Introduction
Mammary tumors are the most common types of cancer
affecting unspayed bitches or bitches that are spayed late in life
(1–5). This disease represents ~52% of all
neoplasms affecting bitches, and 41–53% of mammary tumors are
histopathologically diagnosed as malignant (1–7). Studies
conducted in Brazil between 2010 and 2012 have demonstrated that
the incidence rate of malignant mammary tumors ranges from 60–82%
(8,9). Therefore, studying mammary neoplasms is
of great importance due to the high rates of mortality and
morbidity that affect this animal population (10,11).
Clinical and histological prognostic factors for
mammary tumors have been previously studied and have been suggested
to be important parameters in determining the possible outcome and
progression of the disease (3,12). The
most important clinical characteristics are tumor size, lymph node
status and the presence of distant metastasis (13). However, they are still incipient and
cannot be used as precise and reliable predictive and/or prognostic
factors, particularly in the early stages of the disease (10).
Previous histological classifications for canine
mammary tumors described in the literature failed to identify
potential biological differences among histological subtypes,
particularly in patients with the same disease status that have the
same anatomical, morphological and grade histological structure
(14–18). A number of researchers have suggested
new histopathological classifications, or have made attempts to
modify the existing ones, resulting in subjectivity during
interpretation (14–18). In addition, due to many different
classification systems, oncologists have found it difficult to
select the one that best defines and characterizes the disease as
well as its progression (10).
Cell phenotypes can be used as a new tool that
contributes to the understanding of tumor biology (10), so cancer cell immunostaining should
be emphasized as an effective tool in the clinical setting. A
number of immunohistochemical markers have been proposed and a
panel of markers has been already established for use in the
clinical prognosis of women with breast cancer (19–21).
Importantly, the analysis of this panel of markers can identify a
specific phenotypic tumor profile, which is associated with a
particular biological behavior (22,23).
This panel includes the estrogen receptor (ER) and progesterone
hormone receptor (PR), human epidermal growth factor receptor 2
(HER-2)/neu oncogene, the cell proliferation marker Ki-67 and p53
(10). However, in veterinary
medicine, there are few studies currently focusing on this subject
and the published results are conflicting (24–26).
Therefore, the objective of molecular research in
breast cancer, in addition to defining specific biological
profiles, is to identify novel potential therapeutic targets in
order to increase the control and the treatment of this disease.
Previous findings have provided important prognostic and predictive
information, as well as a better understanding of the complex
molecular mechanisms underlying tumorigenesis (22,27). As
a result, different treatment approaches were optimized and thus,
the ability to identify molecular subtypes has become an important
element for the management of breast cancer (28).
By combining the protein expression of ERs, PRs and
HER-2, three markers widely used in the diagnosis of breast cancer
in women, it is possible to identify the luminal groups A, luminal
B, HER-2-positive and triple-negative (22). This new form of classification has
gained popularity and credit among the scientific community as it
has been discovered that the groups are associated with specific
treatments (29–31). The luminal group presents a favorable
prognosis and responds to hormone therapy, whereas monoclonal
therapy is indicated in the HER-2 group (32,33). The
targeted therapies against HER-2 positive tumors are very
effective, both in the adjuvant and the metastatic setting
(34,35). Trastuzumab is a humanized monoclonal
antibody that improves response rates, decreases the progression of
the disease and improves survival time when used alone or added to
chemotherapy in metastatic breast cancer (36). The triple-negative group has worse
prognosis and requires specific treatments (37,38). It
is expected that patients with this profile will not benefit from
the use of trastuzumab or hormonal therapies, which results in
challenges when prescribing and administering treatments in
patients with triple-negative mammary tumor (39,40).
In addition, E-cadherin is considered an important
prognostic immunohistochemical marker. This molecule is a cell
adhesion protein and an important regulator of the epithelial
phenotype. The low expression of E-cadherin is associated with the
malignant progression of the disease (41), and the low expression of this protein
occurs in processes involved in the epithelial-to-mesenchymal
transition (EMT). EMT promotes the acquisition of mesenchymal
characteristics in epithelial cancer cells with the invasion and
spreading of cancer cells (41,42).
Canine mammary tumors have been observed to be significantly
associated with the loss of E-cadherin expression, resulting in a
poor prognosis (43).
Therefore, the purpose of the present study was to
define a phenotypic immunohistochemical classification for canine
mammary tumors in four groups: i) luminal A (ER+ and/or
PR+, and HER-2−); ii) luminal B
(ER+ and/or PR+, and HER-2+); iii)
HER-2 positive (ER−, PR− and
HER-2+); and iv) triple-negative (ER−,
PR− and HER-2−). In addition, the present
study investigated E-cadherin expression in these phenotypes in
order to examine the association between tumor classification and
prognosis also in the canine species.
Materials and methods
Experimental group
The experimental group consisted of 110 bitches,
enrolled between January 2011 and December 2013, of different
breeds and ages (3–15 years). In all dogs examined, the mammary
cancer developed spontaneously. All patients were treated in the
Obstetric and Reproduction Department of the Veterinary Hospital
Governador Laudo Natel of Faculdade de Ciências Agrárias e
Veterinárias/Universidade Estadual Paulista, Jaboticabal, Brazil,
and the veterinary clinics of São José do Rio Preto (São Paulo,
Brazil). The owners of each dog provided written consent for the
use of the samples, and the present study was approved by the The
Ethics Committee on Animal Experimentation of the Faculty of
Medicine of São José do Rio Preto prior to the study start. All
dogs were treated surgically, and none of the dogs received any
additional anticancer treatment prior to or following
mastectomy.
