Introduction
Aspirin and other non-steroidal anti-inflammatory
drugs (NSAIDs) have been demonstrated to decrease the incidence,
progression and mortality of several types of cancer in randomized
trials (1–4). As previously reported,
phosphatidylcholine-associated NSAIDs (PC-NSAIDs) may be more
effective than NSAIDs alone in inhibiting tumor growth in
vitro and in vivo, in addition to their improved
gastrointestinal safety relative to unmodified NSAIDs (5–8).
Several observational studies have demonstrated that
NSAIDs were associated with decreased risk, recurrence and
mortality in breast cancer (9–11);
however, other cohort studies observed no effect (12,13). A
number of randomized controlled trials are currently underway that
aim to investigate the effect of aspirin on the treatment and
secondary prevention of breast cancer (14–16);
these results should shed light on the inconsistent epidemiological
data.
Breast tumors are classified into subtypes based on
a number of factors, including hormone receptor status and
amplification of the human epidermal growth factor receptor 2
(HER2) gene. These subtypes may reveal variations in cyclooxygenase
(COX) expression (17,18) or other factors that impact their
susceptibility to NSAID therapy. Limited observational and in
vitro studies have investigated the response of specific breast
cancer subtypes to NSAID therapy, but a consistent pattern has not
yet been unveiled (19–22).
To the best of our knowledge, the antitumor effects
of PC-NSAIDs in breast cancer have not been previously reported.
Likewise, the potent NSAID, indomethacin, has been largely
disregarded in breast cancer research, although it has been
demonstrated to be highly effective against the growth of colon and
pancreatic cancer both in vitro and in vivo (5,23).
Therefore, the present study was performed in order to investigate
the inhibitory effect of NSAIDs and PC-NSAIDs in cell lines
representing three major breast cancer subtypes. Antitumor activity
was measured as a decrease in cell number following culture with
aspirin, indomethacin and the PC-associated preparations of the two
drugs.
Materials and methods
Preparation of test drugs
For the NSAID trials, aspirin was purchased from
Solvay Pharmaceuticals and indomethacin from Spectrum Chemicals,
Ltd. PC (S-100 from Lipoid LLC) was dissolved in chloroform (Thermo
Fisher Scientific, Inc.), which was then evaporated under nitrogen
at 22°C. NSAID and PC stock solutions were prepared by diluting
each drug in serum-free culture medium and sonicating for 20 min at
30°C in a bath-type sonicator. Aspirin-PC was prepared by combining
the aforementioned stock solutions of aspirin and PC in serum-free
medium at an equal mass ratio, and sonicating for an additional 10
min. Indomethacin-PC was prepared by combining indomethacin and PC
at a 1:2 mass ratio, dissolved in acetone (Thermo Fisher
Scientific, Inc.). The acetone was then removed by vacuum
processing using a rotary evaporator and stock solution prepared by
dilution and sonication, as aforementioned. Complete descriptions
of the drug preparation procedures are available in previous
publications under patent (5,24,25).
Cell culture
Human cell lines were selected and are presented in
Table I. MCF-7, SK-BR-3 and
MDA-MB-231 cells were obtained from the laboratory of Dr Jeffrey
Chang at McGovern Medical School at UTHealth (Houston, TX, USA).
MCF-7 and MDA-MB-231 cells were cultured in DMEM with 5% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.), and SK-BR-3
cells were cultured in McCoy's medium with 5% FBS. Cells were
combined with each test drug and plated in 24 well plates at an
initial density of 4,000 cells/well (MCF-7 and MDA-MB-231) or 5,000
cells/well (SK-BR-3). Drug concentrations ranged from 0–180 µg/ml
for aspirin/aspirin-PC, and 0–50 µM for
indomethacin/indomethacin-PC. Concentrations were selected based on
previous studies that determined the toxic and therapeutic ranges
for each drug (5,6). Control wells of PC alone were run for
each drug and cell line. Cells were incubated for 8 days at 37°C in
5% CO2, with one change of medium on day 4. At least 4
replicate wells were plated for each drug and cell line.
 | Table I.Characteristics of the breast cancer
cell lines selected for use in the present study. |
Table I.
