Introduction
Endometrial carcinoma is the most common malignancy
of female genital organs in developed countries, and the incidence
is recently increasing (1). The
standard primary treatment is composed of surgery with or without
postoperative chemotherapy and/or radiotherapy based on
stratification by the risks for recurrence. Endometrial carcinoma
is conventionally categorized into two major classes, namely type I
and II. Type I tumors are generally characterized by endometrioid
histology, precancerous atypical hyperplasia, perimenopausal
incidence, obesity, superficial myometrial invasion, favorable
prognosis, and frequent PTEN mutations (2,3). Type II
tumors are generally characterized by non-endometrioid histology,
precancerous intraepithelial carcinoma arising in atrophic
endometrium, older age, postmenopausal status, reduced weight, deep
myometrial invasion, poor disease prognosis, and frequent
TP53 mutations. The tumor suppressor protein p53 functions
as the ‘guardian of the genome’ by inducing cell cycle arrest,
senescence, and apoptosis in response to oncogene activation, DNA
damage, and other stress signals. Loss of p53 function occurs in
the majority of human tumors by mutation of TP53 or by
inactivation of the p53 signal transduction pathway. The majority
of the mutations result in the expression of a p53 protein that has
lost wild-type functions and exerts a dominant-negative regulation
over the remaining wild-type p53 proteins. However, it has recently
become apparent that mutant p53 further acquires oncogenic
functions different to those resulting from loss of wild-type
function (4). The majority of the
mutant p53 proteins acquire oncogenic properties, such as invasion,
metastasis, increased proliferation, and cell survival. Recently, a
number of molecular agents targeting mutant p53 have been developed
(5–9), and the efficacies for various types of
malignancy are currently being examined in clinical trials.
However, the precise prognostic significance of p53 aberration in
endometrial carcinoma remains to be clarified. In the present
study, we investigated the impact of the abnormal accumulation of
p53 in tumors on the outcome of patients with the disease. The
findings provide novel and useful implications for genome-directed
individualized management of endometrial carcinoma.
Materials and methods
Patients and specimens
The Ethics Committee of the University of Tsukuba
Hospital approved the study protocol. All patients diagnosed with
endometrial carcinoma, who received surgery in the Department of
Obstetrics and Gynecology at the University of Tsukuba Hospital
between 1999 and 2009, were identified by our database. A total of
221 consecutive patients were included in the present study, and
their medical records were retrospectively reviewed. The median
follow-up duration was 132 months (range, 3–209 months). The