Following excision of the tumor, the patients were
followed up for 1–18 months. During the follow-up time, the
veterinarians evaluated tumor metastasis and recurrence, as well as
the cause of mortality in the animals. For histopathological
diagnosis, the tumor biopsies collected were classified according
to Goldschmidt et al (18).
The parameters employed for the classification of
clinical tumor staging were in accordance with the
Tumor-Node-Metastasis (TNM) system established by the World Health
Organization for canine mammary gland tumors (44,45).
Immunohistochemistry (IHC)
The IHC procedure was performed following the method
described by Lopes et al (46). Tumor samples were embedded into
paraffin blocks and cut into 3-µm sections. The samples were
prepared on silanized glass slides prior to deparaffinization. The
sections were rehydrated in an ascending range of alcohol
concentrations and incubated with 3% hydrogen peroxide for 30 min.
Antigen retrieval was performed by heating at 95°C in buffer for 35
min. The slides were incubated with bovine serum albumin (BSA). The
slides were incubated at 4°C overnight with the primary antibodies
(Table I). After being washed with
phosphate-buffered saline (PBS) for 15 min, incubation was carried
out with Starr Trek Universal HRP Detection kit (Medical Biocare,
Concord, CA, USA), consisting of the secondary antibody
‘anti-mouse, rabbit and goat immunoglobulin with biotin’ for 1 h
and ‘streptoavidin complex with peroxidase’ for 30 min, followed by
washes with PBS for 15 min. Subsequently, 0.5%
3,3′-diaminobenzidine tetrahydrochloride was applied to the slides
for 2–5 min at 20–22°C. The slides were counterstained with Harris'
hematoxylin for 40 min. Negative controls were obtained by omitting
the primary antibody.
 | Table I.Antibodies and dilutions. |
Table I.
Antibodies and dilutions.
| Antibody | Specificity | Clone | Dilution | Supplier |
|---|
| E-cadherin | (Mouse) | 36/Ecad | 1:2,000 | BD Biosciences |
| ER | Monoclonal
(mouse) | 1D5 | 1:150 | Santa Cruz
Biotechnology, Inc. |
| PR | Monoclonal
(rabbit) | SP42 | 1:400 | Abcam |
| HER-2/neu | Polyclonal
(rabbit) | C18 | 1:800 | Santa Cruz
Biotechnology, Inc. |
The slides were scanned using the Panoramic Digital
Slide Scanner (magnification, ×40; 3DHISTECH®) and
Panoramic Viewer software (3DHISTECH®) (47).
Analysis and interpretation of
IHC
For the ERs and PRs, the immunoreactivity index of
Allred et al (48) was
applied. The final score was calculated according to the quantity
of marked cells and the intensity of the staining. The scores
ranged between 0 and 8. Samples with a score of 0–1 were considered
negative, and samples with a score of 2–8 were considered positive.
The scores and criteria used to quantify the labeled cells were: i)
0, no labeling; ii) 1, labeling in <1% of cells; iii) 2, 1–10%;
3, 11–33%; iv) 4, 34–66%; and 5, 67–100%. The criteria and scores
used to determine the intensity of immunostaining were: i) Absent,
0; ii) weak, 1; iii) moderate, 2; and iv) strong, 3. The staining
intensity was determined by eye. The sum of these criteria for each
sample determined the final score.
HER-2/neu exhibited membrane staining in >10% of
neoplastic cells, and the intensity of the staining was assessed
according to a previously described semi-quantitative method
(49). The criteria used for the
score were as follows: i) 0, no staining; ii) 1, weak, incomplete
membranous staining; iii) 2, moderate, complete membranous staining
in at least 10% of tumor cells; and iv) 3, strong membranous
staining in at least 10% of tumor cells. The staining intensity was
determined by eye. Cases with a score of 0–1 were considered
negative, whereas scores of 2–3 were considered to exhibit HER-2
positive.
Expression of E-cadherin was performed using the
modified semi-quantitative immunoreactive score scale according to
Remmele and Stegner (50).
Immunostaining signal of E-cadherin was observed in the cytoplasmic
membrane. The method described by Remmele and Stegner takes into
account both the proportion of positively stained cells and the
intensity of the staining. The criteria used to quantify the
labeled cells were: i) 0, no labeling; ii) 1, labeling in ≤10% of
cells; iii) 2, 11–50%; iv) 3, 51–80%; and v) 4, 81–100%. The
criteria used to determine the intensity of immunostaining were: i)
Zero, no staining; ii) ‘+’, weak staining; iii) ‘++’, moderate
staining; and iv) ‘+++’, strong staining. The multiplication of the
criteria for each sample determined the final score.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism (version 5.0; GraphPad Software, Inc.) software. Raw
data were initially subjected to descriptive analyses to determine
the normal distribution. Any associations between immunostaining
intensity of E-cadherin and the mammary cancer phenotypes of the
patients within each group were analyzed using the χ2
test. The survival curve was constructed using the Kaplan-Meier
method and the differences between the curves were evaluated using
a log-rank test and hazard function. P<0.05 was considered to
indicate a statistically significant result.
Results
Phenotype characterization
In the present study, the luminal A phenotype was
the most frequently observed (38.2%), followed by luminal B
(37.3%), triple-negative (15.4%) and HER-2 positive (9.1%).