Characteristics of the breast cancer
cell lines selected for use in the present study.
| Cell line | Estrogen
receptor | Progesterone
receptor | HER2/neu
amplification | Subtype term |
|---|
| MCF-7 | + | + | − | Luminal A |
| SK-BR-3 | − | − | + | HER2-enriched |
| MDA-MB-231 | − | − | − | Triple
negative |
MTT assay
On day 8, an MTT assay was performed in order to
assess cell number. MTT reagent (Sigma-Aldrich; Merck KGaA) was
added to the wells at a concentration of 0.5 mg/ml. Cells were then
incubated for 4 h at 37°C. MTT and culture medium were removed and
a solvent (90% isopropanol, 0.2% sodium dodecyl sulfate and 0.01 M
HCl) was added to extract the formazan product. The plates were
read at 570 nm. The average optical density reading for each well
was converted to a ratio relative to untreated control wells from
the same plate.
ELISA
Cell culture supernatant was collected on day 4 and
assayed for prostaglandin E2 (PGE2) using the PGE2 ELISA
kit-monoclonal from Cayman Chemical Company (cat. no. 514010). PGE2
activity was used as a marker of COX-2 activity.
Statistical analysis
Data are presented as the mean optical density
reading relative to the untreated control. StatView software
(version 5.01; SAS Institute Inc.) was used for the statistical
analysis. One-way analysis of variance, with a Tukey-Kramer
post-hoc test, was used to analyze the decrease in proliferation at
each drug dosage vs. the untreated control, and to compare the
decrease in cell proliferation as a result of each drug tested
(NSAID, PC-NSAID and PC). P<0.05 was considered to indicate a
statistically significant difference. Significant decreases in
proliferation relative to the control for each drug are indicated
by an asterisk (*) in the figures. Significant differences between
tested drugs are indicated by a caret (^) in the figures.
Results
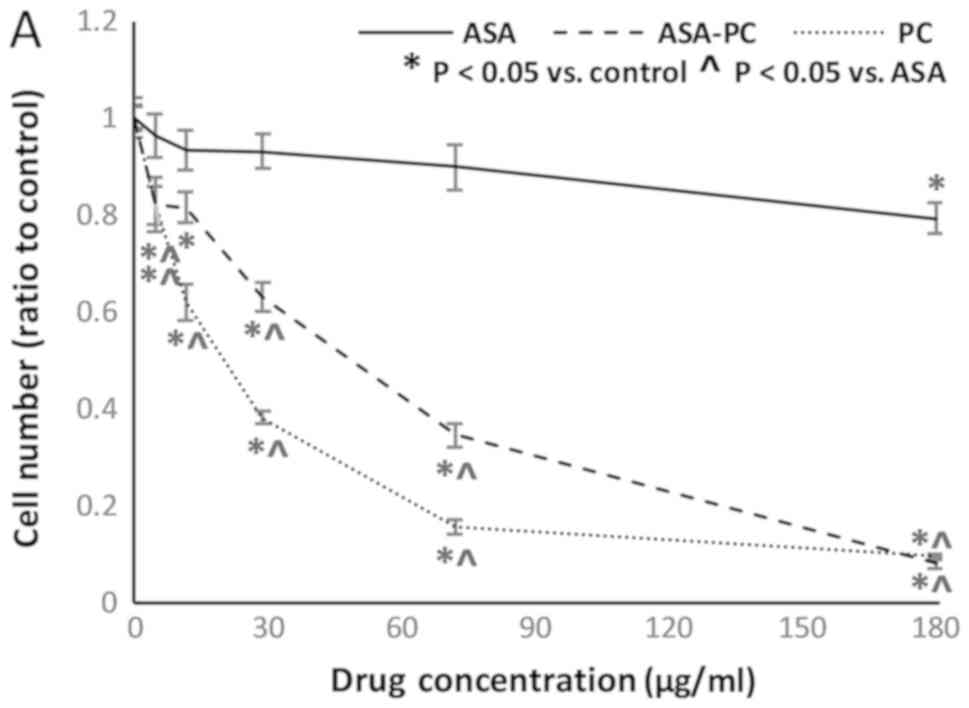
MCF-7 (Luminal A) cell culture
MCF-7 cells, the estrogen receptor positive
(ER+)/progesterone receptor positive (PR+) cell line, were
unaffected by aspirin even at the highest concentration of 180
µg/ml (Fig. 1A). However, they were
strongly inhibited by aspirin-PC at much lower doses. A control
with PC alone demonstrated similar effectiveness, significantly
reducing cell number even at the lowest dose of 5 µg/ml. Thus, PC
was determined to have a marked anti-tumor effect in this cell
line, which potentially accounted for all the observed effects of
aspirin-PC. According to the relative percentage of the decrease in
cell number vs. control, indomethacin was the more effective of the
two NSAIDs in the MCF-7 line across their respective dose ranges,
with modest but significant reduction of cell number at doses as
low as 12 µM, and an ~50% decrease in cell number at the highest
dose of 50 µM (Fig. 1B).
