Introduction
Phosphatase and tension homolog (PTEN), also known
as mutated in multiple advanced cancer 1, is a dual-specificity
phosphatase that was first identified in 1997 (1). PTEN is one of the most studied tumor
suppressors, as it is frequently mutated in various types of
cancer, including breast cancer, prostate cancer and brain tumors
(2,3). PTEN somatic mutations in sporadic
tumors and PTEN germline mutations have also been demonstrated to
result in the occurrence of inherited syndromes, including Cowden,
Bannayan-Riley-Ruvalcaba and Proteus-like syndromes (4). PTEN is involved in numerous cellular
processes associated with tumorigenesis, including cell
proliferation, cell survival, cell migration, genome stability and
DNA replication (5–8). In addition to these anti-tumor effects,
emerging evidence has demonstrated that PTEN could be associated
with other biological functions. For instance, the deletion of PTEN
in hepatocytes can increase glycogen and fatty acid synthesis by
regulating glycogen synthase kinase-3β, fatty acid synthase,
glucose 6-phosphatase as well as phosphoenolpyruvate carboxykinase;
resulting in fatty liver and insulin hypersensitivity (9). Furthermore, the inhibition of PTEN can
rescue the normal synaptic function in cellular and animal models
of Alzheimer's disease and restore cognition in patients with
Alzheimer's disease (10). PTEN also
effects antiviral innate immunity by activating interferon
regulatory factor 3 (11). However,
despite numerous evidence demonstrating the multiple functions of
PTEN in numerous diseases, the multifaceted roles of PTEN remain
unclear.
The thyroid is an important endocrine organ that
synthesizes and secretes thyroxine, which is a hormone involved in
numerous biological functions, such as metabolism, homeostasis and
development, by binding and altering the transcriptional regulatory
properties of its receptors (12–14).
Thyroid disorders, including hypothyroidism, goiter and thyroid
tumor are very common, and their incidence has significantly
increased in the last decade. It has been estimated that 1.5
billion people will be at risk of thyroid disorders by 2013
worldwide (15). The incidence of
thyroid cancer has increased by 211% between 1975 and 2013 in the
United States (16). Somatic
mutations of PTEN are not common in thyroid disorders; however,
loss of heterozygosity at 10q23 is found in 20–60% of all cases of
thyroid cancer, although it varies depending on the histological
type (17–19). PTEN expression is also reduced in a
series of thyroid tumor-derived cell lines and in sporadic human
benign and malignant thyroid tumors (20–22).
Cowden syndrome, which is an inherited syndrome caused by a
germline mutation of PTEN, is also characterized by a high risk of
thyroid disorders, including multinodular goiter, thyroid adenoma
and carcinoma (23). In addition,
loss of PTEN in the thyroid of mice results in goiter and
follicular adenomas (24). These
findings suggest that PTEN mutation or deficiency may result in
thyroid disorders. However, the role of PTEN in thyroid diseases
remains unknown.
PAX8 is a member of the paired box gene family of
proteins encoding evolutionary conserved transcription factors that
control the development of various organs (25). PAX8 is expressed in the developing
kidney, neural tube as well as in the developing and adult thyroid
(26). Previous studies have
indicated that PAX8 is important for the morphogenesis,
differentiation and function of the thyroid gland (27,28),
which suggested that PAX8 might be a mediator for the function of
PTEN in thyroid cells.
To determine the role and underlying mechanism of
PTEN in thyroid disorders, PTEN expression was knocked down in the
human thyroid follicular epithelial cell line Nthy-Ori 3-1. Western
blotting, Matrigel tube formation assay and iodide uptake assay
were subsequently performed to determine the role of PTEN in
thyroid disorders.
Materials and methods
Cell culture, transfection and
chemicals
The human thyroid follicular epithelial cell line
Nthy-Ori 3-1 was obtained from the European Collection of
Authenticated Cell Cultures. Cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% fetal bovine serum (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd.) and penicillin (100 U/ml)-streptomycin (0.1
mg/ml) (Gibco; Thermo Fisher Scientific, Inc.) and placed at 37°C
in a humidified incubator containing 5% CO2.
