Introduction
Osteosarcoma is a malignant bone tumor that occurs
in children and young people with an incidence of 2–5 per million
and in older adults with an incidence of 1.5–5 per million
(1,2). The incidence rate and occurrence age of
osteosarcoma in children and young people is relatively stable with
little geographic variation, whereas they vary in older patients
(1,2). The treatment strategy of osteosarcoma
is commonly based on surgical resection combined with systemic
chemotherapy, including neoadjuvant chemotherapy, followed by
restoration of limb function (3–5). In
addition, the five-year overall survival rate of patients with
osteosarcoma has significantly increased from <30 to >70%,
which may be due to the use of neoadjuvant chemotherapy (6).
The use of chemotherapy for patients with
osteosarcoma consists of adjuvant and neoadjuvant chemotherapy,
including doxorubicin, cisplatin, methotrexate or cyclophosphamide
(7–9). Chemotherapy induces tumor necrosis,
promotes surgical resection and inhibits micrometastasis (10–14).
However, drug resistance reduces the effect of chemotherapy
(15,16). Refining the chemotherapy regimen to
improve the prognosis in patients with osteosarcoma remains
challenging for scientists (15,16).
Radiotherapy is an adjuvant treatment for
osteosarcoma that can inhibit tumor cell activity, reduce the local
recurrence rate and prolong the overall survival of patients with
osteosarcoma (17–20). Radiotherapy can be offered to
patients with inoperable tumors or patients who cannot tolerate
chemotherapy (14,21,22).
The poor prognosis of patients with osteosarcoma has
remained a persistent problem in the last decades, in particular
for patients with inoperable tumors or metastasis, even when
chemotherapy duration is prolonged, the dose is increased, or an
immune treatment is adopted (6).
Considering the young age of patients with osteosarcoma, the
malignant nature of osteosarcoma, the absence of one single
specific therapeutic method, the significant side effects and poor
overall effects, it is crucial to develop a novel and effective
therapy with low toxic effects to treat patients with osteosarcoma
(23).
Engert et al (24) reported the presence of the BRCAness
phenomenon in osteosarcoma and demonstrated that poly (ADP-ribose)
polymerase inhibitors targeting breast cancer 1/2 (BRCA1/2)
mutations in patients with breast cancer can also inhibit
osteosarcoma cell proliferation, which suggests that the
BRCA gene could be associated with the occurrence and
development of osteosarcoma (24–27).
At present, the combination of neoadjuvant
chemotherapy and surgery remains the first-line treatment applied
to patients with osteosarcoma. The combination of radiotherapy and
chemotherapy has been used for patients with metastasis or
recurrence, patients unsuitable for surgery and patients refusing
surgery (14,28). Furthermore, it has been demonstrated
that the combined use of radiotherapy and chemotherapy can benefit
the survival of patients with osteosarcoma and increase the rate of
limb salvage (29). The present
study investigated the effect of the combined radiation and
cisplatin treatment on the malignant osteosarcoma cell line MG-63
and the BRCA1-associated signaling pathways. The findings from the
present study may provide a basis for the clinical application of
radiation and cisplatin therapy for osteosarcoma.
Materials and methods
Cell line and reagents
The MG-63 osteosarcoma cell line was purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. The bicinchoninic acid (BCA) protein assay kit was
purchased from Beijing Biomedical Co., Ltd. PVDF membranes were
purchased from EMD Millipore. Skimmed milk powder was purchased
from Sangon Biotech (Shanghai) Co., Ltd.
Cell culture and determination of cell
proliferation
The osteosarcoma cell line MG-63 was cultured in
H-Dulbecco's Modified Eagle medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Biological lndustries) and 1%
antibiotics penicillin and streptomycin (Beijing Solarbio Science
& Technology Co., Ltd.) and placed at 37°C in a humidified
incubator containing 5% CO2. Cells
(2×103/well in 100 µl) in the logarithmic growth stage
were seeded in a 96-well plate and cultured overnight. Cells were
then treated by radiation (0, 0.5, 1, 1.5 and 2 Gy) and/or
cisplatin (0, 5, 10, 20 and 40 µg/ml) at 37°C for 24 h. For
combined treatment, radiation was applied first and followed by
cisplatin treatment. Following 12 h culture, cell proliferation was
determined using a Cell Counting Kit-8 (CCK-8; 7seaPharm
Technology, Co. Ltd.) according to the manufacturer's protocol. The
absorbance was measured at 450 nm with a microplate reader.
Determination of cell apoptosis
MG-63 cells in the logarithmic growth stage were
seeded in a 6-well plate at a density of 2×105/2 ml/well
and cultured overnight. Cells were treated by radiation and/or
cisplatin as aforementioned. Following 12 h culture, cells were
collected, and apoptosis was determined using Annexin V/propidium
iodide (PI) (BD Biosciences; cat. no. 559763) according to the
manufacturer's instructions. Briefly, cells were washed twice with
cold PBS and resuspended in 1X Binding Buffer (BD Biosciences; cat.
no. 51-66121E) at the concentration of 1×106 cells/ml.
The cell suspension (100 µl, 1×105 cells) was
transferred into a 5 ml culture tube. Annexin V-PE (5 µl; BD
Biosciences; cat. no. 51-65875X) and 5 µl 7-Amino-actinomycin D (BD
Biosciences; cat. no. 51-68981E) were added. The solution was
gently mixed and incubated for 15 min at room temperature in the
dark. Binding Buffer (400 µl) was added to each tube. Cells were
analyzed by flow cytometry within 1 h. The results were analyzed
using CytExpert 1.2 software (Beckman Coulter, Inc.).
Determination of cell cycle
MG-63 cells in the logarithmic growth stage were
seeded in a 6-well plate at a density of 2×105/2 ml/well
and cultured overnight. Cells were treated by radiation and/or
cisplatin as aforementioned. Following 12 h culture, cells were
collected in 500 µl of 0.1% Triton X-100 PBS buffer containing 12.5
µl PI and 10 µl RNase A and incubated in a CO2 incubator
at 37°C for 30 min. Cell cycle distribution was determined using an
EPICS-XL flow cytometer (Beckman Coulter, Inc.). The results were
analyzed using CytExpert 1.2 software.
Examination of cell migration
MG-63 cells in the logarithmic growth stage were
seeded in a 24-well Transwell (pore size, 8 µm) insert at a density
of 5×103/200 µl/well. The upper and lower chambers were
filled with 1 ml serum-free medium and 1 ml of 10% FBS-containing
medium, respectively. Following 12 h culture, cells in the upper
chamber were treated with radiation and/or cisplatin as
aforementioned, and medium with 10% FBS was added to the lower
chamber for 12 h. Migrated cells were fixed with 1 ml of 100%
methanol for 20 min at room temperature. After washing with PBS,
cells were incubated with 1 ml of 0.5% crystal violet staining
solution at 37°C for 30 min. After washing with PBS, stained cells
were examined using a light microscope (magnification, ×400;
TH4-100; Olympus Corporation) and counted in five random fields of
the images. The means of cell number per field in each treatment
group were calculated and compared.
Determination of mRNA expression
levels by reverse transcription-quantitative PCR (RT-qPCR)
MG-63 cells in the logarithmic growth stage were
seeded in a 6-well plate at a density of 2×105/2 ml/well
and cultured overnight. Cells were treated by radiation and/or
cisplatin as aforementioned for 12 h. Cells were collected, and
total RNA was extracted using a RaPure Total RNA Micro kit
(Guangzhou Magen Biotechnology Co., Ltd.) according to the
manufacturer's protocol. cDNA was synthesized using M-MLV (Promega
Corporation) according to the manufacturer's protocol. RT-qPCR
reactions were performed using a Stratagene Mx3000P (Agilent
Technologies, Inc.) and SYBR Premix (Takara Bio, Inc.) according to
manufacturer's protocol. The thermocycling conditions of the real
time PCR were as follows: 95°C for 30 sec, 40 cycles at 95°C for 10
sec and 60°C for 30 sec, then 60°C for 60 sec and 95°C for 15 sec.
GAPDH was used as the reference gene. The primers were provided by
Beijing Biomedical Co. Ltd. and designed as follows: BRCA1 forward,
5′-GCTGCTGCTCATACTACTG-3′ and reverse, 5′-CCACATCTCCTCTGACTTC-3′;
p53 forward, 5′-ACCACCATCCACTACAACTAC-3′ and reverse,
5′-ACAAACACGCACCTCAAA-3′; and GAPDH forward,
5′-ATCCCATCACCATCTTCC-3′ and reverse, 5′-TGACCCTTTTGGCTCCCC-3′. The
relative expressions levels were normalized to endogenous controls
and were expressed as 2−ΔΔCq (30).