Indomethacin-PC was highly effective at limiting MCF-7 cell number,
reaching a decrease of 58% at 8 µM and almost complete elimination
of cells at 50 µM. In contrast to the experiment with aspirin-PC,
in which the PC-NSAID and PC alone showed very similar results, the
effect of indomethacin-PC was greater than that of either the NSAID
or PC alone. It is unclear whether the decreases in cell number
caused by each drug are attributable to decreased proliferation,
increased apoptosis or both.
MDA-MB-231 (triple negative) cell
culture
MDA-MB-231, the triple negative cell line, exhibited
a very similar response to MCF-7 in the aspirin experiments. Here,
aspirin did cause a minimal decrease in cell number, but only
reached a significant decrease at the highest dose of 180 µg/ml
with 21% fewer cells than control (Fig.
2A). Again, both aspirin-PC and PC alone caused a similar and
marked decrease in cell number even at low doses. Indomethacin was
more effective than aspirin at its highest dose (50 µM), leading to
a 33% decrease in cell number, but had minimal and non-significant
effects at lower doses. Indomethacin-PC was highly effective
against MDA-MB-231 cells (Fig. 2B).
A significant decrease in cell number was observed at doses as low
as 3.2 µM, with a 78% decrease at the 50 µM dose. As in the MCF-7
line, indomethacin-PC was substantially more effective than
indomethacin or PC alone against the MDA-MB-231 tumor cells.
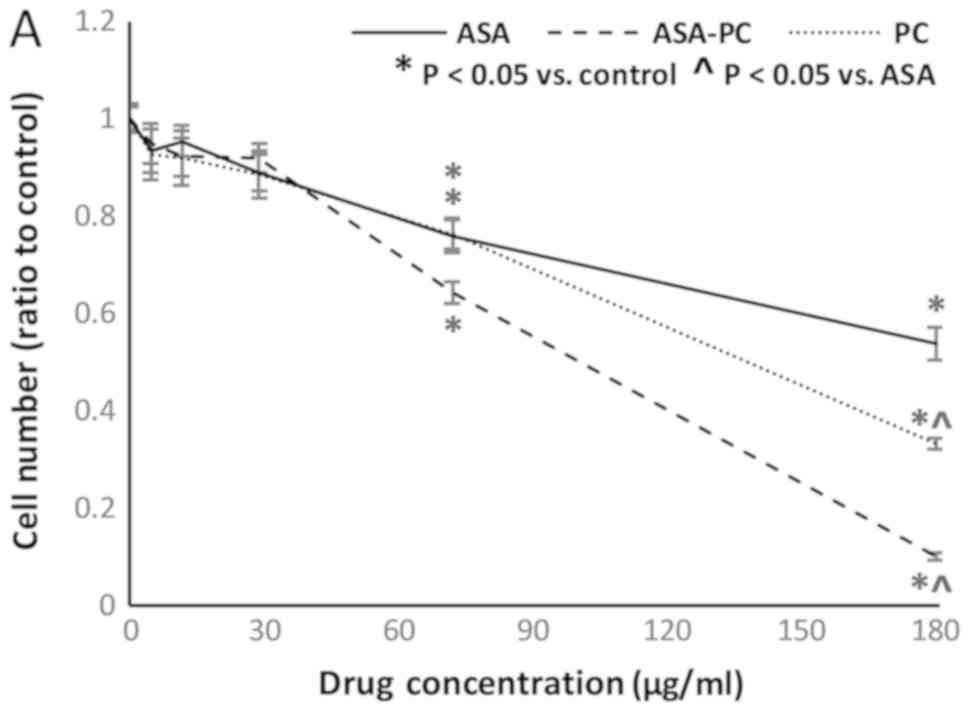
SK-BR-3 (HER2 enriched) cell
culture
SK-BR-3 cells, the HER2/neu+ cell line, were the
most responsive of the three cell lines to aspirin, with a 48%
decrease in cell number at 180 µg/ml (Fig. 3A). This was also the only cell line
in which aspirin-PC was more effective than either aspirin or PC
alone, although this effect was not observed until doses of 72
µg/ml and above were reached. It was also the only cell line in
which aspirin and aspirin-PC were more effective than indomethacin
and indomethacin-PC, respectively, at their maximum doses (Fig. 3B). However, the effects of
indomethacin were observed at lower concentrations, with
significant decreases in cell number at doses as low as 8 µM for
indomethacin and 3.2 µM for indomethacin-PC. Both PC-NSAIDs were
more effective than the NSAID or PC alone in this cell line.
