 and <1,260.0 ng/ml, N=34) and large increase (≥1,260.0
ng/ml, N=12). A statistically significant difference (P=0.012)
between the groups with negative HSP60 levels (mOS 16.4 months) and
with slight increase (mOS 9.6 months) was observed. There was also
a statistically significant difference between the groups with
negative (mOS 16.4 months) and large increase of IGFBP-2 serum
levels (mOS 4.5 months, P=0.007; Fig.
4C).
The cut-off level for serum CEA was determined as
5.0 ug/l. Patients were divided into groups with negative serum
levels of CEA (<5.0 ng/ml, N=27), slight increase (5.0 and
<10.0 ng/ml, N=8) and large increase (≥10.0 ng/ml, N=62). No
significant differences (P=0.089) between the groups with negative
HSP60 levels (mOS 17.2 months) and slight increase (mOS 10.2
months) were observed, nor between the groups with negative (mOS
17.2 months) and large increase of CEA serum levels (mOS 10.4
months, P=0.199; Fig. 4D).
Discussion
Early diagnosis is crucial for successful treatment
even in metastatic CRC with liver involvement. Therefore, new
biological markers for early detection (more sensitive and specific
than CEA and CA19-9) and predictors of prognosis for CRC are
urgently needed in clinical practice.
HSP60 is a key factor involved in inflammation, and
serum HSP60 levels might also be increased in patients with
inflammatory pathologies such as Crohn's disease and ulcerative
colitis (46). Only one previous
study has evaluated the application of HSP60 in the diagnosis and
prognosis of CRC. Hamelin et al published results from 152
patients with localized CRC and 130 healthy controls. HSP60 AUC was
0.7, which was identical to CEA in this group. They suggested that
levels are higher in stage IV compared to I–III, but there is no
information about the number of samples in each stage (16). Our data show similar results of
sensitivity and specificity compared to CEA (AUC 0.856, and 0.905
respectively) in patients with metastatic disease. These results
suggested that serum HSP60 could be a useful biomarker in CRC. In
addition, we present a strong correlation between HSP60 and patient
survival.
CHI3L1 (Chitinase 3-like 1, YKL-40), a highly
conserved glycoprotein produced by cancer cells (including CRC
cells), seems to be a new biomarker in patients with cancer
(16,26). High serum CHI3L1 levels among the
general population are associated with an increased risk of
development (27,28) and death from gastrointestinal cancer
(29). In addition, high serum
CHI3L1 levels before and after operation for CRC are independent
prognostic biomarkers of short overall survival (30–32).
There is only limited data for metastatic CRC. CHI3L1 levels are
different in patients with CRC compared to healthy controls, but
sensitivity is not better than CEA. On the contrary, CHI3L1 levels
correlate with extent of liver involvement in cases without
pulmonary metastases. A difference between overall survival in
patients with higher levels of CHI3L1 compared to lower levels was
not observed.
IGFBP-2 is an extracellular protein that binds IGF-2
and, with a smaller affinity, IGF-1 (33) IGFBP-2 plays an important role in heat
shock protein 27-mediated cancer progression and metastasis
(39). IGFBP-2 serum levels were
reported as significantly elevated in patients with colon cancer in
three studies. Liou et al, presented data from 162 patients
before surgery for CRC with a sensitivity rate of 80.2% and
specificity rate of 64.0%. Higher levels of IGFBP-2 were associated
with an increased risk of mortality (38). Renehan et al, showed data of
92 patients with CRC, but only 29 with distant metastasis. They
reported correlation of serum levels with tumor size and
sensitivity in metastatic disease at a rate of 55% with specificity
at a rate of 92% (42). Kushlinski
et al, showed data for 95 patients, but only 17 with mCRC
colon cancer patients overall (43).
Our cohort shows the same sensitivity and
specificity as previously reported studies. Additionally, our data
show statistically significant correlation between serum levels and
extent of liver involvement. The entry level of IGFBP-2 appears to
be a prognostic factor that strongly correlates with overall
survival. This can be partially explained by the strong correlation
of both markers with liver involvement in patients without
pulmonary metastases. Patients with a sum of liver metastases
larger than 100 mm had higher levels of IGFBP-2 and their liver
reserve was smaller. Another partial explanation could be that
patients with non-resected primary tumor, who have higher levels of
IGFBP-2, were not operated on because of their worse performance
status, thereby possibly being a reason for the worse outcome of
the treatment.