The sequences of the short hairpin (sh)RNA used in
the present study were as follows: PTEN-specific shRNA forward,
5′-GACAAAGCCAACCGATACTTT-3′; PTEN-specific shRNA reverse,
5′-AAAGTATCGGTTGGCTTTGTC-3′; paired box 8 (PAX8)-specific shRNA
forward, 5′-GCAACCATTCAACCTCCCTAT-3′; paired box 8 (PAX8)-specific
shRNA reverse, 5′-ATAGGGAGGTTGAATGGTTGC-3′; control shRNA forward,
5′-TTCTCCGAACGTGTCACGT-3′; and control shRNA reverse,
5′-ACGTGACACGTTCGGAGAA-3′. These shRNAs and the PAX8 overexpression
lentivirus were provided by Suzhou GenePharma Co., Ltd. Nthy-Ori
3-1 cells were seeded into 96-well plates, at a density of
1×104 cells/well, 1 day before viral infection. After
the cells had adhered, lentivirus (25 µl) and the transfection
agent polybrene (5 µg/ml; Suzhou GenePharma Co., Ltd.) were added
to the medium for 12 h. Medium was then replaced by complete
medium, and cells were cultured for a further 72 h, prior to
subsequent experiments.
MG132, a proteasome inhibitor which can inhibit the
degradation of protein mediated by ubiquitin-proteasome pathway,
was purchased from Sigma-Aldrich; Merck KGaA and used at the final
concentration of 20 µM for 4 h at room temperature. Cycloheximide
(CHX; Amresco, LLC) was used at the final concentration of 10
µg/ml.
Immunohistochemistry (IHC)
The thyroid tumor tissue microarray (TMA) slides
containing 12 thyroid tumor tissues and 12 non-neoplastic tissues
were purchased from Alenabio. Slides were deparaffinized in xylene
and then rehydrated in decreasing grades of alcohol (100, 100, 95,
90, 80 and 70%). Antigen retrieval was performed by pressure cooker
in citrate buffer (0.01 M, pH 6.0) and slides were blocked using 5%
goat serum (Vicmed Life Sciences) for 1 h at room temperature.
Slides were then incubated with anti-PTEN antibody (1:200; cat. no.
9188; Cell Signaling Technology, Inc.) overnight at 4°C, and with
peroxidase-conjugated secondary antibody (ready-to-use; cat. no.
PV6001; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.)
for 2 h at room temperature. Diaminobenzidine (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.) was used to detect positive
signals. Images were acquired at ×4 and ×20 magnifications using a
Zeiss light microscope (Carl Zeiss AG). For each specimen, three
high-power fields were analyzed. Two independent observers assessed
the immunohistochemical score blindly. PTEN staining was scored as
0, 1+, 2+, 3+ or 4+ for 0, <25, 25–49, 50–75 or >75% of
positively stained cells, respectively. Positive staining was also
graded according to its intensity from 0 to 3+, representing
negative, weak, moderate and strong staining, respectively.
Subsequently, PTEN protein expression was evaluated by calculating
a final score according to the following formula: Score=intensity ×
positive cell proportion. A final score of 0 to 12 was therefore
assigned.
Western blotting
Total cell protein was extracted from Nthy-Ori 3-1
cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was determined using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Proteins were separated
(30 µg per lane) by SDS-PAGE on a 10% gel, and transferred onto
polyvinylidene fluoride membranes (Merck KGaA). Membranes were
blocked in 5% non-fat milk in TBS-Tween (TBST, 0.05%) for 1 h at
room temperature, and incubated at 4°C overnight with the following
primary antibodies: Rabbit anti-human PTEN (1:2,000; cat. no. 9188;
Cell Signaling Technology, Inc.), rabbit anti-human PAX8 (1:2,000;
cat. no. GTX101583; GeneTex, Inc.), rabbit anti-human sodium/iodide
symporter (NIS; 1:1,000; cat. no. 83816; Abcam), mouse anti-GAPDH
(1:20,000; cat. no. 60004-1-Ig; ProteinTech Group, Inc.), mouse
anti-human thyroglobulin (TG; 1:1,000; cat. no. sc-51708; Santa
Cruz Biotechnology, Inc.) and mouse anti-human thyroid peroxidase
(TPO; 1:2,000; cat. no. sc-376876; Santa Cruz Biotechnology, Inc.).