Fig. 1 presents the phenotypic
profiles according to the immunohistochemical markers ER, PR and
HER2. The mammary tumor group exhibited the most frequent
histopathological classifications: i) Luminal A (29.0% complex
mammary carcinoma, 24.0% tubulopapillary mammary carcinoma and
21.0% mixed mammary carcinoma); and ii) luminal B (37.0% complex
mammary carcinoma, 19.0% tubulopapillary mammary carcinoma, 15.0%
mixed mammary carcinoma and 15.0% solid carcinoma); iii) HER-2
positive (30.0% complex mammary carcinoma, 30% tubulopapillary
mammary carcinoma and 20.0% anaplastic mammary carcinoma); and iv)
triple-negative (29.4% complex mammary carcinoma, 23.5% mixed
mammary carcinoma and 23.5% tubulopapillary mammary carcinoma).
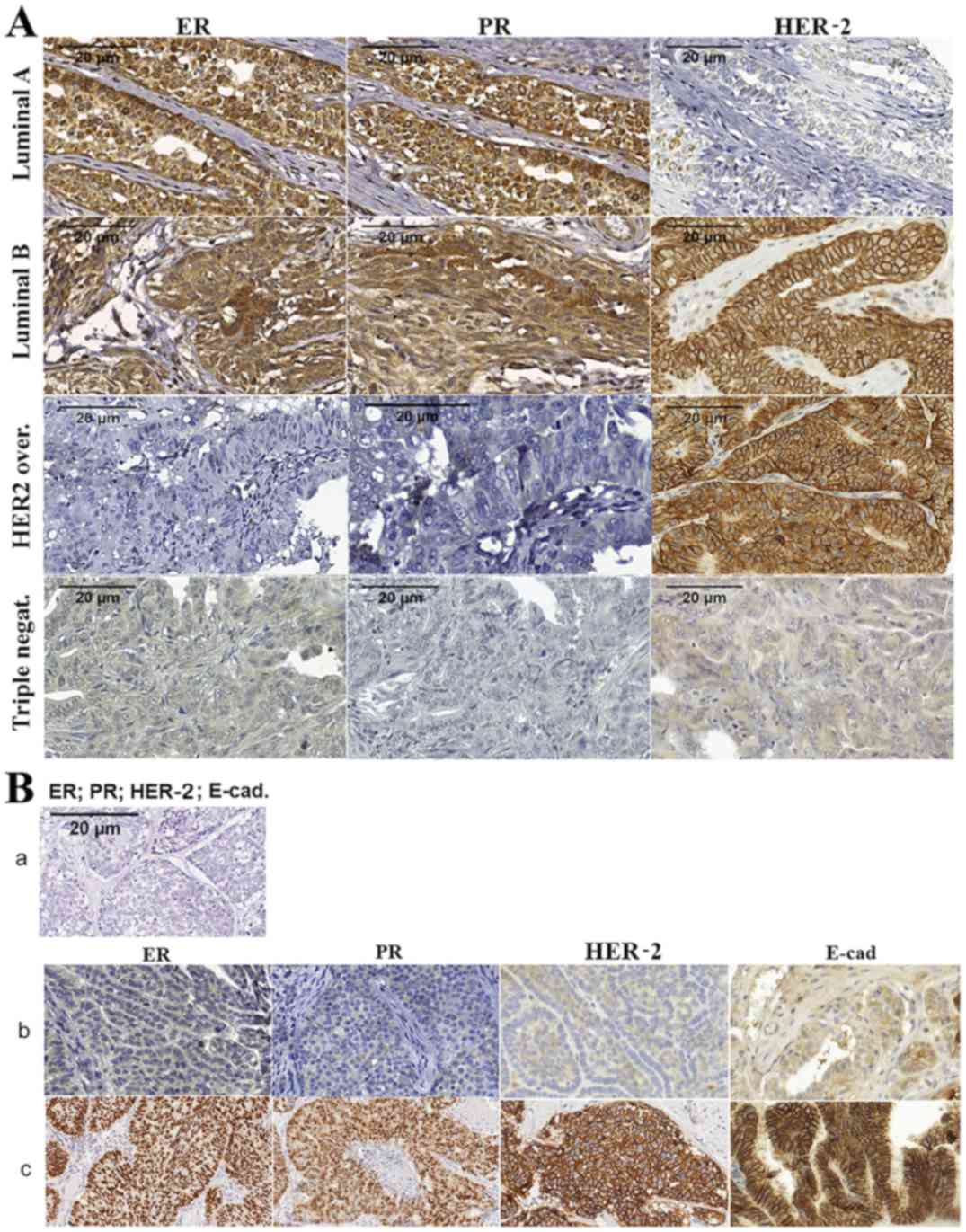 | Figure 1.Immunohistochemical characteristics
of mammary cancer phenotypes in bitches. (A) Photomicrograph of
phenotypic profiles classified by immunohistochemical markers of
ER, PR and HER-2 in canine mammary tumors. 3,3′-diaminobenzidine
chromogen. Scale bar, 20 µm. ER positive and/or PR positive and
HER-2 negative signals are associated with the phenotype Luminal A.
ER positive and/or PR positive and HER-2 positive signals are
associated with the phenotype Luminal B. The phenotype HER-2
upregulated was characterized by ER negative, PR negative and HER-2
positive signals. ER negative, PR negative, and HER-2 negative
signals are associated with the triple negative phenotype. (B)
Photomicrograph of (B-a) negative human breast cancer control and
(B-b) negative to low protein expression of ER, PR, HER-2 and
E-cadherin in canine mammary cancer. (B-c) Human samples positive
for ER, PR, HER-2 and E-cadherin. Magnification, ×40. Laboratory of
Molecular Investigation of Cancer (LIMC), 2019. ER, estrogen
receptor; PR, progesterone receptor; HER-2, human epidermal growth
factor receptor. |
Mammary tumor phenotypes and
clinicopathological characteristics
For the luminal A tumor subtype, only 14.3% (6/42)
of the animals presented cutaneous (2/42) and pulmonary (2/42)
metastasis and recurrence (1/42). Regarding the TNM classification,
the minority of mammary tumors were classified with the worst
prognosis. Regarding the luminal B subtype, 22.0% (9/41) of the
animals presented hepatic (2/41) and pulmonary (5/41) metastasis
and recurrence (5/41). For the TNM classification, stages I and II
(30/41) were significantly higher in number compared with stages
III, IV and V.