follow-up data were retrieved until 2018-7-20. All samples were
obtained with opt-out procedure in accordance with the study
protocol approved by the Ethics Committee of the University of
Tsukuba Hospital. Staging was performed based on the criteria of
the International Federation of Gynecology and Obstetrics (FIGO,
2008) (10). Endometrioid carcinomas
were subclassified into three grades (G1, G2,
and G3) according to the FIGO criteria. The treatment of
the patients was performed as described previously (3). Table I
summarizes the patient characteristics.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Number, n (%),
(n=221) |
|---|
| Median age
(range) | 57 (26–84) |
| FIGO stage |
|
| I | 144 (65) |
|
IA | 110 (50) |
|
IB | 34 (15) |
| II | 17 (8) |
| III | 36 (16) |
|
IIIA | 13 (6) |
|
IIIC | 23 (10) |
| IV | 24 (11) |
|
IVA | 2 (1) |
|
IVB | 22 (10) |
| Histotype |
|
|
Endometrioid | 196 (89) |
|
G1 | 115 (52) |
|
G2 | 56 (25) |
|
G3 | 25 (11) |
|
Serous | 12 (5) |
|
Adenosquamous | 4 (2) |
| Clear
cell | 4 (2) |
| Poorly
differentiated | 1 (0) |
|
Undifferentiated | 1 (0) |
| Mixed
epithelial | 3 (1) |
| Primary
treatment |
|
|
Surgery | 221 (100) |
|
Lymphadenectomy | 171 (77) |
|
Lymph node
sampling | 21 (10) |
|
Lymph node not
removed | 29 (13) |
|
Adjuvant chemotherapy | 60 (27) |
|
TC | 55 (25) |
|
CAP | 4 (2) |
|
Adjuvant radiotherapy | 58 (26) |
Immunohistochemistry
Immunohistochemistry was performed as described
previously (11). The antibodies
used were the following: Anti-human p53 (DO-7) (mouse monoclonal,
1:200; Dako) and anti-human PTEN (6H2.1) (mouse monoclonal, 1:100;
Cascade). The corresponding normal endometrial or stromal tissues
were used as an internal positive control. The negative control
samples comprised samples incubated in the absence of primary
antibody that indicated low background staining. Representative
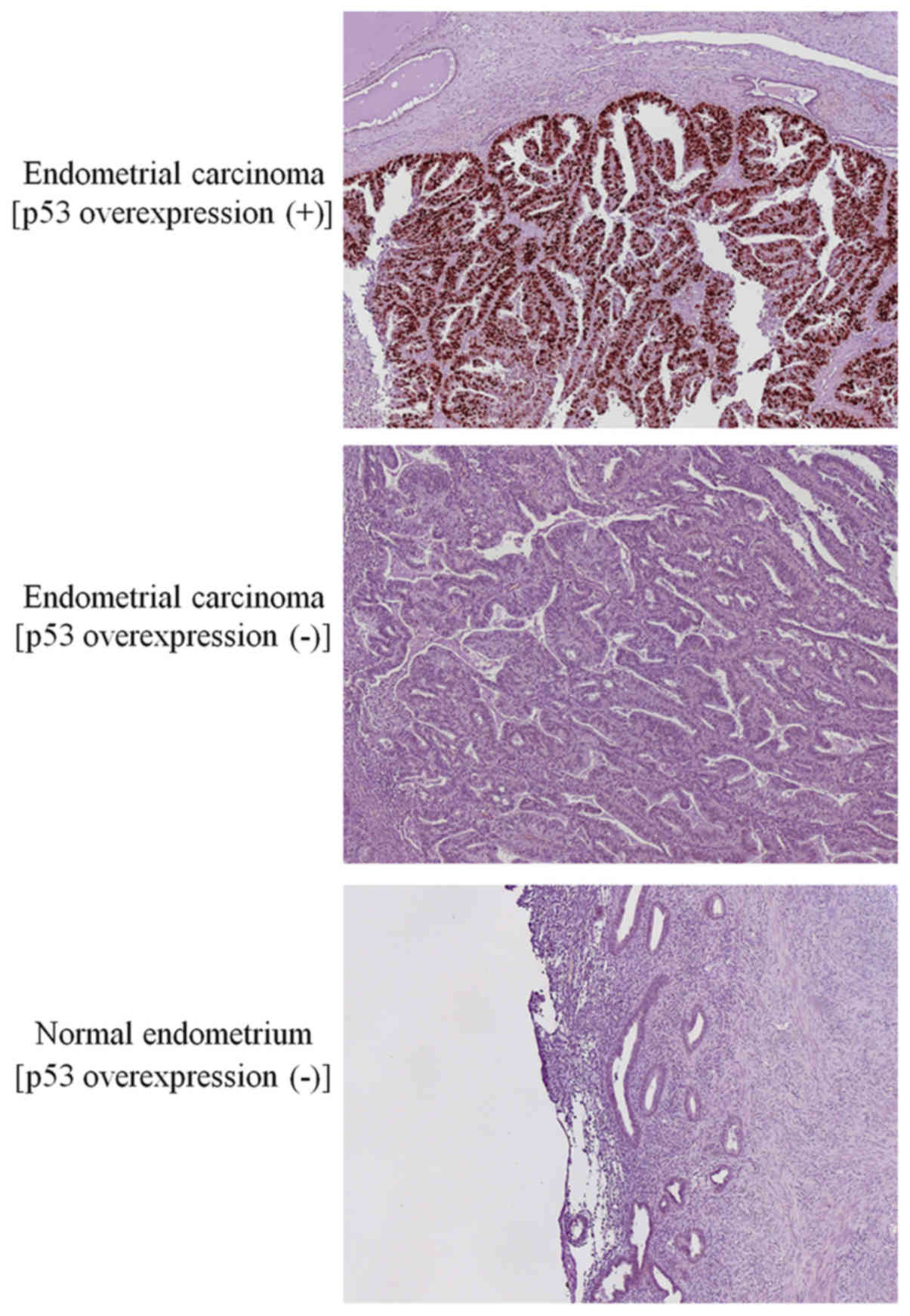
immunostaining images for p53 in endometrial carcinomas and normal
endometria are shown in Fig. 1.
Immunohistochemical (IHC) scoring
P53 and PTEN expression levels were evaluated as
previously described (3,11). Briefly for p53 expression, positive
staining of ≥10% of tumor cells was considered overexpression (+),
and negative or positive staining of <10% of tumor cells was
overexpression (−). The average value from the scores of two
independent observers (AA and TM) blinded to the
clinicopathological variables was used as the final value. Normal
endometrial samples from 15 women were used as control samples, and
100% of the specimens were negative for p53, whereas more than 90%
exhibited a score value of 6 for PTEN expression.
Statistical analysis
The differences in the proportions were evaluated by
the Fisher's exact test. Kaplan-Meier survival curves were
calculated and compared using the log-rank test. The Cox
proportional hazard model was used for the univariate and
multivariate analyses.