Membranes were washed three times with TBST and incubated with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(IgG; 1:10,000; sc-2004; Santa Cruz Biotechnology, Inc.) and goat
anti-mouse IgG (1:20,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. Membranes were
washed three times with TBST and the signal was visualized using
Pierce Enhanced Chemiluminescent-plus substrate (Thermo Fisher
Scientific, Inc.) and analyzed by Image J (version 1.48; National
Institute of Health). GAPDH was used as an internal control.
Matrigel tube formation assay
Nthy-Ori 3-1 cells were seeded at the density of
1×104 cells per well in 96-well plates that were
precoated with 50% Matrigel (BD Biosciences) at 37°C for 30 min.
After 8 h incubation, the tubular-like structures were imaged and
evaluated under a light microscope (magnification, ×100). The
capillary tubes in each image were counted and analyzed for
statistical significance.
Iodide uptake assay
Cells were seeded at a density of 1×105
cells per well in 24-well plates 24 h prior to the assay. Once
cells had reached 75% confluence, the medium was discarded and
cells were washed twice with ice-cold PBS. Subsequently, 0.5 ml
medium containing 3.7 kBq 125I (Xuzhou Atomic High Tech
Pharmaceutical Co., Ltd.) was added into each well and cells were
cultured at 37°C. After 15, 30, 60 or 120 min, cells in each well
were separately lysed with 0.5 ml of 0.3 mol/l NaOH and iodide
uptake was measured using a γ radioimmunoassay counter. A parallel
set of cells treated with the same method were applied for cell
counting assay with the Cell Counting Kit-8 (Nanjing KeyGen Biotech
Co., Ltd.) according to the manufacturer's instructions, and used
for normalization of iodide uptake.
Statistical analysis
Statistical analyses were performed using SPSS
software version 16.0 (SPSS, Inc.). The data are presented as the
mean ± standard deviation of three independent repetitions.
Statistical comparisons were performed using a two-tailed Student's
t-test when comparing two groups, or ANOVA for multiple
comparisons, along with Newman-Keuls test, were used for pairwise
comparisons in multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
PTEN expression is decreased in
thyroid tumors
To study the association between PTEN and thyroid
diseases, the present study determined whether PTEN expression was
different in human thyroid tumors compared with normal thyroid
tissues. IHC staining was performed with TMA slides containing 12
thyroid tumor tissues and 12 non-neoplastic tissues. As presented
in Fig. 1A, non-neoplastic thyroid
tissue exhibited a uniform strong nuclear signal whereas the
cytoplasmic staining was less strong. Furthermore, a significantly
lower PTEN expression was observed in thyroid tumor tissue
(P<0.01; Fig. 1B) compared with
non-neoplastic tissues. These data indicated that PTEN was usually
expressed in normal thyroid tissues but was downregulated in
thyroid tumor tissues, suggesting that PTEN deficiency may lead to
thyroid tumors.
PTEN-knockdown modifies the morphology
and growth pattern of Nthy-Ori 3-1 cells
To determine the function of PTEN expression in
thyroid cells, PTEN-specific shRNA was transfected into Nthy-Ori
3-1 to knockdown PTEN expression. As presented in Fig. 2A, PTEN protein expression was
significantly decreased following 72 h of transfection. In
addition, Nthy-Ori 3-1 cell morphology had changed. Cells
transfected with PTEN-shRNA appeared rounder and flatter compared
with control cells (Fig. 2B). Cells
were then cultured on Matrigel to evaluate their growth pattern.
Cells in the control group grew on the Matrigel with a tubular-like
structure; however, cells in the PTEN-knockdown group presented a
significantly reduced number of tubular-like structures (Fig. 2C). These results suggested that
PTEN-knockdown may induce Nthy-Ori 3-1 cell morphological changes
and affect their growth pattern.