ELISA
In the ELISA assay, native expression of PGE2 was
low for all cell lines, with values ranging from 5–30 pg/ml of PGE2
in the collected supernatant. These values were at the low end of
the ELISA sensitivity and could not be reliably distinguished from
the background signal of the culture medium + 5% FBS. Thus, the
variable effects of the drugs used on each cell line in the present
study could not be attributed to their differences in COX
expression or activity.
Discussion
The results from the present study are consistent
with those from previous publications that demonstrated that NSAIDs
and PC-NSAIDs inhibited the growth of colon, ovarian and pancreatic
cancer cells in vitro (5,6,23). As with the previous studies,
PC-NSAIDs were more effective than the NSAID alone for all cell
lines and drugs tested in the present study, and markedly so in
certain cases. Furthermore, the results from the present study
indicate that the PC component of the complexed drug possesses an
independent anti-tumor action, resulting in an additive or
synergistic drug effect, rather than PC serving merely to deliver
the NSAIDs more efficiently to their site of action. Although
PC-NSAIDs have not yet been evaluated in an in vivo model of
breast cancer, PC-NSAIDs have, in certain cases, exhibited greater
antitumorigenic effects than the NSAID alone in rodent models of
colon and ovarian cancers (5,6).
PC-NSAIDs have an excellent safety profile in vivo, as
demonstrated in both preclinical and clinical trials (8,26–28).
PC-NSAIDs have also been demonstrated as non-toxic to the
non-cancerous gingival epithelium (29), suggesting a tumor-selective action.
Indomethacin-PC looks particularly promising as a therapeutic agent
in breast cancer since it was effective at a low dose in all three
breast cancer cell lines, and it also exhibited a clear synergistic
effect with the PC and drug components.
Although the results from the present study are
quite promising, there were some limitations. First, the drugs used
in the present study were not tested in vivo, although this
will become the focus of future studies. The in vitro
results from the present study, while suggestive, require
additional evidence in order to be confirmed before being applied
clinically. Secondly, the present study was designed with a single
incubation time of 8 days based on previous successful results
studying non-breast cancer cell lines (5,6). This
method does not allow the assessment of time dependency of the
cells' response to the test drugs over the course of the
experiment. Finally, a control line representing normal cells was
not included due to concerns that the available lines were not
truly representative of normal cells and could not be accurately
compared with tumor lines. Immortalized cells, such as the MCF10A
cell line, possess genetic modifications that are typical of cancer
cells rather than normal cells, including telomerase activation
(30), and the MCF10A line was
demonstrated to be phenotypically unrepresentative of normal
mammary tissue (31). Both
immortalized and primary cell lines require potent growth factors
not present in tumor cell media, limiting comparisons. As an
appropriate control was unavailable, it is not known how normal
mammary cells would respond to the drugs tested in the present
study. Further research, particularly in vivo trials, will
be instrumental to address these unresolved questions and further
assess the clinical utility of the tested drugs in breast cancer
treatment.