Regarding HER-2 positive, 80.0% (8/10) of animals
presented cutaneous (1/10) and pulmonary (7/10) metastasis and
60.0% (6/10) exhibited stage III, IV or V TNM classification. In
the triple-negative tumor phenotype, 76.4% (6/10) of animals
presented hepatic (1/17) and pulmonary (12/17) metastasis and 53.0%
(9/17) of tumors exhibited stage III, IV and V TNM
classification.
Table II presents
the distribution of frequency (number of cases) of
clinicopathological characteristics in the phenotypes luminal A,
luminal B, HER-2 positive and triple-negative mammary cancer in
female dogs.
 | Table II.Distribution of frequency of
clinicopathological characteristics in the mammary cancer
phenotypes of female dogs. |
Table II.
Distribution of frequency of
clinicopathological characteristics in the mammary cancer
phenotypes of female dogs.
| Clinical
characteristics | Luminal A, n | Luminal B, n | HER-2 positive,
n | Triple-negative,
n |
|---|
| No
complications | 36 | 32 | 2 | 4 |
| Recurrence | 1 | 2 | 0 | 0 |
| Metastasis |
|
Cutaneous | 2 | 0 | 1 | 0 |
|
Lung | 3 | 5 | 7 | 12 |
|
Liver | 0 | 2 | 0 | 1 |
| TNM |
| I | 15 | 20 | 1 | 3 |
| II | 10 | 10 | 2 | 4 |
|
III | 8 | 2 | 4 | 4 |
| IV | 5 | 6 | 1 | 1 |
| V | 3 | 1 | 0 | 4 |
|
Uninformed | 1 | 2 | 2 | 1 |
Association between mammary tumor
phenotypes and survival
Regarding the luminal A subtype, only three patients
(3/42) died as a result of the disease (pulmonary metastasis). The
others (39/42) were examined during the 18 months of follow-up
without experiencing recurrence. For the luminal B subtype, eight
dogs (8/41) died due to distant metastasis (pulmonary or hepatic).
In two patients (2/41) there was a local tumor recurrence within
the surgical scar. The other dogs (32/41) had no complications
associated with the disease during the 18 months of follow-up. In
the present study, it was observed that the majority of the
patients classified as luminal A and luminal B were included in
stage I of the disease and had longer survival times when compared
with other phenotypes.
In the tumors with HER-2 positive, the mean survival
time was 167 days. A total of seven dogs (7/10) died due to
pulmonary metastasis, and there were no secondary complications
associated with mammary cancer detected in the remaining three
dogs. In the triple-negative tumor phenotype, the mean survival
time associated with this profile was 274 days. A total of three
dogs (3/17) did not exhibit evidence of disease complications, and
14 died from lung and/or liver metastasis. The survival curve of
each phenotype is presented in Fig.
2 (P<0.0001).
Low E-cadherin expression is
associated with poor prognostic outcome
Positive E-cadherin expression was observed in 80%
(n=84) of mammary tumors, whereas no staining was observed in 20%
(n=21) of samples. Regarding the E-cadherin intensity, it was
observed that 15 mammary tumors were scored as ‘+’, 52 samples were
scored as ‘++’ and 17 samples were scored as ‘+++’.
The present study also compared the level of
E-cadherin expression with the assessed phenotypic groups. A
significant association was observed between the intensity of
expression among the four profiles considered (P=0.03). The
majority of samples with luminal A and B phenotypes presented a
score of ‘++’ and ‘+++’. On the other hand, tumors classified as
HER-2 positive and triple-negative primarily presented scores of
‘0’, ‘+’ and ‘++’ (Table III).
 | Table III.Distribution of frequency of
intensity of E-cadherin expression in canine mammary tumors
according to each phenotype. |
Table III.
Distribution of frequency of
intensity of E-cadherin expression in canine mammary tumors
according to each phenotype.
| Immunostaining | Luminal A, n | Luminal B, n | HER-2 positive,
n | Triple-negative,
n |
|---|
| Negative | 6 | 5 | 4 | 7 |
| Weak | 5 | 4 | 3 | 3 |
| Moderate | 23 | 22 | 2 | 5 |
| Strong | 6 | 9 | 0 | 1 |
| Total | 40 | 40 | 9 | 16 |
Discussion
Previous studies have demonstrated that canine
mammary cancer is a heterogeneous disease (24,26,51).
Despite previous studies have tried to define histopathological
classifications able to associating the type of mammary tumors and
prognostic features, these attempts have failed in a number of
ways; particularly in predicting disease characteristics in the
early stages, in the monitoring of progress, and in the assessment
of risk of recurrence and mortality in the absence of treatment
(10,40,41).
The present study suggested that histopathological
classification are not associated with a clear prognosis. It was
observed that the same histopathological subtype may exhibit
different phenotypes and, as a result, different outcomes.
Therefore, it has been suggested that the anatomical and
morphological evaluation of neoplastic lesions should not be
considered as the only diagnostic method. In veterinary medicine,
few studies investigated molecular classifications in combination
with the immunohistochemical markers in canine mammary tumors
(25,26,51). In
addition, there are discrepancies between previous studies, which
are based on a variety of markers and different techniques, making
challenging to perform comparisons among previous methodologies
(24–26,51,52).