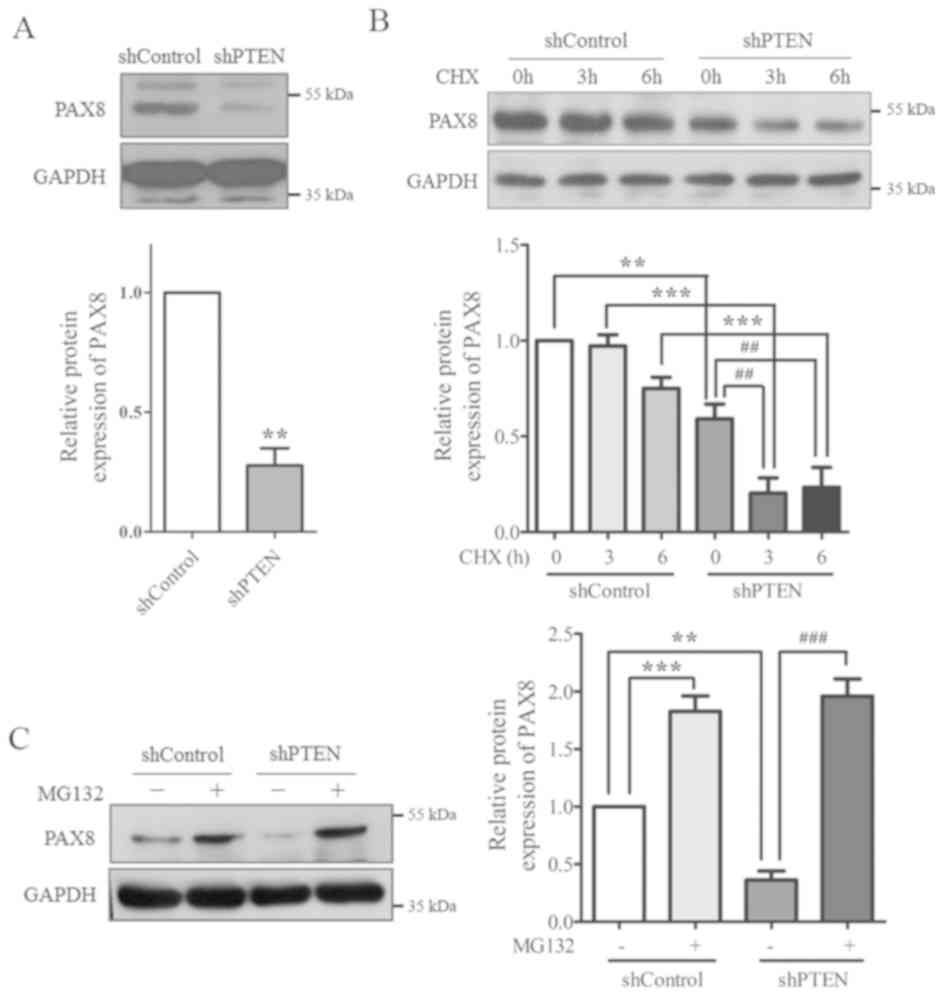
PTEN-knockdown decreases PAX8 protein
level
Since PAX8 is important for the morphogenesis,
differentiation and function of the thyroid gland, it was
hypothesized that PTEN may affect the morphology and growth pattern
of thyroid cells by regulating PAX8. To validate this hypothesis,
PAX8 protein expression levels in the control and PTEN-knockdown
cells were determined using western blotting. The results indicated
that PAX8 protein expression protein was significantly reduced in
PTEN-knockdown cells compared with the control group (Fig. 3A).
To examine the underlying mechanisms, cells were
treated with MG132 (a proteasome inhibitor) and CHX (a protein
synthesis inhibitor) to evaluate the stability of PAX8 protein. The
results from western blotting demonstrated that, following CHX
treatment, PAX8 protein expression was increased in the control
group compared with cells transfected with PTEN-shRNA (Fig. 3B). Following treatment with MG132,
PAX8 protein expression was significantly recovered in the
PTEN-shRNA group (Fig. 3C).
Together, these results suggested that PAX8 stability may be
regulated by PTEN, as PTEN-knockdown increased PAX8
degradation.
PAX8 overexpression in PTEN knocked
down cells restores normal morphology and growth pattern of
Nthy-Ori 3-1 cells
To further validate the hypothesis that a PTEN-PAX8
pathway may exist in the thyroid cells, PAX8 was overexpressed in
PTEN knocked down Nthy-Ori 3-1 cells (Fig. 4A) and PAX8 was knocked down in
Nthy-Ori 3-1 cells (Fig. 4B). The
cell morphology and growth pattern were then evaluated. As
presented in Fig. 4C, PTEN or PAX8
knockdown resulted in a rounder and flatter cell morphology
(Fig. 4C-a and d). However, PAX8
overexpression in PTEN knocked down cells resulted in a thin and
long cell morphology (Fig. 4C-c).