The activity of NSAIDs against breast cancer is
traditionally attributed to inhibition of the COX-2 enzyme, which
is overexpressed in certain forms of breast tumor (32) and may be associated with poor
prognosis (33–35). However, the results from the ELISA in
the present and previous experiments have not convincingly
demonstrated a dominant role for COX inhibition, despite the
effectiveness of the drugs in limiting tumor cell number (5). In the Nurses' Health Study, the
survival benefit of aspirin was revealed to be independent of COX-2
expression in breast tumors (35). A
number of COX-independent mechanisms for the chemopreventive and
chemotherapeutic effects of NSAIDs have been proposed (36). For example, indomethacin can modify
the behavior of cell membranes, potentially affecting intracellular
signaling pathways (37). Sulindac
and other NSAIDs have demonstrated proapoptotic and antiangiogenic
effects via non-COX-mediated mechanisms (36). In addition, new evidence has
suggested that the ability of aspirin to decrease breast cancer
invasion and metastasis may be platelet-mediated (38). This coincides with a previous study,
which demonstrated that activated platelets increased proliferation
of colon cancer in both in vitro and in vivo mouse
models, while treatment with aspirin and aspirin-PC decreased
platelet counts and activation, which was correlated with decreased
tumor burden (24). Platelet effects
may account for the apparent effectiveness of aspirin against
breast cancer in epidemiological studies despite the minimal direct
antitumor effect of the drug in previous in vitro studies,
which were performed in the absence of platelets (5,6,39). The authors of the present study aim
to investigate the role of platelets and NSAID-induced platelet
inhibition in breast cancer in future projects.
The significant and consistent anti-tumor activity
of phosphatidylcholine in the present study was unanticipated. PC
is commonly used to prepare liposomes for the delivery of drugs,
including chemotherapeutic agents, under the assumption that
liposomes may help the drug reach its target tissue while
minimizing systemic toxicity (40).
However, the majority of currently published studies have not used
empty liposomes as a control; a few have attempted this and
demonstrated that liposomes composed of PC alone exhibited
independent antitumor activity (41,42). For
example, empty PC liposomes decreased tumor size and metastasis in
a mouse model of pancreatic cancer (41). In another study, liposomes containing
PC and the omega-3 fatty acid docosahexaenoic acid decreased
metastases from colon and hepatic cancer in mice (42). Dietary PC was also observed to
inhibit the growth of hepatocellular carcinoma in rats by inducing
apoptosis, without impairing liver function (43). The mechanism underlying the potential
anti-tumor effect of PC is currently unclear, but may be
attributable to affecting the activity of phospholipase enzymes,
including phospholipase A2 (PLA2), which is highly expressed in
certain types of breast cancer (44,45). PC
cleavage by PLA2 generates lyso-PC, which is cytotoxic in high
concentrations (46,47) and may accumulate differentially
around PC-exposed tumor cells due to their excessive enzyme
activity. High concentrations of PC may also modify cellular
activity by altering membrane fluidity and signaling pathways
(48–51). Abnormal phospholipase signaling is
known to play a key role in tumorigenesis in a number of different
types of cancer, and has been suggested as a promising pathway for
pharmaceutical intervention (52).
PC may open up this potential, particularly when used in
combination with other effective drugs, such as NSAIDs.
Acknowledgements
The authors would like to thank Dr Dexing Fang from
the McGovern Medical School at UTHealth (Houston, USA), who
assisted with the ELISA procedures and advised on cell culture
techniques, and Dr Elizabeth J. Dial from the McGovern Medical
School at UTHealth (Houston, USA), who advised on drug preparation
and interpretation of the results.
Funding
The present study was funded with discretionary
funds from the authors.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LL designed and directed the present study in
consultation with SB, interpreted the results and contributed to
writing the article. SB performed the cell cultures, ELISA,
statistical analyses and was the primary author of the article.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
LL is a co-founder and shareholder in PLx Pharma
Inc., which is developing PC-NSAIDs for commercial use. SB has
declared no competing interests.