Notably, the main limitation of the present study was the
non-standardization of the subtype and the histological grade of
the neoplasms used.
Therefore, the classification proposed in the
present study was based on four immunohistochemical markers (ER,
PR, HER-2 and E-cadherin) that were used to define the following
phenotypic groups: i) luminal A; ii) luminal B; iii) HER-2
positive; and iv) triple-negative. The use of these markers proved
to be very effective in defining the phenotypic profiles of canine
mammary tumors to ensure an accurate association with prognosis.
This is the main result of the present study, as phenotypic
classifications from previous studies use additional markers to
define the panel (25,26,51).
Collectively, it was observed that the majority of
canine neoplasms were classified as luminal A and luminal B, which
suggested a favorable prognosis. These groups were characterized by
positive expression of ERs and PRs. It has already been previously
established that elevated expression levels of these receptors
occurs in normal mammary glands and benign mammary tumors (3). However, mammary cancer can also exhibit
expression of these receptors, which tends to have an improved
prognosis while the negative staining is associated with the most
malignant mammary tumors (24,52,53).
ERs are steroid receptors located in the cytoplasm
and on the nuclear membrane (54,55). The
activation of cytoplasmic steroidal receptors occurs via the
non-genomic pathway (56).
Non-genomic effects do not depend on gene transcription or protein
synthesis and involve steroid-induced modulation of cytoplasmic
regulatory proteins or cell membrane boundaries (57,58).
Previous studies have demonstrated that the activation of this
pathway influences mammary gland carcinogenesis and disease
progression (59–61).
There are few studies in veterinary literature that
investigated the presence and actions of ER and PR in cytoplasmic
in mammary tumors (62,63). These studies revealed important
information about the heterogeneity of this cancer. However, none
of these focused on the prognostic value of these findings in
mammary cancer. Rutteman et al (62) measured cytoplasmic ERs and PRs in
normal and neoplastic mammary tissues of bitches. Rutteman et
al (62) concluded that the
expression of these receptors was more frequent in normal and
benign neoplasm samples. Due to the results observed, they also
concluded that metastases were less frequently observed in those
with the same receptors. Donnay et al (63) demonstrated that poorly differentiated
malignant tumors in bitches express lower concentrations of ER and
PR in the cytoplasm when compared with healthy glands. Donnay et
al suggested that the expression of ER and PR are greater in
the most differentiated tumors and with less aggressive biological
behavior. In addition, other studies (64–66) have
evaluated nuclear immunoexpression of these same receptors, ER and
RP, and yet the findings corroborate the results of Rutterman et
al (62) and Donay et al
(63).
The expression of the HER-2 protein was demonstrated
to be an efficient prognostic marker (22,24,67). In
the present study, there was an increased mortality rate in the
luminal B group compared with the luminal A. HER-2 positive
phenotype, defined by the absence of ER and PR, as well as the
HER-2 expression, exhibited the worst prognosis compared with the
other groups. The expression of HER-2 protein in canine mammary
tumors was determined by Dutra et al (68) and the results are in line with the
present study, as they demonstrated that upregulation of the HER-2
protein is associated with more aggressive neoplasms. However,
Campos et al (69) observed
no prognostic features associated with this marker.
In the present study, it was observed that
triple-negative tumors are associated with a shorter survival time
and, therefore, a worse prognosis. Kim et al (52) demonstrated that 18.7% of canine
mammary tumors analyzed were triple-negative, and that the
triple-negative phenotype was associated with a poor prognosis.
Other molecular classifications based on
immunohistochemical markers for mammary tumors in female dogs were
described in three previous studies. Gama et al (26) investigated an immunohistochemical
panel based on five markers (ER, PR, HER-2, CK5, p63 and
P-cadherin) and determined five phenotypes: Luminal A, luminal B,
HER-2 positive, basal-like and null phenotype. In line with the
present study, there was a significant difference between the
groups regarding the prognosis and survival time, except for in the
null phenotype. Sassi et al (25) proposed five immunohistochemical
markers for classifying neoplasms in the luminal A, luminal B,
HER-2 positive, basal-like and typical-like phenotypes. However,
they only identified the luminal A subtype, luminal B and
basal-like phenotypes. In contrast with the present study, there
were no statistically significant differences in survival times
between phenotypes (25). Im et
al (51) used six antibodies
(ER, HER-2, CK-14, P63, smooth muscle actin and vimentin) to define
six groups: Luminal A, luminal B, HER-2 positive, basal-like and
typical-like. Im et al (51)
suggested that low ER expression and upregulation of HER-2 were
associated with poor prognosis in canine mammary tumors.
The addition of the E-cadherin antibody to the panel
ensures the classification of neoplastic cells in the epithelial
profile. The association of this marker with the other groups
allows the evaluation of the different behaviors among neoplasms
characterized in the same phenotypic subtype. The present study
demonstrated that the absence or low expression of E-cadherin was
associated with more aggressive and metastatic phenotypes
(triple-negative and HER-2 positive) representing an important
marker that can help to understand and better define the
development and/or progression of the disease. Poorly
differentiated, invasive and metastatic canine mammary carcinomas
exhibited a loss of E-cadherin expression in certain tumor cell
subpopulations, suggesting that altered E-cadherin expression may
be an important process in malignant transformation. This
hypothesis is supported by the observation that the loss of
E-cadherin expression is associated with a shorter overall survival
time and disease-free period (70).
Previous studies have demonstrated that the decrease
in E-cadherin expression is associated with tumors of a higher
invasive grade, and therefore more metastatic (13,43), in
line with the results of the present study. The present study also
observed that the absence or low expression of E-cadherin was found
associated with more aggressive and metastatic phenotypes
(triple-negative and HER-2 positive).