The results from the Matrigel assay demonstrated that PTEN or
PAX8-knockdown alone decreased the tubular-like structures formed
by Nthy-Ori 3-1 cells (Fig. 4D-b and
d), whereas PAX8 reintroduction significantly increased the
formation of the tubular-like structures (Fig. 4D-c). These results suggested that
PAX8 may serve a crucial role in the effect of PTEN on Nthy-Ori 3-1
cell morphology and growth pattern.
PTEN affects Nthy-Ori 3-1 cell
function by regulating PAX8
PAX8 is a key transcription factor that controls the
expression of numerous thyroid-specific proteins, including TG, TPO
and NIS, which are essential for physiological functioning of the
thyroid gland (29). As PAX8 was
downregulated following PTEN-knockdown, the expression levels of
TG, TPO and NIS were also determined. As presented in Fig. 5A, compared with the control cells,
TG, TPO and NIS expression levels in PTEN-knockdown cells were all
decreased, whereas PAX8 overexpression restored the expression of
these proteins. These results suggested that PTEN-knockdown may
also affect Nthy-Ori 3-1 cell function by regulating PAX8.
 | Figure 5.PTEN affects Nthy-Ori 3-1 cell
function by regulating PAX8. (A) Expression of the thyroid-specific
proteins TG, TPO and NIS in shControl, shPTEN and shPTEN+PAX8
groups of cells detected using western blot analysis. GAPDH served
as an internal control. (B) Iodide uptake ability was measured at
different times following the addition of 125I. Data are
presented as the mean ± standard error of mean from at least three
different experiments. *P<0.05, **P<0.01, ***P<0.001 vs.
control. #P<0.05, ###P<0.001 vs.
shPTEN. PAX8, paired box 8; PTEN, phosphatase and tensin homolog;
sh, short hairpin; TG, thyroglobulin; TPO, thyroid peroxidase; NIS,
sodium/iodide symporter; Cpm, counts per minute. |
Iodide uptake is an important characteristic of
thyroid follicular cells and is pivotal for its normal function
(30). Iodide uptake was therefore
assessed in Nthy-Ori 3-1 cells by using an iodide uptake assay. The
results demonstrated that PTEN-knockdown decreased the peak value
of iodide uptake and increased the time period before peak uptake
was reached from 30 to 120 min. Reintroduction of PAX8 into the
PTEN knockdown cells restored the iodide uptake ability of Nthy-Ori
3-1 cells. The peak value of iodide uptake was increased and the
time period before peak uptake was reached decreased from 120 to 60
min (Fig. 5B). These results
indicated that PTEN may disrupt thyroid cell function by regulating
PAX8 expression.
Discussion
PTEN is a dual specificity phosphatase that affects
cell proliferation, cell apoptosis, DNA replication, cell
metabolism and organ development. Numerous studies have reported an
association between PTEN and thyroid disorders (23,24,31);
however, most of these studies demonstrated the inhibitory effects
of PTEN only in thyroid neoplasms. The present study investigated
the non-antitumor effect of PTEN in Nthy-Ori 3-1 cells, and
demonstrated that PTEN-knockdown induced a change in the
morphology, growth pattern and function of thyroid cells. These
results increased understanding of PTEN function and highlighted
how PTEN insufficiency may result in thyroid diseases.
Follicle formation is a typical characteristic of
thyrocytes that is crucial for the physiological functioning of the
thyroid gland. Numerous studies demonstrated by using 3D Matrigel
and tubular-like structure in 2D in vitro cultures that
thyroid cells grow with a follicular-like structure (32–34).
Similarly, Nthy-Ori 3-1 cells were cultured as a monolayer in
Matrigel and the formation of tubular-like structures was observed
in the present study. Following PTEN-knockdown, formation of the
tubular-like structures was partially disrupted, suggesting that
PTEN may be involved in maintaining thyroid cell structure. PAX8
has been demonstrated as a key regulator of follicle formation both
in vivo and in vitro. PAX8-knockout in mice results
in a lack of follicular cells in the thyroid gland (27), and mutations of PAX8 gene in humans
are associated with congenital hypothyroidism (35). Furthermore, it has been reported that
PAX8 can regulate the morphology, differentiation and polarity of
thyrocytes cultured in vitro (28,33,36).