Western blotting
MG-63 cells in the logarithmic growth stage were
seeded in a 6-well plate at a density of 2×105/2 ml/well
and cultured overnight. Cells were treated by radiation and/or
cisplatin as aforementioned for 12 h. Cells were lysed using lysis
buffer (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) and
subjected to a cycle of freezing at −70°C and thawing at 37°C (1 h
per step). The protein concentration was measured using the BCA
kit. Proteins (30 µg) were separated by 12% SDS-PAGE and
transferred onto PVDF membranes. Membranes were blocked with 5%
skimmed milk dissolved in TBS containing 0.05% Tween-20 (TBST) at
37°C for 1 h and incubated with antibodies against BRCA1 (1:700;
Abcam; cat. no. ab238983), p53 (1:700; Abcam; cat. no. ab131442),
Bcl-2 (1:700; Abcam; cat. no. ab196495), Bax (1:700; Abcam; cat.
no. ab53154) and GAPDH (1:700; Abcam; cat. no. ab9485) at 4°C
overnight. Following washing with TBST, membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (1:1,000; ABclonal Biotech Co.,
Ltd.; cat. no. AS011) dissolved in TBST buffer containing 5%
skimmed milk at 37°C for 1 h. Following washing with TBST, enhanced
chemiluminescence reagent (7sea Pharm Technology, Co. Ltd) was used
to detect the signal on the membrane. Relative expression level of
the proteins was normalized to the endogenous control using
Quantity One software (version 4.6.9; Bio-Rad Laboratories,
Inc.).
Statistical analysis
Data were expressed as the means ± standard
deviation of three independent experiments. Statistical analysis
was performed using SPSS software v21.0 (IBM Corp.). Differences
among groups were analyzed using one-way ANOVA followed by Least