Results
IHC analysis demonstrated p53 overexpression in 37
out of 221 patients (17%). P53 overexpression was significantly
associated with non-endometrioid histology, non-G1, post-menopause,
and advanced FIGO stage (III/IV) (P=0.0006, 0.004, 0.03, and 0.025,
respectively, Table II).
 | Table II.Association between
immunohistochemistry results and clinicopathological features. |
Table II.
Association between
immunohistochemistry results and clinicopathological features.
|
| P53
overexpression |
|
|---|
|
|
|
|
|---|
| Clinicopathological
variables | (+) (n=37) (%) | (−) (n=184)
(%) | P-value |
|---|
| Age ≥70 | 10 (27) | 26 (14) | 0.084 |
| Post-menopause | 32 (86) | 125 (68) | 0.028 |
| Null parity | 3 (8) | 34 (18) | 0.151 |
| BMI >30 | 3 (8) | 27 (15) | 0.430 |
| DM | 6 (16) | 33 (18) | >0.999 |
| Endometrioid (vs.
non-endometrioid) | 26 (70) | 170 (92) | <0.001 |
| G1 (vs.
Non-G1) | 11 (30) | 104 (57) | 0.004 |
| MI>1/2 | 15 (41) | 66 (36) | 0.581 |
| LVI | 17 (46) | 67 (36) | 0.353 |
| FIGO stage
III/IV | 16 (43) | 44 (24) | 0.025 |
Survival analysis demonstrated that patients with
p53-overexpressing tumors exhibited significantly poor overall
survival (OS) compared with the patients who did not exhibit p53
overexpression (Fig. 2A,
P<0.000001). Univariate analysis for unfavorable prognostic
factors indicated that the parameters p53 overexpression, age
higher than and/or equal to 70 years (≥70), non-endometrioid
histology, advanced FIGO stage (III/IV), myometrial invasion higher
than ½, and lymphovascular space invasion were significantly
associated with OS (P<0.00001, <0.00001, <0.00001,
<0.00001, <0.00001, and 0.00011, respectively, Table III). Subsequent multivariate
analysis indicated that the parameters p53 overexpression, age ≥70,
non-endometrioid histology, and advanced tumor stage were
significantly associated with OS (P=0.00012, 0.00048, 0.0027, and
0.0015, Table III).
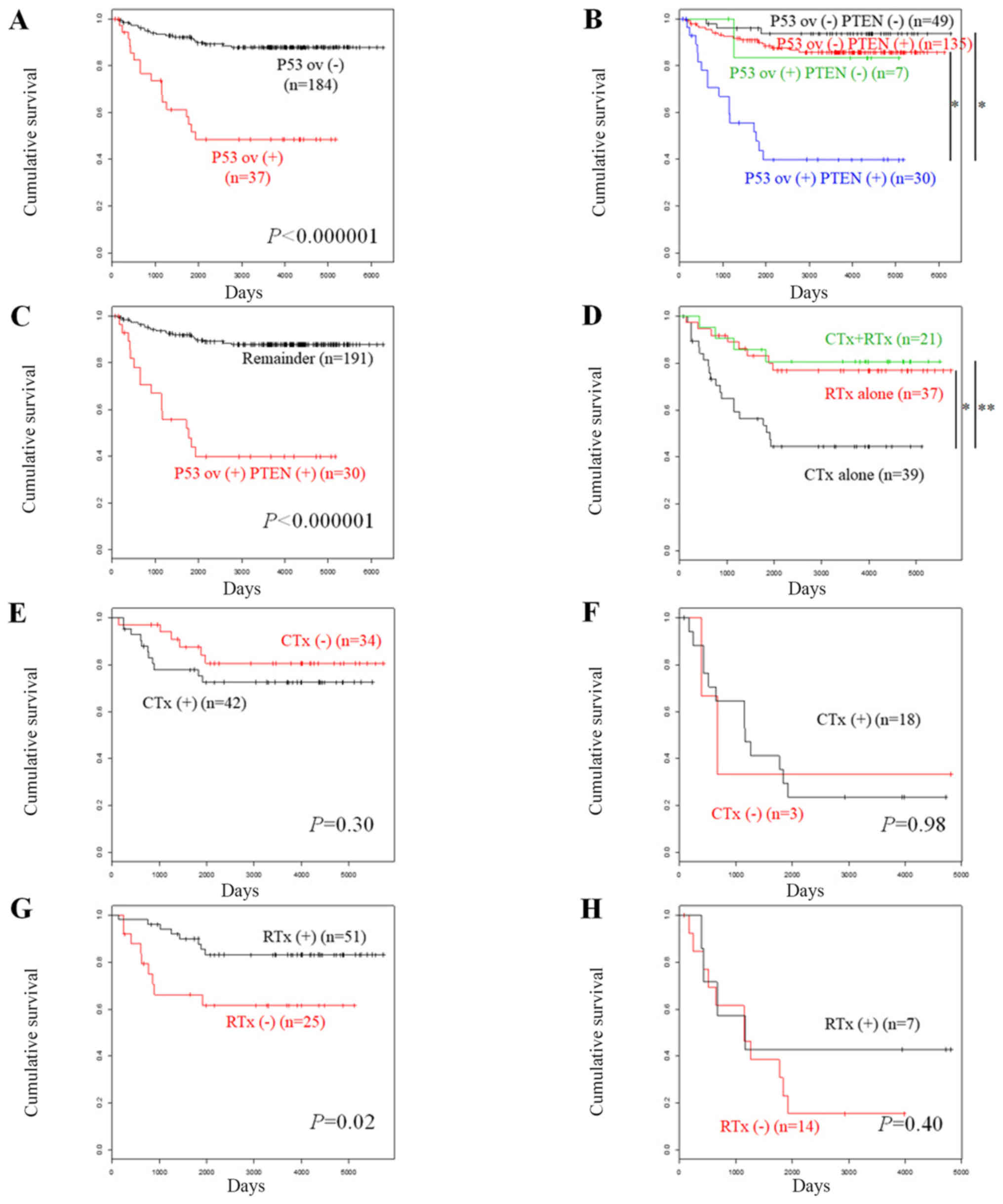 | Figure 2.Kaplan-Meier curves were constructed
in order to assess overall survival according to protein expression
levels in endometrial carcinoma. (A) Patients without p53
overexpression (n=184) vs. those with p53 overexpression (n=37).
(B) Patients with no p53 overexpression and negative PTEN (n=49),
no p53 overexpression and positive PTEN (n=135), p53 overexpression
and negative PTEN (n=7), and p53 overexpression and positive PTEN