On the other hand, we are aware of the limited
number of patients investigated in this study. Further
investigation and data are needed to clarify these promising
results on a larger cohort of patients.
Our data indicated that serum HSP60 could be used as
an effective biomarker for the detection of distant metastasis with
the same sensitivity as CEA and better sensitivity than CA19-9.
Serum IGFBP-2 has a smaller sensitivity than CEA and a similar one
to CA19-9. HSP60 and IGFBP-2 may play an important role in
promoting CRC progression and dissemination. HSP60 and IGFBP-2
levels correlate with extension of liver involvement in patients
without pulmonary metastases, who are the candidates for a curative
liver resection. The entry level of HSP60 and IGFBP-2 appears to be
a prognostic factor that correlates with overall survival.
Acknowledgements
Not applicable.
Funding
The present study was supported by research projects
from the Ministry of Industry and Trade if the Czech Republic
(grant no. TIP FR-TI3/666), Charles University (Czech Republic;
grant no. Progres Q25) and the Ministry of Health (Czech Republic;
grant no. DRO VFN64165).
Availability of data and materials
The datasets used and analysed for the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
MV, LP, MK, TH, TZ and JP conceived and designed the
study. MV, DL, VF and JP recruited patients and obtained samples.
MK performed the laboratory analyses. MV, DL, LP, JP and VF
analysed and interpreted the data, and wrote the manuscript. MK,
TH, TZ, JP and VF revised the paper. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study protocol conformed to the Declaration of
Helsinki, and was approved by the Institutional Ethics Committee of
First Faculty of Medicine of Charles University in Prague (Czech
Republic). Each participating patient had provided signed written
informed consent.
Patient consent for publication
Consent was obtained for the publication of patient
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dusek L, Muzik J, Maluskova D, Májek O,
Pavlik T, Koptíková J, Melichar B, Büchler T, Fínek J, Cibula D, et
al: Cancer incidence and mortality in the Czech Republic. Klin
Onkol. 27:406–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new american joint committee on
cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hendrick JP and Hartl FU: Molecular
chaperone functions of heat-shock proteins. Annu Rev Biochem.
62:349–384. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pockley AG: Heat shock proteins,
inflammation, and cardiovascular disease. Circulation.
105:1012–1017. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henderson B, Fares MA and Lund PA:
Chaperonin 60: A paradoxical, evolutionarily conserved protein
family with multiple moonlighting functions. Biol Rev Camb Philos
Soc. 88:955–987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marino Gammazza A, Rizzo M, Citarrella R,
Rappa F, Campanella C, Bucchieri F, Patti A, Nikolic D, Cabibi D,
Amico G, et al: Elevated blood Hsp60, its structural similarities
and cross-reactivity with thyroid molecules, and its presence on
the plasma membrane of oncocytes point to the chaperonin as an
immunopathogenic factor in hashimoto's thyroiditis. Cell Stress
Chaperones. 19:343–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomasello G, Rodolico V, Zerilli M,
Martorana A, Bucchieri F, Pitruzzella A, Marino Gammazza A, David
S, Rappa F, Zummo G, et al: Changes in immunohistochemical levels
and subcellular localization after therapy and correlation and
colocalization with CD68 suggest a pathogenetic role of Hsp60 in
ulcerative colitis. Appl Immunohistochem Mol Morphol. 19:552–561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cappello F, Caramori G, Campanella C,
Vicari C, Gnemmi I, Zanini A, Spanevello A, Capelli A, La Rocca G,
Anzalone R, et al: Convergent sets of data from in vivo and in
vitro methods point to an active role of Hsp60 in chronic
obstructive pulmonary disease pathogenesis. PLoS One. 6:e282002011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Czarnecka AM, Campanella C, Zummo G and
Cappello F: Mitochondrial chaperones in cancer: From molecular
biology to clinical diagnostics. Cancer Biol Ther. 5:714–720. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cappello F, Bellafiore M, Palma A, David
S, Marcianò V, Bartolotta T, Sciumè C, Modica G, Farina F, Zummo G
and Bucchieri F: 60KDa chaperonin (Hsp60) is over-expressed during
colorectal carcinogenesis. Eur J Histochem. 47:105–110. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Y, Wu Y, Mou Z, Li W, Zou L, Fu T,
Zhang A, Xiang D, Xiao H and Wang X: Proteomics-based
identification of HSP60 as a tumor-associated antigen in colorectal
cancer. Proteomics Clin Appl. 1:336–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bini L, Magi B, Marzocchi B, Arcuri F,
Tripodi S, Cintorino M, Sanchez JC, Frutiger S, Hughes G, Pallini
V, et al: Protein expression profiles in human breast ductal
carcinoma and histologically normal issue. Electrophoresis.