Significant Difference post-hoc analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of radiation and cisplatin on
MG-63 cell proliferation
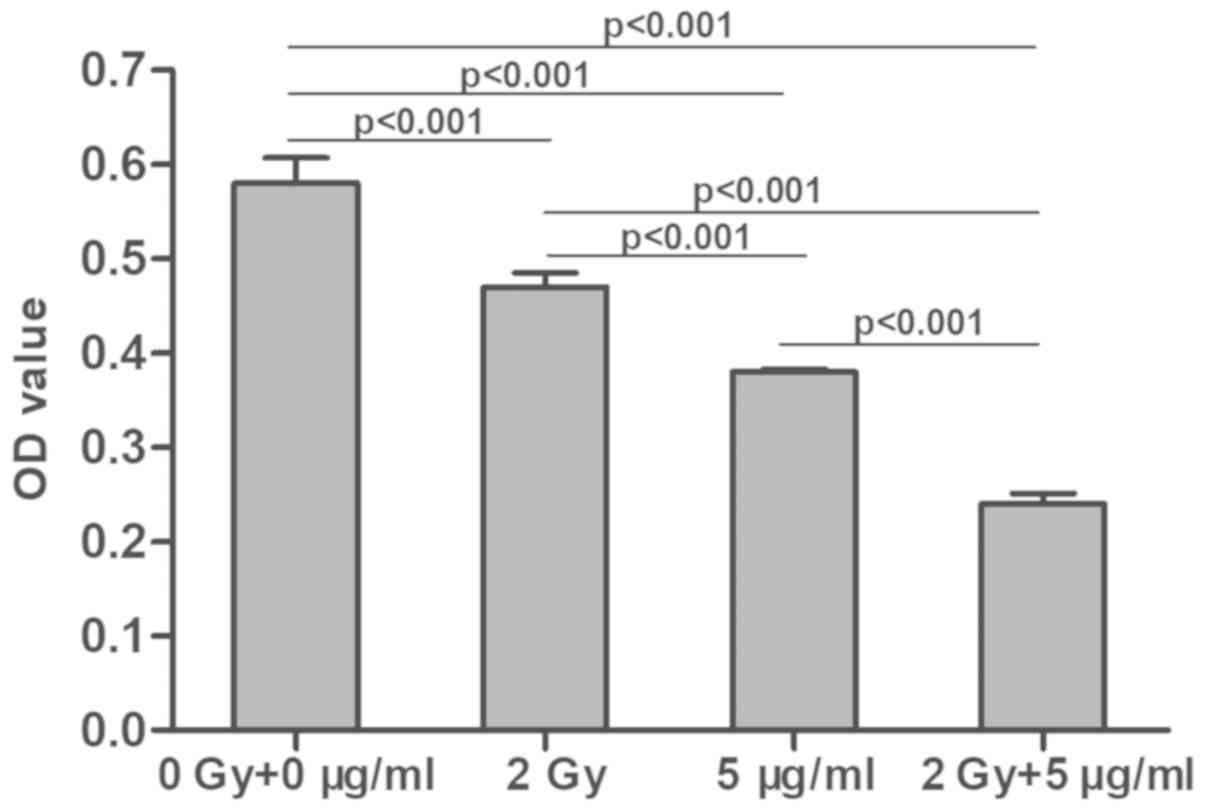
Following treatment with radiation and/or cisplatin,
MG-63 cell proliferation was determined using a CCK-8 assay. The
results demonstrated that the optical density (OD) values in the
combined radiation and cisplatin treatment group were significantly
lower than those in the radiation or cisplatin only groups, which
were also significantly lower compared with the control group
(P<0.001; Fig. 1). The OD values
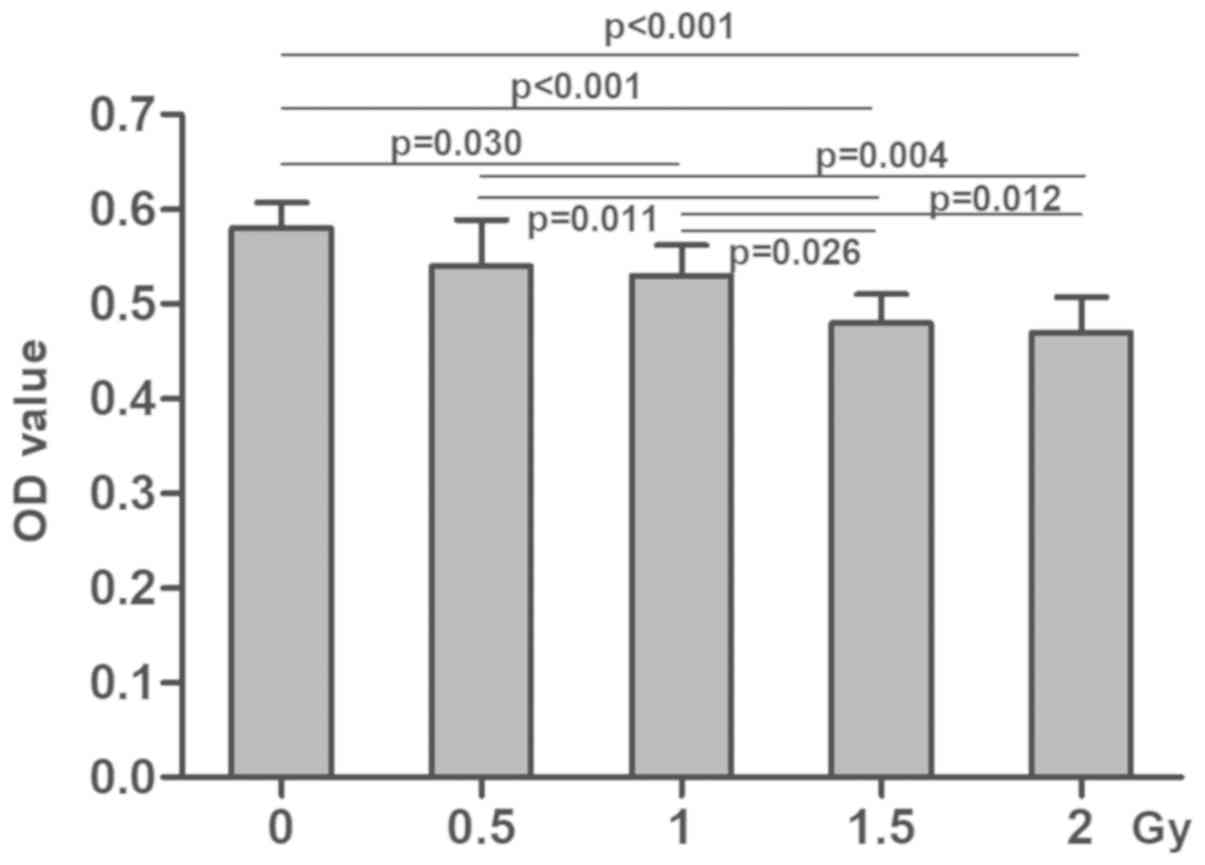
in the radiation groups were decreased in a dose-dependent manner
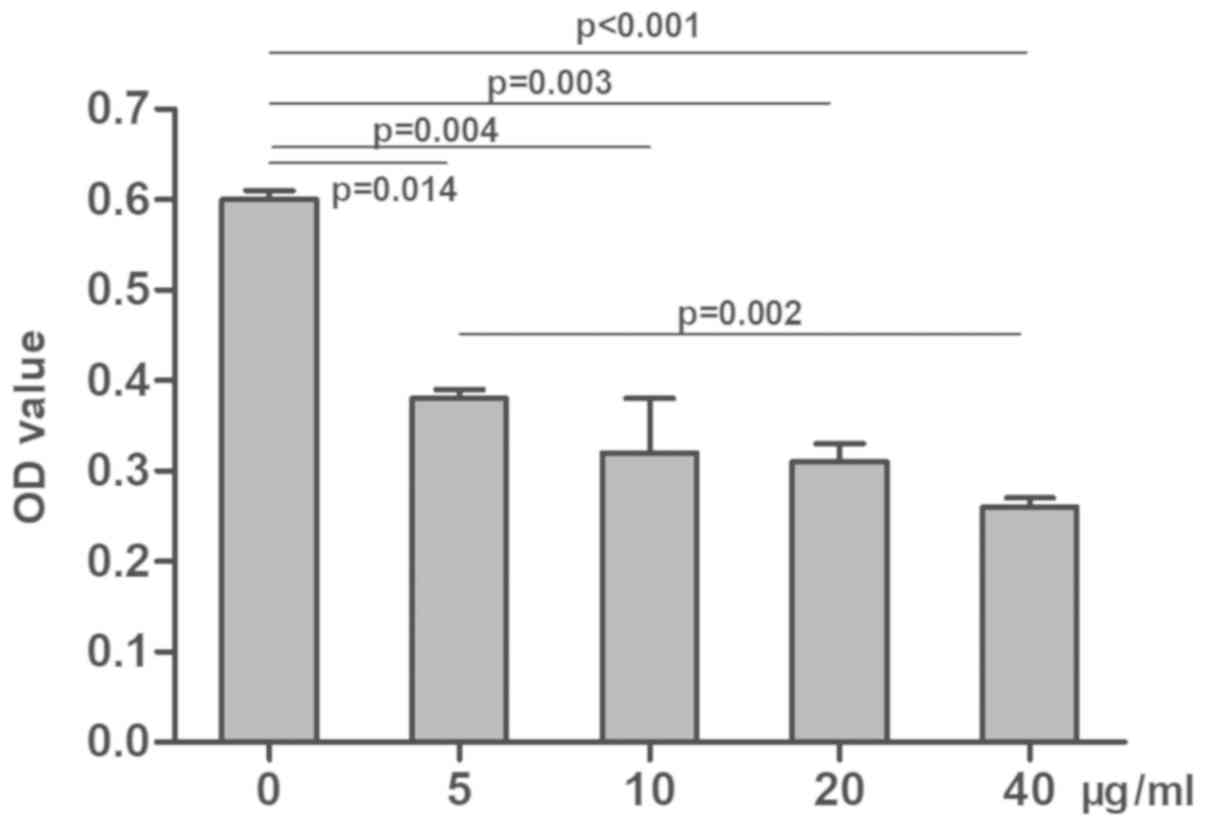
compared with the control group (P<0.05; Fig. 2). Similarly, OD values in the
cisplatin groups were decreased in a dose-dependent manner compared
with the control group (P<0.05; Fig.
3). These results suggested that both radiation and cisplatin
treatment inhibited MG-63 cell proliferation and that the combined
treatment with radiation and cisplatin was even more effective.
Effects of radiation and cisplatin on
MG-63 cell apoptosis
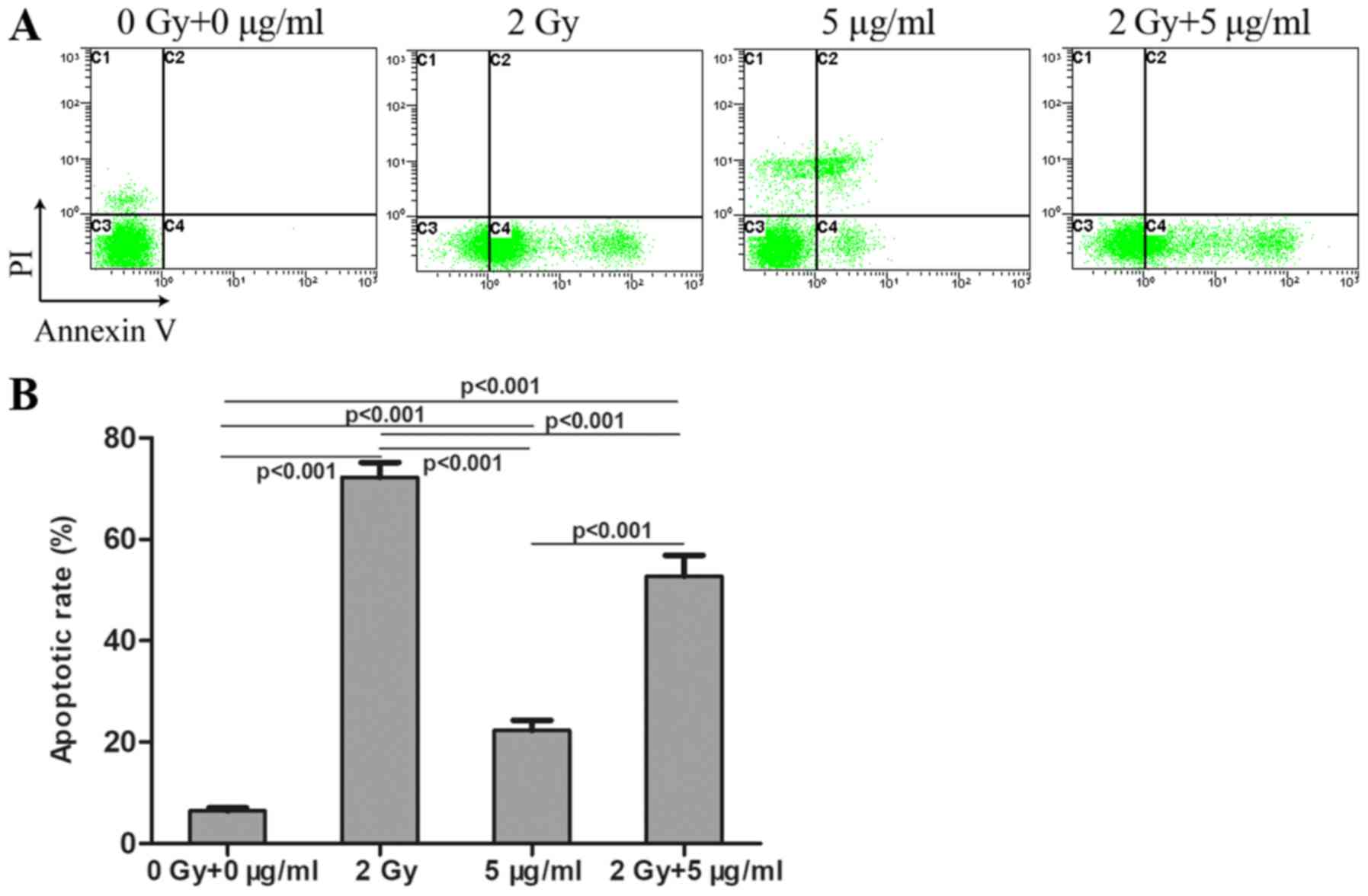
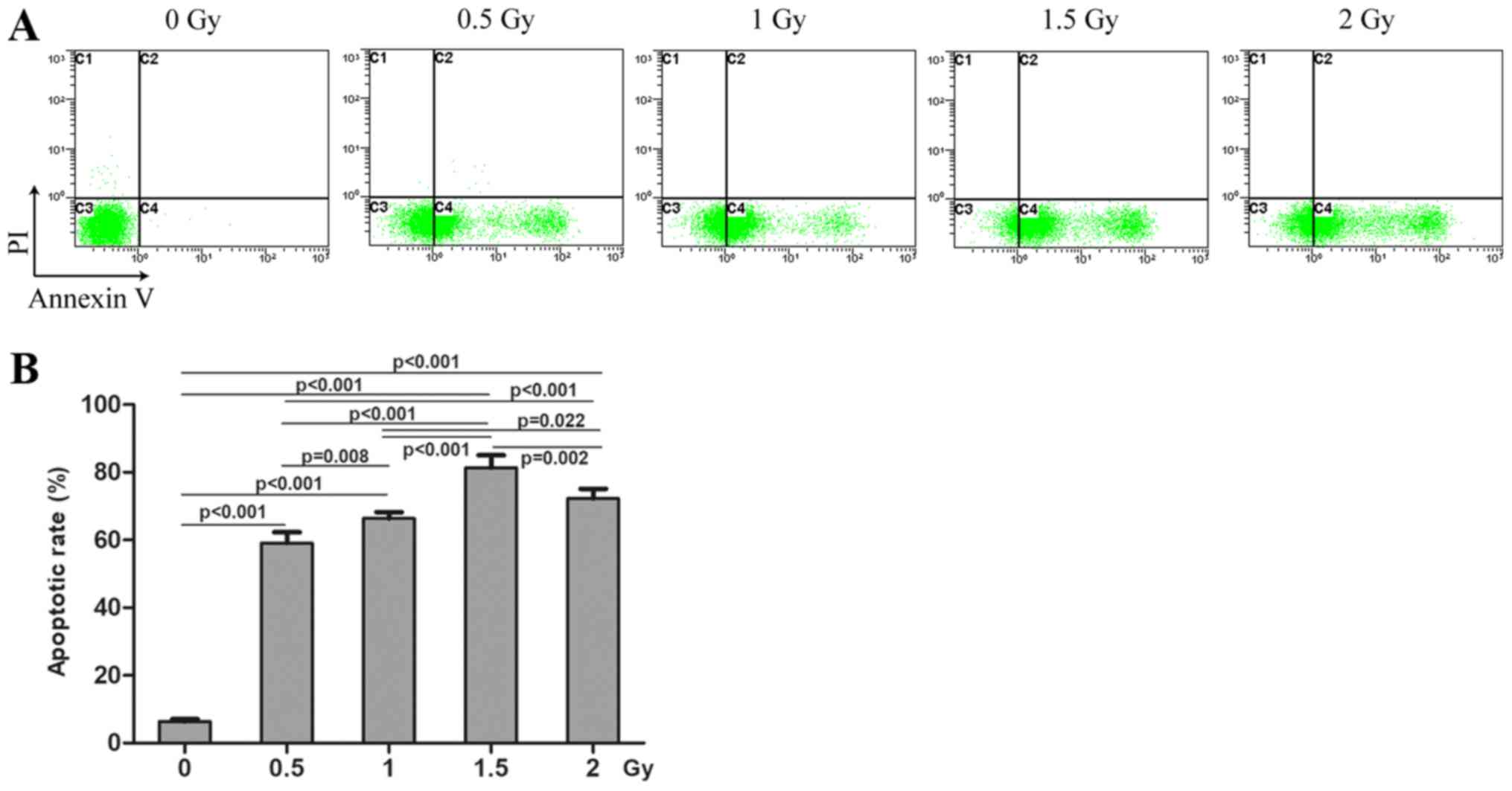
Following treatment with radiation and/or cisplatin,
MG-63 cell apoptosis was determined by flow cytometry using Annexin
V and PI double staining. The results revealed that the apoptosis
rate in the combined radiation and cisplatin treatment group was
significantly higher compared with that in the cisplatin group, but
was lower compared with that in the radiation group (Fig. 4). The apoptosis rates in all these