Therefore, the present study investigated PAX8 expression in
Nthy-Ori 3-1 cells. The results demonstrated that PAX8 expression
was decreased in PTEN knocked down cells, which may be responsible
for the aberrant morphology and growth pattern observed in these
cells.
Although PAX8 has been demonstrated to have a
pivotal role and to be involved in the development and
differentiation of thyroid tissue, how PAX8 is regulated remains
unclear. Thyroid-stimulating hormone may regulate PAX8 synthesis at
the transcriptional level (37).
Furthermore, de Cristofaro et al (38) demonstrated that sumoylation could
control PAX8 protein stability. The present study determined
another potential PAX8 regulatory mechanism involving PTEN.
PTEN-knockdown facilitated PAX8 degradation and decreased its
protein expression. However, the role of PTEN in the regulation of
PAX8 stability requires further investigation.
PAX8 is a member of the paired box gene family of
transcription factors, which regulate the expression of several
thyroid-specific proteins, including TG, TPO and NIS (39). These thyroid-specific proteins are
crucial for normal thyroid function, and their decreased secretion
results in hypothyroidism in humans (40,41). In
the present study, PAX8 was downregulated in PTEN knocked down
cells. As a result, TG, TPO and NIS were also downregulated,
suggesting that PTEN-knockdown may impair the function of thyroid
cells. Iodide uptake is a crucial function of the thyroid (30). Subsequently, an iodide uptake assay
was used in the present study to evaluate the function of thyroid
cells in vitro. The results from the iodide uptake assay
analyzed two important indexes, the peak value of iodide uptake,
which represents the iodide uptake capacity, and the time when the
peak value appeared, which represents the iodide uptake speed. The
results demonstrated that the peak value and peak time were both
disrupted following knockdown of PTEN in Nthy-Ori 3-1 cells. In
addition, reintroduction of PAX8 reversed the effects of
PTEN-knockdown, suggesting that PAX8 may be an important mediator.
No significant difference was observed in the peak value of iodide
uptake between shControl and shPTEN at 60 min, which may be due to
the fact that iodide uptake is a dynamic process. Indeed, when at
60 min, iodide uptake in shControl group had reached its peak and
decreased with time, the iodide uptake in shPTEN group had not yet
reached its peak and was still increasing. These observations may
explain that the difference observed between shControl and shPTEN
at 60 min was smaller than at 15 or 30 min. In addition, the peak
value measured in the shControl group during the three repetitions
at 60 min varied a lot (2,672, 4,652 and 4,080 cpm/105
cells), suggesting a higher intra-group variance. Subsequently,
smaller inter-group difference and higher intra-group variance
induced no significant difference at 60 min. However, this didn't
affect the final conclusion of the present study. Since it was
observed that the shControl group had a higher peak value and
shorter peak time compared with shPTEN group, this demonstrated
that PTEN-knockdown may impair the iodide uptake ability of
Nthy-Ori 3-1 cells. These results suggested that PTEN may have a
crucial role in the physiological functioning of thyroid cells.
In conclusion, the present study investigated the
role of PTEN in the morphology and function of thyroid cells by
using the normal thyroid follicular epithelial cell line Nthy-Ori
3-1. The results demonstrated that PTEN-knockdown in Nthy-Ori 3-1
cells induced important morphological changes, which were
determined to be mediated by PAX8 downregulation. It was also
reported that PTEN-knockdown decreased the protein expression of
several thyroid-specific proteins and attenuated the iodide uptake
ability of thyroid cells. These findings highlighted the importance
of PTEN in the regulation of thyroid cell morphology and function,
and reported that PTEN function may be mediated by PAX8.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81502387 and 81372479), the
Natural Science Foundation of the Jiangsu Higher Education
Institutions (grant no. 18KJB310014) and the Xuzhou Science and
Technology Program (grant number KC18036).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YPW and ZDS conceived and designed the experiments.
ZS drafted the manuscript. ZS, JQL and MYW performed the
experiments, collected the data and analyzed the results. CLO and
XCH contributed to the iodide uptake assay. YPX assisted with
western blotting and Matrigel assay. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hollander MC, Blumenthal GM and Dennis PA:
PTEN loss in the continuum of common cancers, rare syndromes and
mouse models. Nat Rev Cancer. 11:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasheed BK, Stenzel TT, McLendon RE,
Parsons R, Friedman AH, Friedman HS, Bigner DD and Bigner SH: PTEN
gene mutations are seen in high-grade but not in low-grade gliomas.