(n=30). *P<0.000001, as indicated. (C) Patients with p53
overexpression and positive PTEN (n=30) vs. the remaining subjects
(n=191). (D) Patients who received adjuvant chemotherapy alone
(n=39), adjuvant radiotherapy alone (n=37) and both therapies
(n=21). *P=0.004 and **P=0.01, as indicated. (E) Patients without
p53 overexpression, who received adjuvant chemotherapy (n=42) vs.
those who did not receive adjuvant chemotherapy (n=34). (F)
Patients with p53 overexpression, who received adjuvant
chemotherapy (n=18) vs. those who did not receive adjuvant
chemotherapy (n=3). (G) Patients without p53 overexpression, who
received adjuvant radiotherapy (n=51) vs. those who did not receive
adjuvant radiotherapy (n=25). (H) Patients with p53 overexpression,
who received adjuvant radiotherapy (n=7) vs. those who did not
receive adjuvant radiotherapy (n=14). CTx, chemotherapy; ov,
overexpression; RTx, radiotherapy. |
 | Table III.Univariate and multivariate analyses
of prognostic factors for poor overall survival. |
Table III.
Univariate and multivariate analyses
of prognostic factors for poor overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Prognostic
factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| P53 overexpression
(+) [vs. (−)] | 5.71 | 3.00–10.9 | <0.001 | 3.90 | 1.95–7.79 | <0.001 |
| Age ≥70 years (vs.
<70 years) | 5.04 | 2.63–9.64 | <0.001 | 3.38 | 1.71–6.69 | <0.001 |
| Non-endometrioid
(vs. endometrioid) | 5.78 | 2.99–11.2 | <0.001 | 2.93 | 1.45–5.91 | 0.003 |
| FIGO stage III/IV
(vs. I/II) | 8.62 | 4.26–17.4 | <0.001 | 3.75 | 1.66–8.47 | 0.001 |
| MI >1/2 (vs.
≤1/2) | 5.04 | 2.50–10.2 | <0.001 | 2.18 | 0.95–5.00 | 0.067 |
| LVI present (vs.
absent) | 3.75 | 1.92–7.34 | <0.001 | 1.70 | 0.80–3.61 | 0.165 |
In addition, the OS was compared according to the
expression levels of p53 and PTEN. Loss of PTEN expression was a
prognostic indicator for favorable OS in endometrial carcinoma
(3). Patients with p53
overexpression (−) and PTEN (−) tumors were associated with
favorable disease prognosis, followed by those with p53
overexpression (−) and PTEN (+) tumors and those with p53
overexpression (+) and PTEN (−) tumors. The patients with p53
overexpression (+) PTEN (+) tumors exhibited unfavorable prognosis
(Fig. 2B). Patients with p53
overexpression (+) PTEN (+) tumors exhibited significantly lower OS
compared with that noted in the remaining patients (P<0.000001,
Fig. 2C).