Glossary
Abbreviations
Abbreviations:
|
NSAID
|
non-steroidal anti-inflammatory
drug
|
|
PC
|
phosphatidylcholine
|
|
COX
|
cyclooxygenase
|
|
PGE2
|
prostaglandin E2
|
|
PLA2
|
phospholipase A2
|
References
|
1
|
Rothwell PM, Fowkes FG, Belch JF, Ogawa H,
Warlow CP and Meade TW: Effect of daily aspirin on long-term risk
of death due to cancer: Analysis of individual patient data from
randomised trials. Lancet. 377:31–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mills EJ, Wu P, Alberton M, Kanters S,
Lanas A and Lester R: Low-dose aspirin and cancer mortality: A
meta-analysis of randomized trials. Am J Med. 125:560–567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Algra AM and Rothwell PM: Effects of
regular aspirin on long-term cancer incidence and metastasis: A
systematic comparison of evidence from observational studies versus
randomised trials. Lancet Oncol. 13:518–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lichtenberger LM, Phan T, Fang D and Dial
EJ: Chemoprevention with phosphatidylcholine non-steroidal
anti-inflammatory drugs in vivo and in vitro. Oncol
Lett. 15:6688–6694. 2018.PubMed/NCBI
|
|
6
|
Huang Y, Lichtenberger LM, Taylor M,
Bottsford-Miller JN, Haemmerle M, Wagner MJ, Lyons Y, Pradeep S, Hu
W, Previs RA, et al: Antitumor and antiangiogenic effects of
aspirin-PC in ovarian cancer. Mol Cancer Ther. 15:2894–2904. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dial E, Doyen JR and Lichtenberger LM:
Phosphatidylcholine- associated nonsteroidal anti-inflammatory
drugs (NSAIDs) inhibit DNA synthesis and the growth of colon cancer
cells in vitro. Cancer Chemother Pharmacol. 57:295–300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cryer B, Bhatt DL, Lanza FL, Dong JF,
Lichtenberger LM and Marathi UK: Low-dose aspirin-induced
ulceration is attenuated by Aspirin-Phosphatidylcholine: A
randomized clinical trial. Am J Gastroenterol. 106:272–277. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takkouche B, Regueira-Méndez C and Etminan
M: Breast cancer and use of nonsteroidal anti-inflammatory drugs: A
meta-analysis. J Natl Cancer Inst. 100:1439–1447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holmes MD, Chen WY, Li L, Hertzmark E,
Spiegelman D and Hankinson SE: Aspirin intake and survival after
breast cancer. J Clin Oncol. 28:1467–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fraser DM, Sullivan FM, Thompson AM and
Mccowan C: Aspirin use and survival after the diagnosis of breast
cancer: A population-based cohort study. Br J Cancer. 111:623–627.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egan KM, Stampfer MJ, Giovannucci E,
Rosner BA and Colditz GA: Prospective study of regular aspirin use
and the risk of breast cancer. J Natl Cancer Inst. 88:988–993.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menamin ÚM, Cardwell C, Hughes C and
Murray L: Low-dose aspirin use and survival in breast cancer
patients: A nationwide cohort study. Cancer Epidemiol. 48:1582017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Add-aspirin, . A trial assessing the
effects of aspirin on disease recurrence and survival after primary
therapy in common non metastatic solid tumours status: Recruiting.
First posted 2017. ClinicalTrials.gov identifier: NCT02804815.
https://clinicaltrials.gov/ct2/show/NCT02804815
|
|
15
|
Aspirin in preventing recurrence of cancer
in patients with HER2 negative stage II–III breast cancer after
chemotherapy, surgery and/or radiation therapy status, .
Recruiting. First posted 2016. ClinicalTrials.gov identifier:
NCT02927249. https://clinicaltrials.gov/ct2/show/NCT02927249
|
|
16
|
Low dose chemotherapy with aspirin in
patients with breast cancer after neoadjuvant chemotherapy status,
. Unknown status. First posted 2012. ClinicalTrials.gov identifier:
NCT01612247. https://clinicaltrials.gov/ct2/show/NCT01612247
|
|
17
|
Kennedy BM and Harris RE: Cyclooxygenase
and lipoxygenase gene expression in the inflammogenesis of breast
cancer. Inflammopharmacology. 2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu XH and Rose DP: Differential
expression and regulation of cyclooxygenase-1 and −2 in two human
breast cancer cell lines. Cancer Res. 56:5125–5127. 1996.PubMed/NCBI
|
|
19
|
Clarke CA, Canchola AJ, Moy LM, Neuhausen
SL, Chung NT, Lacey JV Jr and Bernstein L: Regular and low-dose
aspirin, other non-steroidal anti-inflammatory medications and
prospective risk of HER-2 defined breast cancer: The california
teachers study. Breast Cancer Res. 19:522017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marshall SF, Bernstein L, Anton-Culver H,
Deapen D, Horn-Ross PL, Mohrenweiser H, Peel D, Pinder R, Purdie
DM, Reynolds P, et al: Nonsteroidal anti-inflammatory drug use and
breast cancer risk by stage and hormone receptor status. J Natl
Cancer Inst. 97:805–812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Smith-Warner SA, Collins LC,
Rosner B, Willett WC and Hankinson SE: Use of aspirin, other
nonsteroidal anti-inflammatory drugs, and acetaminophen and
postmenopausal breast cancer incidence. J Clin Oncol. 30:3468–3477.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amaral MEA, Nery LR, Leite CE, de Azevedo
Junior WF and Campos MM: Pre-clinical effects of metformin and
aspirin on the cell lines of different breast cancer subtypes.