18:2832–2841. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Castilla C, Congregado B, Conde JM, Medina
R, Torrubia FJ, Japón MA and Sáez C: Immunohistochemical expression
of Hsp60 correlates with tumor progression and hormone resistance
in prostate cancer. Urology. 76:1017.e1–e6. 2010. View Article : Google Scholar
|
|
15
|
Cappello F, David S, Rappa F, Bucchieri F,
Marasà L, Bartolotta TE, Farina F and Zummo G: The expression of
HSP60 and HSP10 in large bowel carcinomas with lymph node
metastase. BMC Cancer. 5:1392005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamelin C, Cornut E, Poirier F, Sylvie
Pons S, Beaulieu C, Charrier JP, Haïdous H, Cotte E, Lambert C,
Piard F, et al: Identification and verification of heat shock
protein 60 as a potential serum marker for colorectal cancer. FEBS
J. 278:4845–4859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johansen JS, Schultz NA and Jensen BV:
Plasma YKL-40: A potential new cancer biomarker? Future Oncol.
5:1065–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johansen JS, Høyer PE, Larsen LA, Price PA
and Møllgård K: YKL-40 protein expression in the early developing
human musculoskeletal system. J Histochem Cytochem. 55:1213–1228.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brøchner CB, Johansen JS, Larsen LA, Bak
M, Mikkelsen HB, Byskov AG, Andersen CY and Møllgård K: YKL-40 is
differentially expressed in human embryonic stem cells and in cell
progeny of the three germ layers. J Histochem Cytochem. 60:188–204.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faibish M, Francescone R, Bentley B, Yan W
and Shao R: A YKL-40 neutralizing antibody blocks tumor
angiogenesis and progression: A potential therapeutic agent in
cancers. Mol Cancer Ther. 10:742–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao R: YKL-40 acts as an angiogenic
factor to promote tumor angiogenesis. Front Physiol. 4:1222013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee CG, Da Silva C, Dela Cruz CS, Ahangari
F, Ma B, Kang MJ, He CH, Takyar S and Elias JA: Role of chitin and
chitinase/chitinase-like proteins in inflammation, tissue
remodelling, and injury. Annu Rev Physiol 2011;. 73:479–501. 2011.
View Article : Google Scholar
|
|
23
|
Kawada M, Seno H, Kanada K, Nakanishi Y,
Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E and Chiba T:
Chitinase 3-like-1 promotes macrophage recruitment and angiogenesis
in colorectal cancer. Oncogene. 31:3111–3123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eurich K, Segawa M, Toei-Shimizu S and
Mizoguchi E: Potential role of chitinase 3-like-1 in
inflammation-associated carcinogenic changes of epithelial cells.
World J Gastroenterol. 15:5249–5259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen CC, Llado V, Eurich K, Tran HT and
Mizoguchi E: Carbohydrate-binding motif in chitinase 3-like 1
(CHI3L1/YKL-40) specifically activates akt signaling pathway in
colonic epithelial cells. Clin Immunol. 140:268–275. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee CG, Hartl D, Lee GR, Koller B,
Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et
al: Role of breast regression protein 39 (BRP-39)/Chitinase
3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J
Exp Med. 206:1149–1166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johansen JS, Christensen IJ, Jørgensen LN,
Olsen J, Rahr HB, Nielsen KT, Lurberg S, Brünner N and Nielsen HJ:
Serum YKL-40 in risk assessment for colorectal cancer: A
prospective study of 4,496 subjects at risk of colorectal cancer.
Cancer Epidemiol Biomarkers Prev. 24:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johansen JS, Bojesen SE, Mylin AK,
Frikke-Schmidt R, Price PA and Nordestgaard BG: Elevated plasma
YKL-40 predicts increased risk of gastrointestinal cancer and
decreased survival after any cancer diagnosis in the general
population. J Clin Oncol. 27:572–578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johansen JS, Bojesen SE, Tybjærg-Hansen A,
Mylin AK, Price PA and Nordestgaard BG: Plasma YKL-40 and total and
disease-specific mortality in the general population. Clin Chem.