We further compared OS according to the modalities
of adjuvant therapies in patients who received post-operative
treatment. Patients who received adjuvant chemotherapy alone
indicated significantly lower OS compared with that noted in
patients with adjuvant radiotherapy alone or with both adjuvant
therapies (Fig. 2D, P=0.004 and
0.01, respectively). The effects of the adjuvant therapies on the
disease prognosis were dependent on the p53 status. Adjuvant
chemotherapy did not influence OS in patients without p53
overexpression (Fig. 2E, P=0.30) or
with p53 overexpression (Fig. 2F,
P=1.0). By contrast, adjuvant radiotherapy significantly increased
OS in patients without p53 overexpression (Fig. 2G, P=0.02). This effect was not noted
in patients with p53 overexpression (Fig. 2H, P=0.40). We further conducted
univariate analyses of the effects of the adjuvant therapies on the
OS of the patients with p53 overexpression compared with those
without p53 overexpression (Table
IV). While adjuvant chemotherapy did not influence OS in
patients with or without p53 overexpression [hazard ratio, 0.98
(95% confidence interval, 0.22–4.37) vs. 1.64 (0.61–4.45),
Table IV], adjuvant radiotherapy
increased OS in patients without p53 overexpression, but not in
patients with p53 overexpression [HR, 0.34 (95% CI, 0.13–0.88)
vs. 0.61 (0.19–1.93), Table
IV]. Univariate analysis of various prognostic factors in
patients without p53 overexpression who received adjuvant therapies
demonstrated that with the exception of adjuvant radiotherapy being
significant for improved OS (P=0.026, Table V), the parameters age ≥70,
non-endometrioid histology, and advanced tumor stage were
significant for unfavorable OS (P=0.010, 0.0081, and 0.019,
respectively, Table IV). However,
subsequent multivariate analysis indicated that only the parameter
age ≥70 was a significant and independent prognostic factor for OS
(P=0.039, Table V).
 | Table IV.Univariate analysis of adjuvant
therapy for overall survival in patient subsets with p53
overexpression (+) vs. (−). |
Table IV.
Univariate analysis of adjuvant
therapy for overall survival in patient subsets with p53
overexpression (+) vs. (−).
| Prognostic
factor | Subset | HR | 95% CI | P-value |
|---|
| Adjuvant CTx | p53 ov (+) | 0.98 | 0.22–4.37 | 0.980 |
|
| p53 ov (−) | 1.64 | 0.61–4.45 | 0.328 |
| Adjuvant RTx | p53 ov (+) | 0.61 | 0.19–1.93 | 0.401 |
|
| p53 ov (−) | 0.34 | 0.13–0.88 | 0.026 |
 | Table V.Survival analyses in patients without
p53 overexpression who received adjuvant therapies. |
Table V.
Survival analyses in patients without
p53 overexpression who received adjuvant therapies.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Prognostic
factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age ≥70 years (vs.
<70 years) | 3.50 | 1.34–9.10 | 0.010 | 2.98 | 1.06–8.40 | 0.039 |
| Non-endometrioid
(vs. endometrioid) | 4.63 | 1.49–14.4 | 0.008 | 1.71 | 0.48–6.12 | 0.406 |
| FIGO stage III/IV
(vs. I/II) | 4.44 | 1.27–15.5 | 0.019 | 3.52 | 0.92–13.5 | 0.065 |
| MI >1/2 (vs.
≤1/2) | 2.74 | 0.63–12.0 | 0.181 | – | – | – |
| LVI present (vs.
absent) | 1.91 | 0.71–5.17 | 0.202 | – | – | – |
| Adjuvant CTx
done | 1.64 | 0.61–4.45 | 0.328 | – | – | – |
| Adjuvant RTx
done | 0.34 | 0.13–0.88 | 0.026 | 0.62 | 0.21–1.79 | 0.373 |
Discussion
Wild-type p53 protein is susceptible to
ubiquitin-mediated degradation by the proteasome, whereas mutant
p53 is not, resulting in abnormal accumulation of the protein in
p53-mutant tumors. The IHC analysis conducted in the present study
revealed abnormal accumulation of p53 in 17% of endometrial
carcinomas. This finding was in line with the previously published
frequencies of TP53 mutations in endometrial cancer
(12).
In addition, the association of the IHC data with
the clinicopathological parameters was examined. P53 overexpression
was significantly associated with non-endometrioid histology and
advanced-stage disease (Table II).