The proposed classification based on the phenotypic
subtypes luminal A, luminal B, HER-2 positive and triple-negative
was performed using immunohistochemical markers (ER, PR, HER-2 and
E-cadherin), and proved to be an important prognostic tool for
malignant canine mammary tumors. The classification described in
the present study could be used in the field of oncology to provide
valuable information about the course and evolution of mammary
tumors in canine species. Notably, the main limitation of the
present study was the non-standardization of the subtype and the
histological grade of the neoplasms used.
Acknowledgements
The authors would like to thank Mrs Georgia Modé
Magalhães (Federal Institute of the South of Minas, Muzambinho, MG,
Brazil) and Mr Felipe Augusto Ruiz Sueiro (VETPAT, Campinas, SP,
Brazil) for their technical support.
Funding
The present study was supported by the Conselho
Nacional Científico e Tecnológico (grant no. 140624/2013) and
Fundação de Apoio à Pesquisa e Extensão de São José do Rio Preto
(grant no. 208/2014).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
DAPDCZ conceived and designed the study. GRV
performed the experiments and wrote the paper. JRL translated the
main text of the article into the English language. GBG, MGM, JRL,
ABDN and MTC recruited the patients and collected the samples. LBMS
and RMR performed the immunohistochemistry assays. DAPDCZ revised
and edited the manuscript.
Ethics approval and consent to
participate
The Ethics Committee on Animal Experimentation of
the Faculty of Medicine of São José do Rio Preto (approval no.
3231/2012) approved the present study on August 16, 2012. The
Committee for Ethics in Research on Human Beings of the Faculty of
Medicine of São José do Rio Preto granted the use of the human
tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
HER-2
|
human epidermal growth factor receptor
2
|
|
PR
|
progesterone receptor
|
|
TNM
|
Tumor-Node-Metastasis
|
References
|
1
|
Egenvall A, Bonnett BN, Ohagen P, Olson P,
Hedhammar A and Von Euler H: Incidence of and survival after
mammary tumors in a population of over 80,000 insured female dogs
in Sweden from 1995 to 2002. Prev Vet Med. 69:109–127. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lana SE, Rutteman GR and Withrow SJ:
Tumors of the mammary glandSmall Animal Clinical Oncology. 4th.
Withrow SJ and MacEwen EG: WB Saunders; Missouri: pp. 619–633.
2007, View Article : Google Scholar
|
|
3
|
Sorenmo KU, Worley DR and Goldschmidt MH:
Tumors of the mammary glandSmall Animal Clinical Oncology. 5th.
Withrow SJ, Vail DM and Page RL: Elsevier; Missouri: pp. 537–556.
2013
|
|
4
|
De Nardi AB, Raposo-Ferreira TMM and da
Assunção KA: Neoplasias MamáriasOncologia em Cães e Gatos. 2nd.
Daleck CR and De Nardi AB: Editora Roca; Rio de Janeiro: pp.
726–756. 2016
|
|
5
|
Sleeckx N, de Rooster H, Veldhuis Kroeze
EJ, van Ginneken C and van Brantegem L: Canine mammary tumours, an
overview. Reprod Domest Anim. 46:1112–1131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brody RS, Goldschimidt MA and Rosze J:
Canine mammary gland neoplasia. J Am Anim Hosp Assoc. 19:61–90.
1985.
|
|
7
|
Al Dissi AN, Haines DM, Singh B and Kidney
BA: Immunohistochemical expression of vascular endothelial growth
factor and vascular endothelial growth factor receptor-2 in canine
simple mammary gland adenocarcinomas. Can Vet J. 5:1109–1114.
2010.
|
|
8
|
Oliveira Filho JC, Kommers GD, Masuda EK,
Marques MF, Fighera RA, Irigoyen LF and Barros CL: Estudo
restrospectivo de 1.647 tumores mamários em cães. Pesq Vet Bras.
30:177–185. 2010. View Article : Google Scholar
|
|
9
|
Feliciano MA, Silva AS, Peixoto RVR,
Galera PD and Vicente WR: Estudo clínico, histopatológico e
imunoistoquímico de neoplasias mamárias em cadelas. Arq Bras Med
Vet Zootec. 64:1094–1100. 2012. View Article : Google Scholar
|
|
10
|
Zuccari DA, Pavam MV, Terzian AC, Pereira
RS, Ruiz CM and Andrade JC: Immunohistochemical evaluation of
e-cadherin, Ki-67 and PCNA in canine mammary neoplasias:
Correlation of prognostic factors and clinical outcome. Pesq Vet
Bras. 28:208–215. 2008. View Article : Google Scholar
|
|
11
|
Manuali E, De Giuseppe A, Feliziani K,
Fortes K, Casciari C and Marchesi MC: CA 15-3 cell lines and tissue
expression in canine mammary cancer and the correlation between
serum levels and tumour histological grade. BMC Vet Res. 8:862012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cassali GD, Lavalle GE, de Nardi AB,
Ferreira E, Bertagnolli AC, Estrela-Lima A, Alessi AC, Daleck CR,
Salgado BS, Fernandes CG, et al: Consensus for the diagnosis,
prognosis and treatment of canine mammary tumors-2013. Brazilian J
Vet Pathol. 7:38–69. 2013.
|
|
13
|
Yoshida K, Yoshida S, Choisunirachon N,
Saito T, Matsumoto K, Saeki K, Mochizuki M, Nishimura R, Sasaki N
and Nakagawa T: The relationship between clinicopathological
features and expression of epithelial and mesenchymal markers in
spontaneous canine mammary gland tumors. J Vet Med Sci.