Cancer Res. 57:4187–4190. 1997.PubMed/NCBI
|
|
4
|
Eng C: PTEN: One gene, many syndromes. Hum
Mutat. 22:183–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng J, Liang J, Li J, Li Y, Liang H, Zhao
X, McNutt MA and Yin Y: PTEN controls the DNA replication process
through MCM2 in response to replicative stress. Cell Rep.
13:1295–1303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen WH, Balajee AS, Wang J, Wu H, Eng C,
Pandolfi PP and Yin Y: Essential role for nuclear PTEN in
maintaining chromosomal integrity. Cell. 128:157–170. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang X, Song C, Du X, Zhang C, Liu Y,
Liang L, He J, Lamb K, Shen WH and Yin Y: PTEN stabilizes TOP2A and
regulates the DNA decatenation. Sci Rep. 5:178732015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang G, Li Y, Wang P, Liang H, Cui M, Zhu
M, Guo L, Su Q, Sun Y, McNutt MA and Yin Y: PTEN regulates RPA1 and
protects DNA replication forks. Cell Res. 25:1189–1204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stiles B, Wang Y, Stahl A, Bassilian S,
Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA and Wu
H: Liver-specific deletion of negative regulator Pten results in
fatty liver and insulin hypersensitivity [corrected]. Proc Natl
Acad Sci USA. 101:2082–2087. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knafo S, Sanchez-Puelles C, Palomer E,
Delgado I, Draffin JE, Mingo J, Wahle T, Kaleka K, Mou L,
Pereda-Perez I, et al: PTEN recruitment controls synaptic and
cognitive function in Alzheimer's models. Nat Neurosci. 19:443–453.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li S, Zhu M, Pan R, Fang T, Cao YY, Chen
S, Zhao X, Lei CQ, Guo L, Chen Y, et al: The tumor suppressor PTEN
has a critical role in antiviral innate immunity. Nat Immunol.
17:241–249. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arthur JR and Beckett GJ: Thyroid
function. Br Med Bull. 55:658–668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J and Lazar MA: The mechanism of
action of thyroid hormones. Annu Rev Physiol. 62:439–466. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yen PM: Physiological and molecular basis
of thyroid hormone action. Physiol Rev. 81:1097–1142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mounika B, Brahmaiah B, Ramesh M,
Bhavaneswari K, Anantha Lakshmi T and Nama S: Review on thyroid
disorders. Int J Pharma Res Biosci. 2:197–214. 2013.
|
|
16
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dahia PL, Marsh DJ, Zheng Z, Zedenius J,
Komminoth P, Frisk T, Wallin G, Parsons R, Longy M, Larsson C and
Eng C: Somatic deletions and mutations in the Cowden disease gene,
PTEN, in sporadic thyroid tumors. Cancer Res. 57:4710–4713.
1997.PubMed/NCBI
|
|
18
|
Halachmi N, Halachmi S, Evron E, Cairns P,
Okami K, Saji M, Westra WH, Zeiger MA, Jen J and Sidransky D:
Somatic mutations of the PTEN tumor suppressor gene in sporadic
follicular thyroid tumors. Genes Chromosomes Cancer. 23:239–243.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeh JJ, Marsh DJ, Zedenius J, Dwight T,
Delbridge L, Robinson BG and Eng C: Fine-structure deletion mapping
of 10q22-24 identifies regions of loss of heterozygosity and
suggests that sporadic follicular thyroid adenomas and follicular
thyroid carcinomas develop along distinct neoplastic pathways.
Genes Chromosomes Cancer. 26:322–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruni P, Boccia A, Baldassarre G, Trapasso
F, Santoro M, Chiappetta G, Fusco A and Viglietto G: PTEN
expression is reduced in a subset of sporadic thyroid carcinomas:
Evidence that PTEN-growth suppressing activity in thyroid cancer
cells mediated by p27kip1. Oncogene. 19:3146–3155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gimm O, Perren A, Weng LP, Marsh DJ, Yeh
JJ, Ziebold U, Gil E, Hinze R, Delbridge L, Lees JA, et al:
Differential nuclear and cytoplasmic expression of PTEN in normal
thyroid tissue, and benign and malignant epithelial thyroid tumors.