Furthermore, survival analyses indicated that p53 overexpression
was a significant and independent prognostic factor for poor OS
(Table III). These findings
suggested that tumors harboring p53 aberrations may have aggressive
biological behavior, such as rapid progression. This effect may
contribute to the prognostic impact of p53 with regard to the poor
patient survival. We further compared OS according to the p53/PTEN
expression of the patients. Previously we reported that negative
PTEN expression is a prognostic indicator for favorable OS in
endometrial carcinoma (3). Patients
with p53 overexpression (+) PTEN (+) tumors exhibited considerably
lower OS compared with that noted in the remaining patients
(Fig. 2B and C), suggesting that
they may be managed as the highest-risk group with the most
aggressive phenotype.
The comparison of OS according to the modalities of
the adjuvant therapies in the patients receiving post-operative
treatment indicated that the improvement in their survival by
adjuvant radiotherapy correlated with their p53 overexpression (−)
status, while adjuvant chemotherapy did not improve OS irrespective
of the p53 status (Fig. 2E-H,
Table IV). Furthermore, univariate
analysis in patients without p53 overexpression who received
adjuvant therapies revealed that adjuvant radiotherapy, but not
adjuvant chemotherapy, was a significant prognostic factor for
improved OS (Table V). These
findings suggested that the effect of p53 on poor prognosis may be
partially mediated by the attenuated radiosensitivity of the tumors
caused due to p53 aberration. The p53 signaling pathway is known to
play critical roles in determining radiosensitivity by diverse
mechanisms of actions (13). It has
been reported that p53 mutations increase radioresistance in
certain types of tumor cells (14–16).
Moreover, p53 status is associated with the disease outcome
following radiotherapy in patients with specific types of
malignancy (17,18). Taken collectively, the data suggest
that p53 expression may serve as a radiosensitivity biomarker for
endometrial carcinoma. Although the p53 pathway is known to
contribute to chemoresistance in certain types of tumors, the
present study did not support this hypothesis. This may be
explained by the tissue-specific induction of the p53 target genes
(19,20), whereby chemosensitivity and
radiosensitivity may be different depending on the type of
tumor.
Accumulating mutant p53 proteins are attractive
targets for molecular therapy as TP53 is the most frequently
mutated gene in human malignancies. Current strategies for
targeting mutant p53 are focusing on the destabilization or
inactivation of its mutant form, or the reactivation of wild-type
p53 function. Destabilization of mutant p53 has been addressed
mainly by targeting heat shock proteins via histone deacetylase
enzymes in order to rescue MDM2-dependent degradation of mutant p53
(7,8). Disruption of mutant p53 function may be
achieved by preventing its interaction with other transcription
factors. For example, the molecule RETRA has been shown to inhibit
the mutant p53-p73 interaction and to restore p73 function
(9). A number of compounds or
peptides that result in the reactivation of wild-type function in
mutant p53 have also been reported. Among them, two small
molecules, namely PRIMA-1 (p53 reactivation and induction of
massive apoptosis) and its potent methylated analog,
APR-246/PRIMA-1MET, have been reported to convert mutant
p53 to a wild-type conformation, thereby restoring its
sequence-specific DNA binding and transcriptional activation
(6,21–23).
PRIMA-1 or APR-246/PRIMA-1MET induce apoptosis in tumors
with both wild-type and mutant p53 (24–27),
which may be explained by the observation that both unfolded mutant
p53 and unfolded wild-type p53 are refolded by PRIMA-1 (28). These compounds further activate
caspase enzymes, leading to cytochrome c release from the
mitochondria (29). The activity of
the compounds can be enhanced by combined administration of
conventional chemotherapeutics as well as molecular targeting
agents, including cisplatin, carboplatin, doxorubicin, docetaxel,
and olaparib (30–32). APR-246 was the first mutant
p53-restoring drug, which entered clinical trials, and exhibited
optimal tolerability (5,6). Currently, two phase II studies are
ongoing in recurrent high-grade serous ovarian cancer with positive
p53 IHC staining. One involves the treatment of platinum-sensitive
disease with combined administration of carboplatin and pegylated
liposomal doxorubicin hydrochloride (PLD) (PiSARRO; NCT02098343),
and the other is conducted for platinum-resistant disease with
combined PLD (PiSARRO-R; NCT03268382). The findings of the present
study suggested that molecular therapeutics that focus on
p53-targeting may sensitize p53-overexpressing tumors to adjuvant
radiotherapy. This potentially leads to the improvement of patient
survival in subjects with poor prognosis. The development and
clinical applications of efficacious molecular agents targeting p53
are warranted in the near future.
The present study contains specific limitations.
Firstly, IHC overexpression of p53 was used as a surrogate for p53
mutation, whereas its mutations were not examined. Secondly, the
present study was conducted in a single institution, and the sample
size was relatively small. Further studies are required to
strengthen the current findings. Finally, the retrospective study
design can cause potential bias, suggesting that the results must
be verified by prospective trials.