76:1321–1327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cassali GD, Lavalle GE, De Nardi AB,
Ferreira E, Bertagnolli AC, Lima AE, Alessi AC, Daleck CR, Salgado
B, Fernandes CG, et al: Consensus for the diagnosis, prognosis and
treatment of canine mammary tumors. Brazilian J Vet Pathol.
4:153–180. 2011.
|
|
15
|
Hampe JF and Misdorp W: Tumors and
dysplasias of the mammary gland. Bull World Health Organ.
50:111–133. 1974.PubMed/NCBI
|
|
16
|
Monlux AW, Roszel JF, Macvean DW and
Palmer TW: Classification of epithelial canine mammary tumours in a
defined population. Vet Pathol. 14:194–217. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Misdorp W: Histologic classification and
further characterization of tumors in domestic animals. Adv Vet Sci
Comp Med. 20:191–221. 1976.PubMed/NCBI
|
|
18
|
Goldschmidt M, Peña L, Rasotto R and
Zappulli V: Classification and grading of canine mammary tumors.
Vet Pathol. 48:117–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaha DC: Significance of
immunohistochemistry in breast cancer. World J Clin Oncol.
5:382–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: A collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med. 7:e10002792010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dai X, Xiang L, Li T and Bai Z: Cancer
hallmarks, biomarkers and breast cancer molecular subtypes. J
Cancer. 7:1281–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perou CM and Børresen-Dale AL: Systems
biology and genomics of breast cancer. Cold Spring Harb Perspect
Biol. 3:a0032932011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peña L, Gama A, Goldschmidt MH, Abadie J,
Benazzi C, Castagnaro M, Díez L, Gärtner F, Hellmén E, Kiupel M, et
al: Canine mammary tumors: A review and consensus of standard
guidelines on epithelial and myoepithelial phenotype markers, HER2,
and hormone receptor assessment using immunohistochemistry. Vet
Pathol. 51:127–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sassi F, Benazzi C, Castellani G and Sarli
G: Molecular-based tumour subtypes of canine mammary carcinomas
assessed by immunohistochemistry. BMC Vet Res. 6:52010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gama A, Alves A and Schmitt F:
Identification of molecular phenotypes in canine mammary carcinomas
with clinical implications: Application of the human
classification. Virchows Arch. 453:123–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cirqueira MB, Moreira MARM, Soares LR and
Freitas-Júnior R: Subtipos moleculares do câncer de mama. Femina.
39:499–503. 2011.
|
|
29
|
Prat A, Parker JS, Karginova O, Fan C,
Livasy C, Herschkowitz JI, He X and Perou CM: Phenotypic and
molecular characterization of the claudin-low intrinsic subtype of
breast cancer. Breast Cancer Res. 12:R682010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meisel JL, Venur VA, Gnant M and Carey L:
Evolution of targeted therapy in breast cancer: Where precision
medicine began. Am Soc Clin Oncol Educ Book. 38:78–86. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rivenbark AG, O'Connor SM and Coleman WB:
Molecular and cellular heterogeneity in breast cancer: Challenges
for personalized medicine. Am J Pathol. 183:1113–1124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ignatiadis M and Sotiriou C: Luminal
breast cancer: From biology to treatment. Nat Rev Clin Oncol.
10:494–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arteaga CL, Sliwkowski MX, Osborne CK,
Perez EA, Puglisi F and Gianni L: Treatment of HER2-positive breast
cancer: Current status and future perspectives. Nat Rev Clin Oncol.
9:16–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fabi A, Malaguti P, Vari S and Cognetti F:
First-line therapy in HER2 positive metastatic breast cancer: Is
the mosaic fully completed or are we missing additional pieces? J
Exp Clin Cancer Res. 35:1042016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Figueroa-Magalhães MC, Jelovac D, Connolly
R and Wolff AC: Treatment of HER2-positive Breast Cancer. Breast.
23:128–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mendes D, Alves C, Afonso N, Cardoso F,
Passos-Coelho JL, Costa L, Andrade S and Batel-Marques F: The
benefit of HER2-targeted therapies on overall survival of patients
with metastatic HER2-positive breast cancer-a systematic review.
Breast Cancer Res. 17:1402015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharma P: Biology and management of
patients with triple-negative breast cancer. Oncologist.
21:1050–1062. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lebert JM, Lester R, Powell E, Seal M and
McCarthy J: Advances in the systemic treatment of triple-negative
breast cancer. Curr Oncol. 25 (Suppl 1):S142–S150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vieira DS, Dufloth RM, Schmitt FC and
Zeferino LC: Breast cancer: New concepts in classification. Rev
Bras Ginecol Obstet. 30:42–47. 2008.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Braunstein LZ and Taghian AG: Molecular
phenotype, multigene assays, and the locoregional management of
breast cancer. Semin Radiat Oncol. 26:9–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan KK, Matchett KB, McEnhill PM, Dakir
el H, McMullin MF, El-Tanani Y, Patterson L, Faheem A, Rudland PS,
McCarron PA and El-Tanani M: Protein deregulation associated with
breast cancer metastasis. Cytokine Growth Factor Rev. 26:415–423.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baranwal S and Alahari SK: Molecular
mechanisms controlling E-cadherin expression in breast cancer.
Biochem Biophys Res Commun. 384:6–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matos AJ, Lopes C, Carvalheira J, Santos
M, Rutteman GR and Gärtner F: E-cadherin expression in canine
malignant mammary tumours: Relationship to other
Clinico-pathological variables. J Comp Pathol. 134:182–189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sorenmo K: Canine mammary gland tumors.