Invest New Drugs. 36:782–796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta R, Fang D and Lichtenberger LM:
Mo1985-evaluation of the inhibitory efficacy and potency of
PC-NSAIDs on pancreatic cancer cells in culture. Gastroenterol. 154
(Suppl 6):S–872. 2018. View Article : Google Scholar
|
|
24
|
Lichtenberger LM, Fang D, Bick RJ,
Poindexter BJ, Phan T, Bergeron AL, Pradhan S, Dial EJ and Vijayan
KV: Unlocking aspirin's chemopreventive activity: Role of
irreversibly inhibiting platelet cyclooxygenase-1. Cancer Prev Res
(Phila). 10:142–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lichtenberger LM: Purified
phospholipid-non-steroidal anti-inflammatory drug associated
compositions and methods for preparing and using same. US patent
8,802,656 B2. Filed October 12, 2005; issued August 12 2014.
|
|
26
|
Lichtenberger LM, Wang ZM, Romero JJ,
Ulloa C, Perez JC, Giraud MN and Barreto JC: Non-steroidal
anti-inflammatory drugs (NSAIDs) associate with zwitterionic
phospholipids: Insight into the mechanism and reversal of
NSAID-induced gastrointestinal injury. Nat Med. 1:154–158. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lichtenberger L, Romero JJ and Dial EJ:
Gastrointestinal safety and therapeutic efficacy of parenterally
administered phosphatidylcholine-associated indomethacin in rodent
model systems. Br J Pharmacol. 157:252–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lanza FL, Marathi UK, Anand BS and
Lichtenberger LM: Clinical trial: Comparison of
ibuprofen-phosphatidylcholine and ibuprofen on the gastrointestinal
safety and analgesic efficacy in osteoarthritic patients. Aliment
Pharmacol Ther. 28:431–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lichtenberger LM, Dial EJ and Fang D: Use
of PC-NSAIDs to protect gingival cells from injury due to cytotoxic
agents. FASEB J. 31:993.9. 2017.
|
|
30
|
Qu Y, Han B, Yu Y, Yao W, Bose S, Karlan
BY, Giuliano AE and Cui X: Evaluation of MCF10A as a reliable model
for normal human mammary epithelial cells. PLoS One.
10:e01312852015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Newbold RF: The significance of telomerase
activation and cellular immortalization in human cancer.
Mutagenesis. 17:539–550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Howe LR: Inflammation and breast cancer.
Cyclooxygenase/prostaglandin signaling and breast cancer. Breast
Cancer Res. 9:2102007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chikman B, Vasyanovich S, Lavy R, Habler
L, Tolstov G, Kapiev A, Halevy A and Sandbank J: COX2 expression in
high-grade breast cancer: Evidence for prognostic significance in
the subset of triple-negative breast cancer patients. Med Oncol.
31:9892014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ristimäki A, Sivula A, Lundin J, Lundin M,
Salminen T, Haglund C, Joensuu H and Isola J: Prognostic
significance of elevated cyclooxygenase-2 expression in breast
cancer. Cancer Res. 62:632–635. 2002.PubMed/NCBI
|
|
35
|
Holmes MD, Chen WY, Schnitt SJ, Collins L,
Colditz GA, Hankinson SE and Tamimi RM: COX-2 expression predicts
worse breast cancer prognosis and does not modify the association
with aspirin. Breast Cancer Res Treat. 130:657–662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gurpinar E, Grizzle WE and Piazza GA:
NSAIDs inhibit tumorigenesis, but how? Clin Cancer Research.