In conclusion, the present study demonstrated that
p53 overexpression was associated with non-endometrioid histology,
post-menopause, and advanced stage, and that patients with
p53-overexpressing tumors exhibited worse OS compared with those
without p53 overexpression. Univariate and multivariate analyses
indicated that p53 overexpression was a significant and independent
prognostic factor for poor OS. Adjuvant radiotherapy correlated
with improved OS in patients without p53 overexpression compared
with that noted in p53-overexpressing patients, and was found to be
a significant favorable prognostic factor in patients without p53
overexpression who received post-operative treatments. The current
findings provide significant applications for the genome-based
individualized management of endometrial carcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Grant-in-Aid
for Scientific Research (grant no. 16K11129) from the Ministry of
Education, Culture, Sports, Science and Technology (Tokyo,
Japan).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AA performed all experiments and drafted the
manuscript. TM designed the study, analyzed the data, and revised
the manuscript. KF, YH, KN, AS, NT, MS, HO and TS contributed to
the study conception and interpretation of data. TS critically
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All samples were obtained with an opt-out procedure
in accordance with the study protocol approved by the Ethics
Committee of the University of Tsukuba Hospital (approval no.
H26-118). The study was performed in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M and Bray F: GLOBOCAN
2018. International Agency for Research on Cancer, Lyon, France.
2018.
|
|
2
|
Minaguchi T, Yoshikawa H, Oda K, Ishino T,
Yasugi T, Onda T, Nakagawa S, Matsumoto K, Kawana K and Taketani Y:
PTEN mutation located only outside exons 5, 6, and 7 is an
independent predictor of favorable survival in endometrial
carcinomas. Clin Cancer Res. 7:2636–2642. 2001.PubMed/NCBI
|
|
3
|
Akiyama-Abe A, Minaguchi T, Nakamura Y,
Michikami H, Shikama A, Nakao S, Sakurai M, Ochi H, Onuki M,
Matsumoto K, et al: Loss of PTEN expression is an independent
predictor of favourable survival in endometrial carcinomas. Br J
Cancer. 109:1703–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muller PA and Vousden KH: P53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deneberg S, Cherif H, Lazarevic V,
Andersson PO, von Euler M, Juliusson G and Lehmann S: An open-label
phase I dose-finding study of APR-246 in hematological
malignancies. Blood Cancer J. 6:e4472016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lehmann S, Bykov VJ, Ali D, Andrén O,
Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A,
et al: Targeting p53 in vivo: A first-in-human study with
p53-targeting compound APR-246 in refractory hematologic
malignancies and prostate cancer. J Clin Oncol. 30:3633–3639. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li D, Marchenko ND and Moll UM: SAHA shows
preferential cytotoxicity in mutant p53 cancer cells by
destabilizing mutant p53 through inhibition of the HDAC6-Hsp90
chaperone axis. Cell Death Differ. 18:1904–1913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan W, Liu S, Xu E, Zhang J, Zhang Y and
Chen X: Histone deacetylase inhibitors suppress mutant p53
transcription via histone deacetylase 8. Oncogene. 32:599–609.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kravchenko JE, Ilyinskaya GV, Komarov PG,
Agapova LS, Kochetkov DV, Strom E, Frolova EI, Kovriga I, Gudkov
AV, Feinstein E and Chumakov PM: Small-molecule RETRA suppresses
mutant p53-bearing cancer cells through a p73-dependent salvage
pathway. Proc Natl Acad Sci USA. 105:6302–6307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abe A, Minaguchi T, Ochi H, Onuki M, Okada
S, Matsumoto K, Satoh T, Oki A and Yoshikawa H: PIK3CA
overexpression is a possible prognostic factor for favorable
survival in ovarian clear cell carcinoma. Hum Pathol. 44:199–207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berchuck A and Boyd J: Molecular basis of
endometrial cancer. Cancer. 76:2034–2040. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Shen L and Sun LQ: The regulation
of radiosensitivity by p53 and its acetylation. Cancer Lett.
363:108–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Concin N, Zeillinger C, Stimpfel M,
Schiebel I, Tong D, Wolff U, Reiner A, Leodolter S and Zeillinger
R: P53-dependent radioresistance in ovarian carcinoma cell lines.