three treatment groups were significantly higher compared with the
control group. MG-63 cell apoptosis rates were significantly
increased in a dose-dependent manner in the radiation treatment
groups, compared with the control group (P<0.05; Fig. 5). MG-63 cell apoptosis rates were
also significantly increased in a dose-dependent manner in the
cisplatin treatment groups compared with the control group
(P<0.05; Fig. 6).
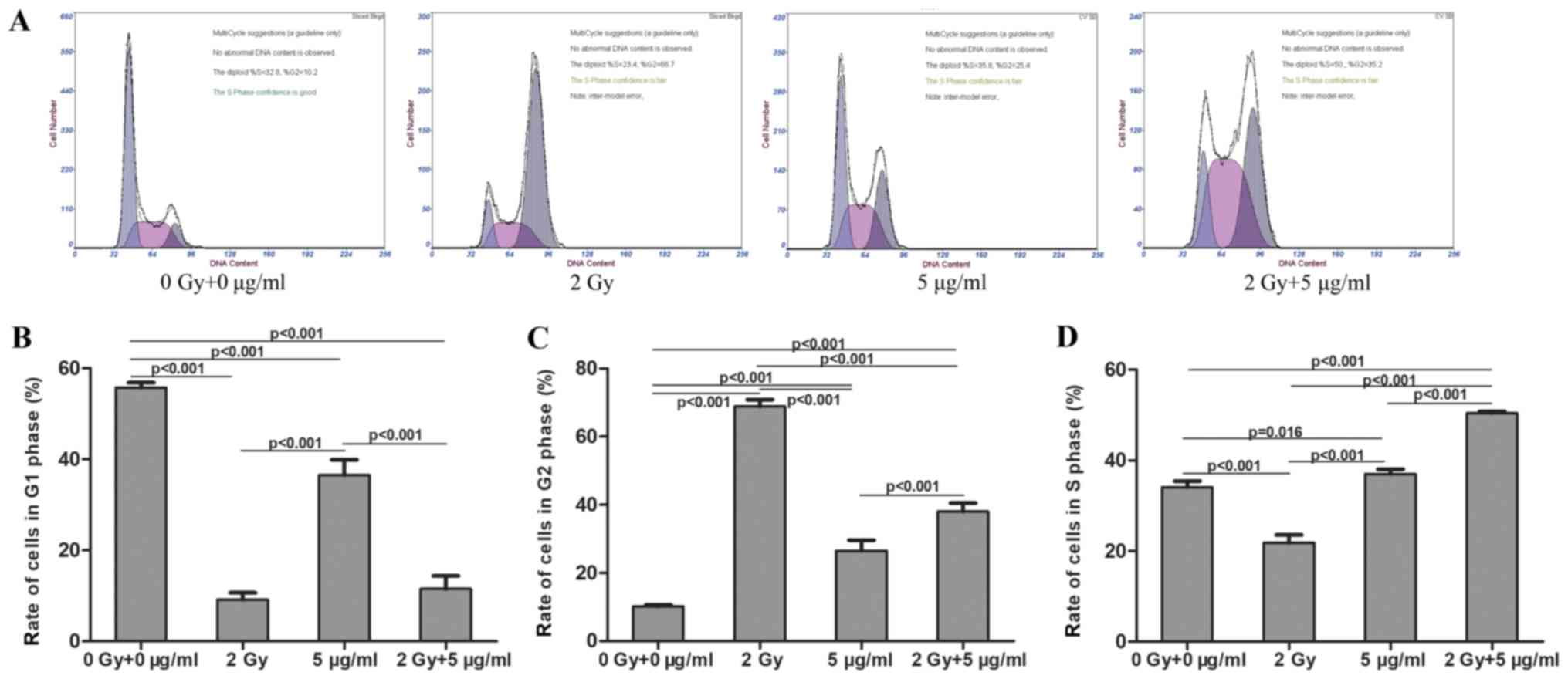
Effects of radiation and cisplatin on
MG-63 cell cycle
MG-63 cells were treated with radiation and/or
cisplatin, and the cell cycle distribution was determined by flow
cytometry. The results demonstrated that the ratio of cells in the
G1 phase was significantly decreased in the radiation,
cisplatin and combined radiation and cisplatin treatment groups,
compared with the control group. The ratio of cells in the
G1 phase was significantly decreased in the combined
radiation and cisplatin treatment group, compared with the
cisplatin group. There was no significant difference in the ratio
of cells in G1 phase between the combined radiation and
cisplatin treatment group and the radiation groups (Fig. 7A and B). The ratio of cells in
G2 phase was significantly increased in the radiation,
cisplatin and combined radiation and cisplatin treatment groups,
compared with the control group. The ratio of cells in
G2 phase was significantly increased in the combined
radiation and cisplatin treatment group compared with the cisplatin
group. The ratio of cells in G2 was significantly
decreased in the combined radiation and cisplatin treatment group
compared with the radiation group (Fig.