20:1104–1113. 2014. View Article : Google Scholar
|
|
37
|
Zhou Y, Plowman SJ, Lichtenberger LM and
Hancock JF: The anti-inflammatory drug indomethacin alters
nanoclustering in synthetic and cell plasma membranes. J Biol Chem.
285:35188–35195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johnson KE, Ceglowski JR, Roweth HG,
Forward JA, Tippy MD, El-Husayni S, Kulenthirarajan R, Malloy MW,
Machlus KR, Chen WY, et al: Aspirin inhibits platelets from
reprogramming breast tumor cells and promoting metastasis. Blood
Adv. 3:198–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lichtenberger LM and Vijayan KV: Are
platelets the primary target of aspirin's remarkable anticancer
activity? Cancer Res. 79:3820–3823. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sercombe L, Veerati T, Moheimani F, Wu SY,
Sood AK and Hua S: Advances and challenges of liposome assisted
drug delivery. Front Pharmacol. 6:2862015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Graeser R, Bornmann C, Esser N, Ziroli V,
Jantscheff P, Unger C, Hopt UT, Schaechtele C, Von Dobschuetz E and
Massing U: Antimetastatic effects of liposomal gemcitabine and
empty liposomes in an orthotopic mouse model of pancreatic cancer.
Pancreas. 38:330–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ichihara H, Zako K, Komizu Y, Goto K and
Ueoka R: Therapeutic effects of hybrid liposomes composed of
phosphatidylcholine and docosahexaenoic acid on the hepatic
metastasis of colon carcinoma along with apoptosis in vivo. Biol
Pharm Bull. 34:901–905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sakakima Y, Hayakawa A, Nagasaka T and
Nakao A: Prevention of hepatocarcinogenesis with
phosphatidylcholine and menaquinone-4: In vitro and in vivo
experiments. J Hepatol. 47:83–92. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Steiner MR: Localization and
characterization of phospholipase A2 in mouse mammary gland derived
cells. Arch Biochem Biophys. 286:293–299. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rillema JA, Osmialowski EC and Linebaugh
BE: Phospholipase A 2 activity in
9,10-dimethyl-1,2-benzanthracene-induced mammary tumors of rats.
Biochim Biophys Acta. 617:150–155. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lutz J, Augustin AJ, Jäger LJ, Bachmann D
and Brandl M: Acute toxicity and depression of phagocytosis in vivo
by liposomes: Influence of lysophosphatidylcholine. Life Sci.
56:99–106. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang MC, Lee JJ, Chen YJ, Lin SI, Lin LD,
Jein-Wen Liou E, Huang WL, Chan CP, Huang CC and Jeng JH:
Lysophosphadylcholine induces cytotoxicity/apoptosis and IL-8
production of human endothelial cells: Related mechanisms.
Oncotarget. 8:106177–106189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Barenholz Y: Sphingomyelin-lecithin
balance in membranes: Composition, structure and function
relationshipsPhysiology of Membrane Fluidity. 1. Shinitzky M: CRC
Press; Florida: pp. 131–174. 1984
|
|
49
|
Cossins AR and Sinensky M: Adaptation of
membranes to temperature, pressure and exogenous lipidsPhysiology
of Membrane Fluidity. 2. Shinitzky M: CRC Press; Florida: pp. 1–20.
1984
|
|
50
|
Van Blitterswijk WJ: Alterations in lipid
fluidity in the plasma membrane of tumor cellsPhysiology of
Membrane Fluidity. 2. Shinitzky M: CRC Press; Florida: pp. 53–84.
1984
|
|
51
|
Lichtenberger LM, Zhou Y, Jayaraman V,
Doyen JR, O'Neil RG, Dial EJ, Volk DE, Gorenstein DG, Boggara MB
and Krishnamoorti R: Insight into NSAID-induced membrane
alterations, pathogenesis and therapeutics: Characterization of
interaction of NSAIDs with phosphatidylcholine. Biochim Biophys
Acta. 1821:994–1002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Park JB, Lee CS, Jang JH, Ghim J, Kim YJ,
You S, Hwang D, Suh PG and Ryu SH: Phospholipase signalling
networks in cancer. Nat Rev Cancer. 12:782–792. 2012. View Article : Google Scholar : PubMed/NCBI
|