Cancer Lett. 150:191–199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dey S, Spring PM, Arnold S, Valentino J,
Chendil D, Regine WF, Mohiuddin M and Ahmed MM: Low-dose
fractionated radiation potentiates the effects of Paclitaxel in
wild-type and mutant p53 head and neck tumor cell lines. Clin
Cancer Res. 9:1557–1565. 2003.PubMed/NCBI
|
|
16
|
Ohnishi K, Inaba H, Yasumoto J, Yuki K,
Takahashi A and Ohnishi T: C-terminal peptides of p53 molecules
enhance radiation-induced apoptosis in human mutant p53 cancer
cells. Apoptosis. 9:591–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishikawa H, Mitsuhashi N, Sakurai H,
Maebayashi K and Niibe H: The effects of p53 status and human
papillomavirus infection on the clinical outcome of patients with
stage IIIB cervical carcinoma treated with radiation therapy alone.
Cancer. 91:80–89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skinner HD, Sandulache VC, Ow TJ, Meyn RE,
Yordy JS, Beadle BM, Fitzgerald AL, Giri U, Ang KK and Myers JN:
TP53 disruptive mutations lead to head and neck cancer treatment
failure through inhibition of radiation-induced senescence. Clin
Cancer Res. 18:290–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
El-Deir WS: The role of p53 in
chemosensitivity and radiosensitivity. Oncogene. 22:7486–7495.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lozano G: The enigma of p53. Cold Spring
Harb Symp Quant Biol. 81:37–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aryee DN, Niedan S, Ban J, Schwentner R,
Muehlbacher K, Kauer M, Kofler R and Kovar H: Variability in
functional p53 reactivation by PRIMA-1(Met)/APR-246 in ewing
sarcoma. Br J Cancer. 109:2696–2704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saha MN, Jiang H, Yang Y, Reece D and
Chang H: PRIMA-1Met/APR-246 displays high antitumor activity in
multiple myeloma by induction of p73 and Noxa. Mol Cancer Ther.
12:2331–2341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shchors K, Persson AI, Rostker F, Tihan T,
Lyubynska N, Li N, Swigart LB, Berger MS, Hanahan D, Weiss WA and
Evan GI: Using a preclinical mouse model of high-grade astrocytoma
to optimize p53 restoration therapy. Proc Natl Acad Sci USA.
110:E1480–E1489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ali D, Jonsson-Videsater K, Deneberg S,
Bengtzén S, Nahi H, Paul C and Lehmann S: APR-246 exhibits
anti-leukemic activity and synergism with conventional
chemotherapeutic drugs in acute myeloid leukemia cells. Eur J
Haematol. 86:206–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nahi H, Lehmann S, Mollgard L, Bengtzen S,
Selivanova G, Wiman KG, Paul C and Merup M: Effects of PRIMA-1 on
chronic lymphocytic leukaemia cells with and without hemizygous p53
deletion. Br J Haematol. 127:285–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nahi H, Merup M, Lehmann S, Bengtzen S,
Möllgård L, Selivanova G, Wiman KG and Paul C: PRIMA-1 induces
apoptosis in acute myeloid leukaemia cells with p53 gene deletion.
Br J Haematol. 132:230–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao W, Chen M, Zhao X, Kumar R, Spinnler
C, Thullberg M, Issaeva N, Selivanova G and Strömblad S:
PRIMA-1Met/APR-246 induces wild-type p53-dependent suppression of
malignant melanoma tumor growth in 3D culture and in vivo. Cell
Cycle. 10:301–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lambert JM, Gorzov P, Veprintsev DB,
Söderqvist M, Segerbäck D, Bergman J, Fersht AR, Hainaut P, Wiman
KG and Bykov VJ: PRIMA-1 reactivates mutant p53 by covalent binding
to the core domain. Cancer Cell. 15:376–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen J, Vakifahmetoglu H, Stridh H,
Zhivotovsky B and Wiman KG: PRIMA-1MET induces mitochondrial
apoptosis through activation of caspase-2. Oncogene. 27:6571–6580.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohell N, Alfredsson J, Fransson A,
Uustalu M, Byström S, Gullbo J, Hallberg A, Bykov VJ, Björklund U
and Wiman KG: APR-246 overcomes resistance to cisplatin and
doxorubicin in ovarian cancer cells. Cell Death Dis. 6:e17942015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Synnott NC, Murray A, McGowan PM, Kiely M,
Kiely PA, O'Donovan N, O'Connor DP, Gallagher WM, Crown J and Duffy
MJ: Mutant p53: A novel target for the treatment of patients with
triple-negative breast cancer? Int J Cancer. 140:234–246. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deben C, Lardon F, Wouters A, Op de Beeck
K, Van den Bossche J, Jacobs J, Van Der Steen N, Peeters M, Rolfo
C, Deschoolmeester V and Pauwels P: APR-246 (PRIMA-1(MET)) strongly
synergizes with AZD2281 (olaparib) induced PARP inhibition to
induce apoptosis in non-small cell lung cancer cell lines. Cancer
Lett. 375:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|