7A and C). The ratio of cells in S phase was significantly
increased in the cisplatin and combined radiation and cisplatin
treatment groups, and was decreased in the radiation group,
compared with the control group. The ratio of cells in S phase was
significantly increased in the combined radiation and cisplatin
treatment group compared with the cisplatin group and the radiation
group (Fig. 7A and D). Consistently,
the ratios of cells in G1 (Fig. 8A and B) and S (Fig. 8A and D) phases were significantly
decreased and the ratio of cells in G2 phase (Fig. 8A and C) was significantly increased
in the radiation treatment group. The ratios of cells in
G1 (Fig. 9A and B) were
significantly decreased and the ratios of cells in G2
phase (Fig. 9A and C) and S phase
(Fig. 9A and D) were significantly
increased in the cisplatin treatment group. These results revealed
that treatment with radiation resulted in G2 phase
arrest in MG-63 cells, and treatment with cisplatin or combined
radiation and cisplatin resulted in both G2 phase arrest
and S phase arrest. The effects of cisplatin on both G2
phase arrest and S phase arrest were less clear than those of
combined radiation and cisplatin treatment.
Effects of radiation and cisplatin on
MG-63 cell migration
MG-63 cells were treated with radiation and/or
cisplatin, and MG-63 cell migration was determined by Transwell
assays. The results demonstrated that the number of invasive cells
was lower in the combined radiation and cisplatin treatment group
compared with the radiation or cisplatin treatment groups, which
were lower than that in the control group (Fig. 10). The number of invasive cells was
significantly decreased in a dose-dependent manner in the radiation
treatment groups, compared with the control group (P<0.05;
Fig. 11). The number of invasive
cells was significantly decreased in a dose-dependent manner in the
cisplatin treatment group, compared with the control group
(P<0.05; Fig. 12).
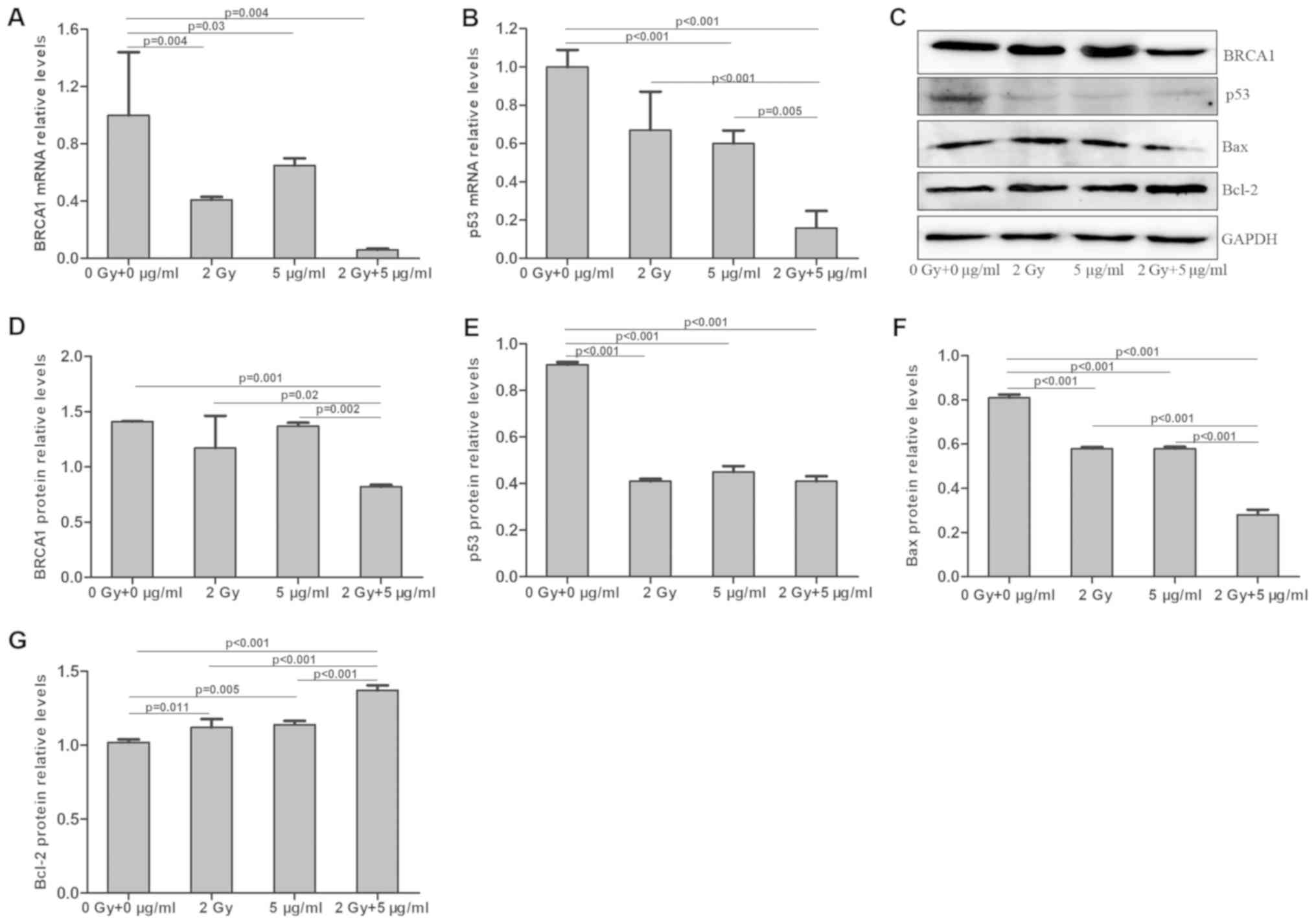
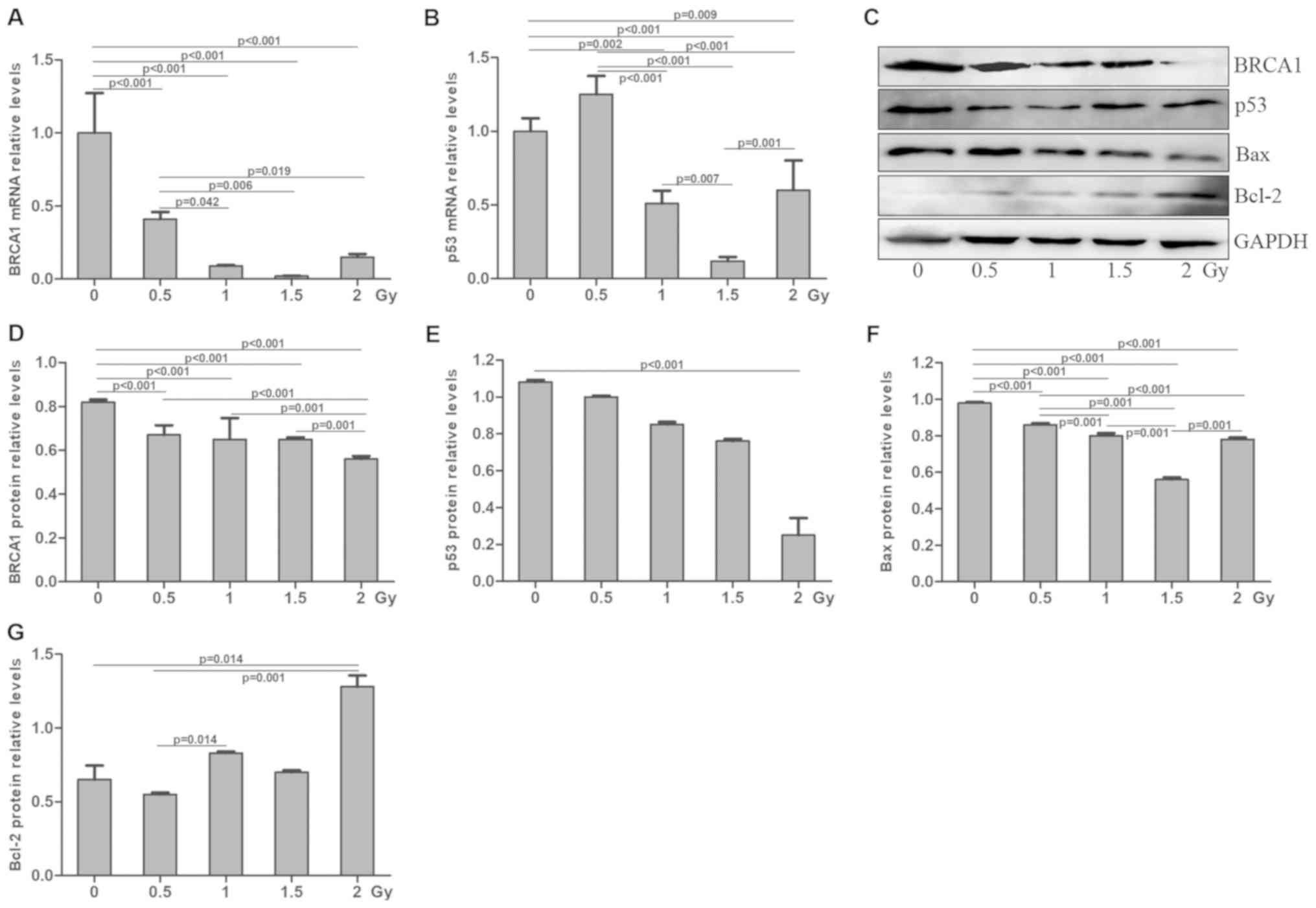
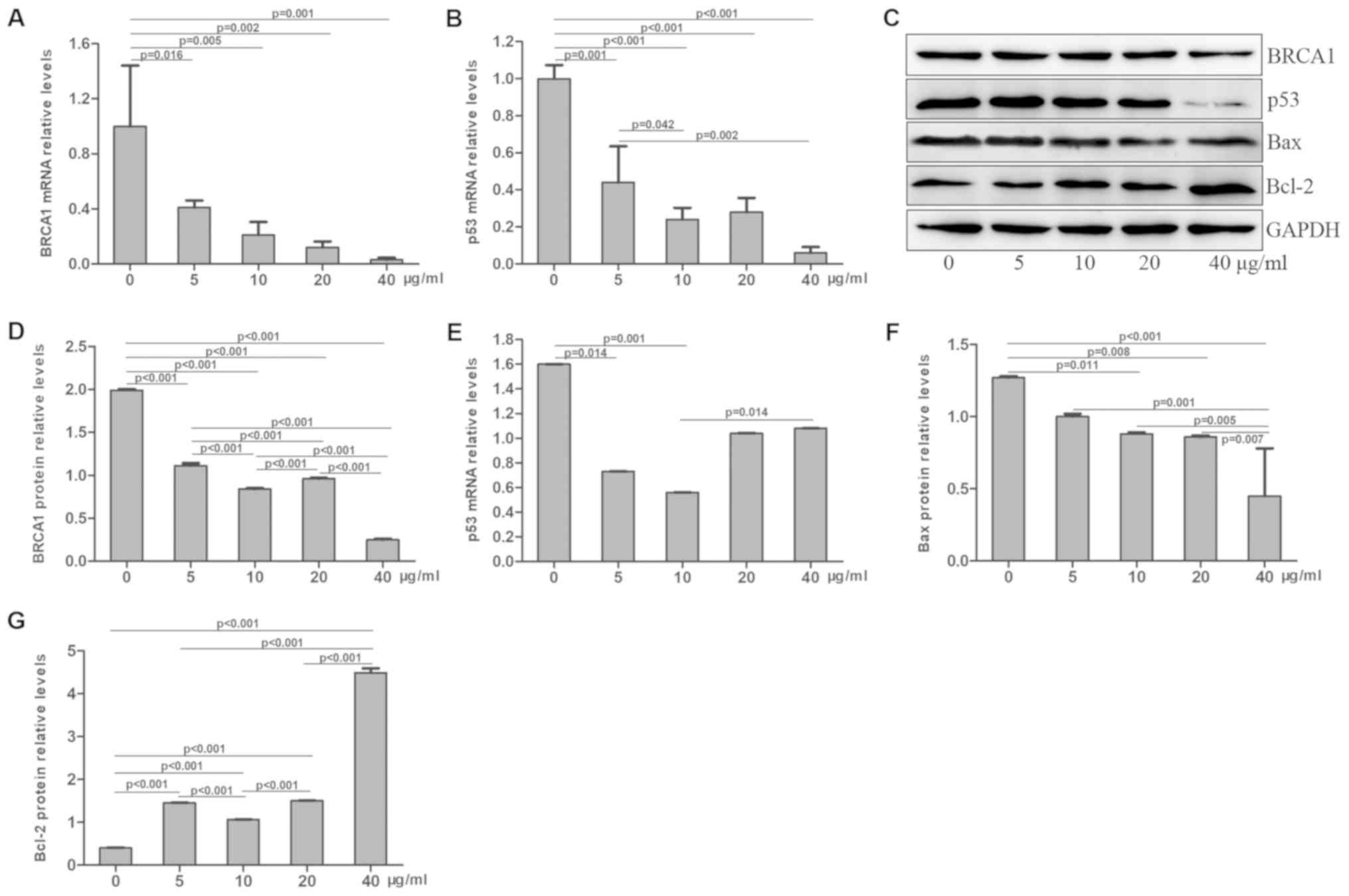
Effects of radiation and cisplatin on
BRCA1 and p53 expression in MG-63 cells
Following treatment with radiation and/or cisplatin,
the mRNA and protein expression levels of BRCA1 and p53 were
determined in MG-63 cells by RT-qPCR and western blotting,
respectively. The results demonstrated that BRCA1 mRNA level was
significantly decreased in the combined radiation and cisplatin
treatment group, compared with those in the radiation or cisplatin
treatment groups, and the BRCA1 mRNA levels in these three
treatment groups were lower than that of the control group
(P<0.05; Fig. 13A). BRCA1 mRNA
level was significantly decreased in the radiation treatment group
in a dose-dependent manner, compared with the control group
(P<0.05; Fig. 14A). BRCA1 mRNA
level was significantly decreased in the cisplatin treatment group
in a dose-dependent manner compared with the control group