Am J Pathol. 156:1693–1700. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Loreto C, Tell G, Pestrin M, Pandolfi
M, Damante G and Puglisi F: PTEN and Egr-1 expression in thyroid
proliferative lesions. Cancer Lett. 224:105–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farooq A, Walker LJ, Bowling J and Audisio
RA: Cowden syndrome. Cancer Treat Rev. 36:577–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeager N, Klein-Szanto A, Kimura S and Di
Cristofano A: Pten loss in the mouse thyroid causes goiter and
follicular adenomas: Insights into thyroid function and Cowden
disease pathogenesis. Cancer Res. 67:959–966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dahl E, Koseki H and Balling R: Pax genes
and organogenesis. Bioessays. 19:755–765. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plachov D, Chowdhury K, Walther C, Simon
D, Guenet JL and Gruss P: Pax8, a murine paired box gene expressed
in the developing excretory system and thyroid gland. Development.
110:643–651. 1990.PubMed/NCBI
|
|
27
|
Mansouri A, Chowdhury K and Gruss P:
Follicular cells of the thyroid gland require Pax8 gene function.
Nat Genet. 19:87–90. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pasca di Magliano M, Di Lauro R and
Zannini M: Pax8 has a key role in thyroid cell differentiation.
Proc Natl Acad Sci USA. 97:13144–13149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Damante G and Di Lauro R: Thyroid-specific
gene expression. Biochim Biophys Acta. 1218:255–266. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carrasco N: Iodide transport in the
thyroid gland. Biochim Biophys Acta. 1154:65–82. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tiozzo C, Danopoulos S, Lavarreda-Pearce
M, Baptista S, Varimezova R, Al Alam D, Warburton D, Virender R, De
Langhe S, Di Cristofano A, et al: Embryonic epithelial Pten
deletion through Nkx2.1-cre leads to thyroid tumorigenesis in a
strain-dependent manner. Endocr Relat Cancer. 19:111–122. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mauchamp J, Mirrione A, Alquier C and
André F: Follicle-like structure and polarized monolayer: Role of
the extracellular matrix on thyroid cell organization in primary
culture. Biol Cell. 90:369–380. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koumarianou P, Goméz-López G and
Santisteban P: Pax8 controls thyroid follicular polarity through
cadherin-16. J Cell Sci. 130:219–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nitsch L, Tramontano D, Ambesi-Impiombato
FS, Quarto N and Bonatti S: Morphological and functional polarity
of an epithelial thyroid cell line. Eur J Cell Biol. 38:57–66.
1985.PubMed/NCBI
|
|
35
|
Macchia PE, Lapi P, Krude H, Pirro MT,
Missero C, Chiovato L, Souabni A, Baserga M, Tassi V, Pinchera A,
et al: PAX8 mutations associated with congenital hypothyroidism
caused by thyroid dysgenesis. Nat Genet. 19:83–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Riesco-Eizaguirre G, Wert-Lamas L,
Perales-Patón J, Sastre-Perona A, Fernández LP and Santisteban P:
The miR-146b-3p/PAX8/NIS regulatory circuit modulates the
differentiation phenotype and function of thyroid cells during
carcinogenesis. Cancer Res. 75:4119–4130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mascia A, Nitsch L, Di Lauro R and Zannini
M: Hormonal control of the transcription factor Pax8 and its role
in the regulation of thyroglobulin gene expression in thyroid
cells. J Endocrinol. 172:163–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Cristofaro T, Mascia A, Pappalardo A,
D'Andrea B, Nitsch L and Zannini M: Pax8 protein stability is
controlled by sumoylation. J Mol Endocrinol. 42:35–46. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stuart ET and Gruss P: PAX: Developmental
control genes in cell growth and differentiation. Cell Growth
Differ. 7:405–412. 1996.PubMed/NCBI
|
|
40
|
Targovnik HM, Citterio CE and Rivolta CM:
Iodide handling disorders (NIS, TPO, TG, IYD). Best Pract Res Clin
Endocrinol Metab. 31:195–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Long W, Lu G, Zhou W, Yang Y, Zhang B,
Zhou H, Jiang L and Yu B: Targeted next-generation sequencing of
thirteen causative genes in Chinese patients with congenital
hypothyroidism. Endocr J. 65:1019–1028. 2018. View Article : Google Scholar : PubMed/NCBI
|