56:1580–1591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cintin C, Johansen JS, Christensen IJ,
Price PA, Sørensen S and Nielsen HJ: High serum YKL-40 level after
surgery for colorectal carcinoma is related to short survival.
Cancer. 95:267–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cintin C, Johansen JS, Christensen IJ,
Price PA, Sørensen S and Nielsen HJ: Serum YKL-40 and colorectal
cancer. Br J Cancer. 79:1494–1499. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Zhang Y, Zhu Z, Ha M and Wang Y:
Elevated pretreatment serum concentration of YKL-40: An independent
prognostic biomarker for poor survival in patients with colorectal
cancer. Med Oncol. 31:852014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clemmons DR: Insulin-like growth factor
binding proteins and their role in controlling IGF actions.
Cytokine Growth Factor Rev. 8:45–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu H and Rohan T: Role of the insulin-like
growth factor family in cancer development and progression. J Natl
Cancer Inst. 92:1472–1489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pollak MN, Schernhammer ES and Hankinson
E: Insulin-like growth factors and neoplasia. Nat Rev Cancer.
4:505–518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schütt BS, Langkamp M, Rauschnabel U,
Ranke MB and Elmlinger MW: Integrin-mediated action of insulin-like
growth factor binding protein-2 in tumor cells. J Mol Endocrinol.
32:859–868. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jogie-Brahim S, Feldman D and Oh Y:
Unraveling insulin-like growth factor binding protein-3 actions in
human disease. Endocr Rev. 30:417–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liou JM, Shun CT, Liang JT, Chiu HM, Chen
MJ, Chen CC, Wang HP, Wu MS and Lin JT: Plasma insulin-like growth
factor-binding protein-2 levels as diagnostic and prognostic
biomarkers of colorectal cancer. J Clin Endocrinol Metab.
95:1717–1725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schoen RE, Tangen CM, Kuller LH, Burke GL,
Cushman M, Tracy RP, Dobs A and Savage PJ: Increased blood glucose
and insulin, body size, and incident colorectal cancer. J Natl
Cancer Inst. 91:1147–1154. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hung CS, Huang CY, Lee CH, Chen WY, Huang
MT, Wei PL and Chang YJ: IGFBP2 plays an important role in heat
shock protein 27-mediated cancer progression and metastasis.
Oncotarget. 8:54978–54992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cohen P, Peehl DM, Stamey TA, Wilson KF,
Clemmons DR and Rosenfeld RG: Elevated levels of insulin-like
growth factor-binding protein-2 in the serum of prostate cancer
patients. J Clin Endocrinol Metab. 76:1031–1035. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Renehan AG, Jones J, Potten CS, Shalet SM
and O'Dwyer ST: Elevated serum insulin-like growth factor (IGF)-II
and IGF binding protein-2 in patients with colorectal cancer. Br J
Cancer. 83:1344–1350. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kushlinskii NE, Gershtein ES, Nikolaev AA,
Delektorskaya VV, Korotkova EA, Dvorova EA and Kostyleva OI:
Insulin-like growth factors (IGF), IGF-binding proteins (IGFBP),
and vascular endothelial growth factor (VEGF) in blood serum of
patients with colorectal cancer. Bull Exp Biol Med. 156:684–688.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee DY, Kim SJ and Lee YC: Serum
insulin-like growth factor (IGF)-I and IGF-binding proteins in lung
cancer patients. J Korean Med Sci. 14:401–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vocka M, Langer D, Petrtyl J, Vockova P,
Hanus T, Kalousova M, Zima T and Petruzelka L: Trefoil factor
family (TFF) proteins as potential serum biomarkers in patients
with metastatic colorectal cancer. Neoplasma. 62:470–477. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rodolico V, Tomasello G, Zerilli M,
Martorana A, Pitruzzella A, Gammazza AM, David S, Zummo G, Damiani
P, Accomando S, et al: Hsp60 and Hsp10 increase in colon mucosa of
Crohn's disease and ulcerative colitis. Cell Stress Chaperones.
15:877–884. 2015. View Article : Google Scholar
|