(P<0.05; Fig. 15A). Similar
results were obtained for the p53 mRNA level (Figs. 13B, 14B and 15B). Furthermore, BRCA1 protein expression
was significantly decreased in the combined radiation and cisplatin
treatment group, compared with the radiation and cisplatin
treatment groups and the control group (P<0.05; Fig. 13C and D). BRCA1 protein expression
was significantly decreased in the radiation treatment group
(Fig. 14C and D) and the cisplatin
treatment group (Fig. 15C and D) in
a dose-dependent manner, compared with the control group
(P<0.05). In addition, p53 protein expression was significantly
decreased in the combined radiation and cisplatin treatment group,
the radiation group and the cisplatin group compared with the
control group (P<0.05; Fig. 13C and
E). The p53 protein expression was decreased in the radiation
treatment group (Fig. 14C and E)
and the cisplatin treatment group (Fig.
15C and E), compared with the control group (P<0.05). These
results revealed that the combined treatment with radiation and
cisplatin induced a decrease in the mRNA and protein expression
levels of BRCA1 and p53 in MG-63 cells. The combination of
radiation and cisplatin exhibited a more potent inhibitory effect
on p53 protein expression compared with BRCA1 protein expression in
MG-63 cells.
Effects of radiation and cisplatin on
Bax and Bcl-2 levels in MG-63 cells
MG-63 cells were treated with radiation and/or
cisplatin and the Bax and Bcl-2 protein levels were determined by
western blotting. The results demonstrated that Bax expression was
significantly decreased in the radiation, cisplatin and combined
radiation and cisplatin treatment groups, compared with the control
group (P<0.001), and that Bax expression was lower in the
combined radiation and cisplatin treatment group than in the
radiation or cisplatin only treatment groups (P<0.001; Fig. 13C and F). Bax protein expression was
significantly decreased in the radiation treatment group (Fig. 14C and F) and the cisplatin treatment
group (Fig. 15C and F) in a
dose-dependent manner compared with the control group (P<0.05).
Furthermore, Bcl-2 protein expression was significantly increased
in the radiation, cisplatin and combined radiation and cisplatin
treatment groups, compared with the control group (P<0.001). In
addition, Bcl-2 expression was significantly higher in the combined
radiation and cisplatin treatment group, compared with those of the
radiation and cisplatin treatment groups (P<0.05; Fig. 13C and G). Bcl-2 protein expression
was significantly increased in the radiation treatment group
(Fig. 14C and G) and the cisplatin
treatment group (Fig. 15C and G) in
a dose-dependent manner compared with the control group
(P<0.05). These results indicated that the combined treatment
with radiation and cisplatin exhibited a more potent inhibitory
effect on Bax protein expression and an inductive effect on Bcl-2
protein expression, compared with radiation and cisplatin
treatments alone in MG-63 cells.
Discussion
The present study demonstrated that the combined
treatment of radiation and cisplatin significantly inhibited MG-63
cell proliferation in a more potent way compared with radiation or
cisplatin treatments alone. Furthermore, the three treatments
increased the apoptosis rates of MG-63 cells, induced MG-63 cell
arrest in the G2 phase and significantly decreased the
migratory capacity of MG-63 cells. In addition, the apoptosis rate
in the combined radiation and cisplatin treatment group was higher
than that in the cisplatin group, but lower than that in the
radiation group. Furthermore, the combined treatment of radiation
and cisplatin resulted in MG-63 cell arrest in the S phase and in a
lower number of migratory cells compared with radiation of
cisplatin treatment alone. These results suggested that combining
radiation and cisplatin treatment may have a more potent
therapeutic effect on MG-63 osteosarcoma compared with radiation or
cisplatin treatments alone.
Radiation exerts detrimental effects on tumor cells
through direct breaking of DNA strands, lipids and proteins and
indirect bystander effects, resulting in DNA damage, chromosomal
instability, gene mutation and apoptosis (31,32).
Cisplatin induces the formation of platinum-DNA adducts, which
results in the breakage and damage of single- and double-stranded
DNA and the inhibition of tumor cell division, leading to tumor
cell death (33,34). Therefore, combined treatment of
radiation and cisplatin may cause inhibition of proliferation and
division of tumor cells through several molecular and cellular
antitumor mechanisms, including enhanced apoptosis and cell cycle
arrest, as previously demonstrated for the treatment of head and
neck cancer and cervical cancer (35–37).
Similarly, the results from the present study demonstrated that the
combined treatment of radiation and cisplatin exhibited superior
therapeutic effects on osteosarcoma MG-63 cells compared with
radiation or cisplatin treatments alone. These findings may be due
to mutually enhanced effects of radiation and cisplatin resulting
from various molecular and cellular antitumor mechanisms.
The present study demonstrated that combining
radiation and cisplatin was more potent in inhibiting MG-63 cell
proliferation and migration compared with radiation or cisplatin
treatments alone. The dose used in the present study is 2.0 Gy
radiation + 5 g/ml cisplatin. However, the effect of combined
radiation and cisplatin treatment on cell cycle G2
arrest and apoptosis is less than those of radiation treatment and
greater than those of cisplatin treatment. These results suggested
that the regulation of MG-63 cell apoptosis and cell cycle by the
combined treatment of radiation and cisplatin may be due to a
different mechanism compared with radiation or cisplatin treatments
alone, and may therefore require further investigation.
The results of the present study revealed that
combined treatment with radiation and cisplatin resulted in
decreased mRNA and protein expression levels of BRCA1 and p53.
Furthermore, the combined treatment of radiation and cisplatin
exhibited a more potent inhibitory effect on p53 expression in
MG-63 cells compared with BRCA1 expression. In addition, the
combined treatment of radiation and cisplatin was more potent in
decreasing Bax protein expression and increasing Bcl-2 protein
expression compared with radiation and cisplatin treatments alone
in MG-63 cells. These findings suggested that the BRCA1-p53
signaling pathway may mediate the effects of combined treatment of
radiation and cisplatin on MG-63 cells. BRCA1 is a tumor suppressor
gene involved in multiple cell signaling pathways, including the
damaged DNA repair pathway and cell cycle regulation (38). The low expression levels and high
rates of mutation of BRCA1 can decrease DNA repair capacity in
cancer cells, including ovarian cancer and breast cancer cells,
resulting in cell insensitivity to platinum and other platinum
drugs (39–43). In addition, DNA is the main target of
radiation, and BRCA1 mutation is associated with radiation
sensitivity (44–46). Therefore, BRCA1 expression may be
negatively associated with the effects of platinum drugs and
radiation on tumor cells, which was demonstrated in the present
study. Therefore, determining how low BRCA1 expression may be
associated with the effect of radiation and cisplatin treatment on
osteosarcoma requires further investigation.
Bax is a pro-apoptotic protein and Bcl-2 is an
anti-apoptotic protein. They mediate the intrinsic apoptosis
pathway by controlling mitochondrial outer membrane integrity
(47,48). The results of the present study
demonstrated that combining radiation and cisplatin had a more
potent effect in decreasing Bax protein expression and increasing
Bcl-2 protein expression compared with radiation or cisplatin
treatments alone in MG-63 cells. Previous studies reported
inconsistent findings on a rat tumor model of human small cell lung
cancer where Bcl-2 expression was increased in cisplatin-resistant
subline (GLC4-CDDP) following combined treatment (49), in esophageal squamous cell carcinoma
where neither Bcl-2 nor Bax expression were associated with the
efficacy of therapy (50), and in
non-small cell lung cancer where high expression of Bcl-2 in tumors
was significantly associated with longer survival duration
(51). The heterogeneous nature of
the tumor and the numerous apoptotic pathways involved in cancer
may account for these differences (52–54).
The current study presented some limitations.
Firstly, the present study only examined the effect of single doses
of cisplatin and radiation and of combined treatment in only one
cell line and at only one time point. Additional cell lines,
multiple doses of treatment and more combinations will be examined
in future studies. Secondly, this study only measured cell
proliferation using a CCK-8 assay to determine treatment efficacy.
The assessment of colony formation to detect cell viability
following therapy will be conducted in future studies. Thirdly,
increased levels of the tumor suppressor p53 are usually induced by
radiation through DNA damage; however, the present study revealed
that the p53 level was decreased following treatment with radiation
and cisplatin. The underlying mechanism require further
investigation.
In conclusion, treatment with radiation and
cisplatin, alone or in combination, inhibited cell proliferation
and migration, induced cell cycle arrest in G2 phase,
stimulated cell apoptosis, decreased the expression levels of BRCA1
and p53, decreased Bax protein expression and increased Bcl-2
protein expression in MG-63 cells, suggesting that the BRCA1-p53
signaling pathway may serve a crucial role. Furthermore, combined
treatment with radiation and cisplatin exhibited more potent
effects in inducing these phenomena compared with radiation or
cisplatin treatments alone. These findings suggested that combining
radiation and cisplatin may be considered a good approach for the
treatment of osteosarcoma and that the BRCA1 level may be used to
evaluate treatment efficacy in MG-63 cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jilin
Scientific and Technological Development Program (grant no.
20180520109JH) and the Science and Technology project of the
Education Department of Jilin Province during the ‘13th Five-Year
Plan’ (grant no. JJKH20180203KJ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HBS, HYW, BW and LNZ designed the study. HBS, HYW,
BW, ZFW, LZW, FQL, JDW and LNZ collected and analyzed the data.
HBS, HYW, BW and LNZ drafted and wrote the manuscript. HBS and LNZ
critically revised the manuscript for intellectual content. All
authors provided intellectual input to the study and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Han L, He Z, Li X, Yang S, Yang J,
Zhang Y, Li D, Yang Y and Yang Z: Advances in limb salvage
treatment of osteosarcoma. J Bone Oncol. 10:36–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGuire J, Utset-Ward TJ, Reed DR and
Lynch CC: Re-calculating! Navigating through the osteosarcoma
treatment roadblock. Pharmacol Res. 117:54–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friebele JC, Peck J, Pan X, Abdel-Rasoul M
and Mayerson JL: Osteosarcoma: A meta-analysis and review of the
literature. Am J Orthop (Belle Mead NJ). 44:547–553.
2015.PubMed/NCBI
|
|
7
|
Bacci G and Lari S: Adjuvant and
neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov.
86:253–268. 2001.PubMed/NCBI
|
|
8
|
Carrle D and Bielack SS: Current
strategies of chemotherapy in osteosarcoma. Int Orthop. 30:445–451.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrari S and Serra M: An update on
chemotherapy for osteosarcoma. Expert Opin Pharmacother.
16:2727–2736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bacci G, Ferrari S, Longhi A, Picci P,
Mercuri M, Alvegard TA, Saeter G, Donati D, Manfrini M, Lari S, et
al: High dose ifosfamide in combination with high dose
methotrexate, adriamycin and cisplatin in the neoadjuvant treatment
of extremity osteosarcoma: Preliminary results of an Italian
Sarcoma Group/Scandinavian Sarcoma Group pilot study. J Chemother.
14:198–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bodmer N, Walters DK and Fuchs B:
Pemetrexed, a multitargeted antifolate drug, demonstrates lower
efficacy in comparison to methotrexate against osteosarcoma cell
lines. Pediatr Blood Cancer. 50:905–908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duffaud F, Egerer G, Ferrari S, Rassam H,
Boecker U and Bui-Nguyen B: A phase II trial of second-line
pemetrexed in adults with advanced/metastatic osteosarcoma. Eur J
Cancer. 48:564–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Machak GN, Tkachev SI, Solovyev YN,
Sinyukov PA, Ivanov SM, Kochergina NV, Ryjkov AD, Tepliakov VV,
Bokhian BY and Glebovskaya VV: Neoadjuvant chemotherapy and local
radiotherapy for high-grade osteosarcoma of the extremities. Mayo
Clin Proc. 78:147–155. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: Current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozaki T, Flege S, Kevric M, Lindner N,
Maas R, Delling G, Schwarz R, von Hochstetter AR, Salzer-Kuntschik
M, Berdel WE, et al: Osteosarcoma of the pelvis: Experience of the
Cooperative Osteosarcoma Study Group. J Clin Oncol. 21:334–341.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozaki T, Flege S, Liljenqvist U, Hillmann
A, Delling G, Salzer-Kuntschik M, Jürgens H, Kotz R, Winkelmann W
and Bielack SS: Osteosarcoma of the spine: Experience of the
Cooperative Osteosarcoma Study Group. Cancer. 94:1069–1077. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohamad O, Imai R, Kamada T, Nitta Y and
Araki N; Working Group for Bone and Soft Tissue Sarcoma, : Carbon
ion radiotherapy for inoperable pediatric osteosarcoma. Oncotarget.
9:22976–22985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsunobu A, Imai R, Kamada T, Imaizumi T,
Tsuji H, Tsujii H, Shioyama Y, Honda H and Tatezaki S; Working
Group for Bone and Soft Tissue Sarcomas, : Impact of carbon ion
radiotherapy for unresectable osteosarcoma of the trunk. Cancer.
118:4555–4563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Delaney G, Jacob S, Featherstone C and
Barton M: The role of radiotherapy in cancer treatment: Estimating
optimal utilization from a review of evidence-based clinical
guidelines. Cancer. 104:1129–1137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwarz R, Bruland O, Cassoni A, Schomberg
P and Bielack S: The role of radiotherapy in oseosarcoma. Cancer
Treat Res. 152:147–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu D, Chen K, Bai Y, Zhu X, Chen Z, Wang
C, Zhao Y and Li M: Screening of diagnostic markers for
osteosarcoma. Mol Med Rep. 10:2415–2420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Engert F, Kovac M, Baumhoer D, Nathrath M
and Fulda S: Osteosarcoma cells with genetic signatures of BRCAness
are susceptible to the PARP inhibitor talazoparib alone or in
combination with chemotherapeutics. Oncotarget. 8:48794–48806.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li B and Ye Z: Epigenetic alterations in
osteosarcoma: Promising targets. Mol Biol Rep. 41:3303–3315. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Turner N, Tutt A and Ashworth A: Hallmarks
of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 4:814–819. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lord CJ and Ashworth A: BRCAness
revisited. Nat Rev Cancer. 16:110–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JA, Paik EK, Seo J, Kim DH, Lim JS,
Yoo JY and Kim MS: Radiotherapy and gemcitabine-docetaxel
chemotherapy in children and adolescents with unresectable
recurrent or refractory osteosarcoma. Jpn J Clin Oncol. 46:138–143.
2016.PubMed/NCBI
|
|
29
|
Dinçbaş FO, Koca S, Mandel NM, Hiz M,
Dervişoğlu S, Seçmezacar H, Oksüz DC, Ceylaner B and Uzel B: The
role of preoperative radiotherapy in nonmetastatic high-grade
osteosarcoma of the extremities for limb-sparing surgery. Int J
Radiat Oncol Biol Phys. 62:820–828. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marín A, Martín M, Liñán O, Alvarenga F,
López M, Fernández L, Büchser D and Cerezo L: Bystander effects and
radiotherapy. Rep Pract Oncol Radiother. 20:12–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lewanski CR and Gullick WJ: Radiotherapy
and cellular signalling. Lancet Oncol. 2:366–370. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muggia F: Platinum compounds 30 years
after the introduction of cisplatin: Implications for the treatment
of ovarian cancer. Gynecol Oncol. 112:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marcu L, van Doorn T and Olver I:
Cisplatin and radiotherapy in the treatment of locally advanced
head and neck cancer-a review of their cooperation. Acta Oncol.
42:315–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Petrelli F, De Stefani A, Raspagliesi F,
Lorusso D and Barni S: Radiotherapy with concurrent cisplatin-based
doublet or weekly cisplatin for cervical cancer: A systematic
review and meta-analysis. Gynecol Oncol. 134:166–171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jacinto JK, Co J, Mejia MB and Regala EE:
The evidence on effectiveness of weekly vs triweekly cisplatin
concurrent with radiotherapy in locally advanced head and neck
squamous cell carcinoma (HNSCC): A systematic review and
meta-analysis. Br J Radiol. 90:201704422017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhong Q, Chen CF, Li S, Chen Y, Wang CC,
Xiao J, Chen PL, Sharp ZD and Lee WH: Association of BRCA1 with the
hRad50-hMre11-p95 complex and the DNA damage response. Science.
285:747–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Caestecker KW and Van de Walle GR: The
role of BRCA1 in DNA double-strand repair: Past and present. Exp
Cell Res. 319:575–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J and Powell SN: The role of the
BRCA1 tumor suppressor in DNA double-strand break repair. Mol
Cancer Res. 3:531–539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song H, Cicek MS, Dicks E, Harrington P,
Ramus SJ, Cunningham JM, Fridley BL, Tyrer JP, Alsop J,
Jimenez-Linan M, et al: The contribution of deleterious germline
mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian
cancer in the population. Hum Mol Genet. 23:4703–4709. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ramus SJ and Gayther SA: The contribution
of BRCA1 and BRCA2 to ovarian cancer. Mol Oncol. 3:138–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ratanaphan A: A DNA repair BRCA1 estrogen
receptor and targeted therapy in breast cancer. Int J Mol Sci.
13:14898–14916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chechlinska M and Nowak R: The sensitivity
of BRCA1 mutation carriers to ionising radiation: Questions of
methodology. Breast Cancer Res Treat. 115:4332009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi M, Ma F, Liu J, Xing H, Zhu H, Yu J
and Yang M: A functional BRCA1 coding sequence genetic variant
contributes to prognosis of triple-negative breast cancer,
especially after radiotherapy. Breast Cancer Res Treat.
166:109–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hallam S, Govindarajulu S, Huckett B and
Bahl A: BRCA1/2 mutation-associated breast cancer, wide local
excision and radiotherapy or unilateral mastectomy: A systematic
review. Clin Oncol (R Coll Radiol). 27:527–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Flórez MM, Fêo HB, da Silva GN, Yamatogi
RS, Aguiar AJ, Araújo JP Jr and Rocha NS: Cell cycle kinetics,
apoptosis rates and gene expressions of MDR-1, TP53, BCL-2 and BAX
in transmissible venereal tumour cells and their association with
therapy response. Vet Comp Oncol. 15:793–807. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng Q, Wang B, Gao J, Xin N, Wang W,
Song X, Shao Y and Zhao C: CD155 knockdown promotes apoptosis via
AKT/Bcl-2/Bax in colon cancer cells. J Cell Mol Med. 22:131–140.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fokkema E, De Vries EG, Groen HJ, Meijer C
and Timens W: Expression of apoptosis-related proteins and
morphological changes in a rat tumor model of human small cell lung
cancer prior to and after treatment with radiotherapy, carboplatin,
or combined treatment. Virchows Arch. 442:349–355. 2003.PubMed/NCBI
|
|
50
|
Miyazaki T, Kato H, Faried A, Sohda M,
Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Ojima H, et al:
Predictors of response to chemo-radiotherapy and radiotherapy for
esophageal squamous cell carcinoma. Anticancer Res. 25:2749–2755.
2005.PubMed/NCBI
|
|
51
|
Jeong SH, Jung JH, Han JH, Kim JH, Choi
YW, Lee HW, Kang SY, Hwang YH, Ahn MS, Choi JH, et al: Expression
of Bcl-2 predicts outcome in locally advanced non-small cell lung
cancer patients treated with cisplatin-based concurrent
chemoradiotherapy. Lung Cancer. 68:288–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hassan C, Afshinnekoo E, Li S, Wu S and
Mason CE: Genetic and epigenetic heterogeneity and the impact on
cancer relapse. Exp Hematol. 54:26–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gentric G, Mieulet V and Mechta-Grigoriou
F: Heterogeneity in cancer metabolism: New concepts in an old
field. Antioxid Redox Signal. 26:462–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dagogo-Jack I and Shaw AT: Tumour
heterogeneity and resistance to cancer therapies. Nat Rev Clin
Oncol. 15:81–94. 2018. View Article : Google Scholar : PubMed/NCBI
|