Vet Clin North Am Small Anim Pract. 33:573–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Owen LN: TNM Classification of Tumours in
Domestic AnimalsWorld Health Organization; Geneva: pp. 531980
|
|
46
|
Lopes JR, Maschio LB, Jardim-Perassi BV,
Moschetta MG, Ferreira LC, Martins GR, Gelaleti GB and De Campos
Zuccari DA: Evaluation of melatonin treatment in primary culture of
canine mammary tumors. Oncol Rep. 33:311–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lopes J, Arnosti D, Trosko JE, Tai MH and
Zuccari D: Melatonin decreases estrogen receptor binding to
estrogen response elements sites on the OCT4 gene in human breast
cancer stem cells. Genes Cancer. 7:209–217. 2016.PubMed/NCBI
|
|
48
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
49
|
Koeppen HK, Wright BD, Burt AD, Quirke P,
McNicol AM, Dybdal NO, Sliwkowski MX and Hillan KJ: Overexpression
of HER2/neu in solid tumours: An immunohistochimical survey.
Histopathology. 38:96–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Remmele W and Stegner HE: Overexpression
of HER2/neu in solid tumours: An immunohistochimical survey.
Pathologe. 8:138–140. 1987.PubMed/NCBI
|
|
51
|
Im KS, Kim NH, Lim HY, Kim HW, Shin JI and
Sur JH: Analysis of a new histological and molecular-based
classification of canine mammary neoplasia. Vet Pathol. 51:549–559.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim NH, Lim HY, Im KS, Kim JH and Sur JH:
Identification of Triple-negative and Basal-like canine mammary
carcinomas using four basal markers. J Comp Pathol. 148:298–306.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
de las Mulas JM, Millán Y and Dios R: A
prospective analysis of immunohistochemically determined estrogen
receptor alpha and progesterone receptor expression and host and
tumor factors as predictors of disease-free period in mammary
tumors of the dog. Vet Pathol. 42:200–212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hewitt SC and Korach KS: Estrogen
receptors: New directions in the new millennium. Endocr Rev.
39:664–675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fuentes N and Silveyra P: Estrogen
receptor signaling mechanisms. Adv Protein Chem Struct Biol.
116:135–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fox EM, Andrade J and Shupnik MA: Novel
actions of estrogen to promote proliferation: Integration of
cytoplasmic and nuclear pathways. Steroids. 74:622–627. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Boonyaratanakornkit V, Hamilton N,
Márquez-Garbán DC, Pateetin P, McGowan EM and Pietras RJ:
Extranuclear signaling by sex steroid receptors and clinical
implications in breast cancer. Mol Cell Endocrinol. 466:51–72.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Vrtačnik P, Ostanek B, Mencej-Bedrač S and
Marc J: The many faces of estrogen signaling. Biochem Med (Zagreb).
24:329–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cortez V, Mann M, Brann DW and Vadlamudi
RK: Extranuclear signaling by estrogen: Role in breast cancer
progression and metastasis. Minerva Ginecol. 62:573–583.
2010.PubMed/NCBI
|
|
60
|
Marino M, Galluzzo P and Ascenzi P:
Estrogen signaling multiple pathways to impact gene transcription.
Curr Genomics. 7:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Poulard C, Treilleux I, Lavergne E,
Bouchekioua-Bouzaghou K, Goddard-Léon S, Chabaud S, Trédan O, Corbo
L and Le Romancer M: Activation of rapid oestrogen signalling in
aggressive human breast cancers. EMBO Mol Med. 4:1200–1213. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Rutteman GR, Misdorp W, Misdorp W,
Blankenstein MA and van den Brom WE: Oestrogen (ER) and progestin
receptors (PR) in mammary tissue of the female dog: Different
receptor profile in non-malignant and malignant states. Br J
Cancer. 58:594–599. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Donnay I, Rauis J, Devleeschouwer N,
Wouters-Ballman P, Leclercq G and Verstegen J: Comparison of
estrogen and progesterone receptor expression in normal and tumor
mammary tissues from dogs. Am J Vet Res. 56:1188–1194.
1995.PubMed/NCBI
|
|
64
|
Peña L, Perez-Alenza MD, Rodriguez-Bertos
A and Nieto A: Canine inflammatory mammary carcinoma:
Histopathology, immunohistochemistry and clinical implications of
21 cases. Breast Cancer Res Treat. 78:141–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Millanta F, Calandrella M, Bari G,
Niccolini M, Vannozzi I and Poli A: Comparison of steroid receptor
expression in normal, dysplastic, and neoplastic canine and feline
mammary tissues. Res Vet Sci. 79:225–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chang CC, Tsai MH, Liao JW, Chan JP, Wong
ML and Chang SC: Evaluation of hormone receptor expression for use
in predicting survival of female dogs with malignant mammary gland
tumors. J Am Vet Med Assoc. 235:391–396. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dutra AP, Granja NV, Schmitt FC and
Cassali GD: c-erbB-2 expression and nuclear pleomorphism in canine
mammary tumors. Brazilian J Med Biol Res. 37:1673–1681. 2004.
View Article : Google Scholar
|
|
69
|
Campos LC, Silva JO, Santos FS, Araújo MR,
Lavalle GE, Ferreira E and Cassali GD: Prognostic significance of
tissue and serum HER2 and MUC1 in canine mammary cancer. J Vet
Diagn Invest. 27:531–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Klopfleisch R, von Euler H, Sarli G, Pinho
SS, Gärtner F and Gruber AD: Molecular carcinogenesis of canine
mammary tumors: News from an old disease. Vet Pathol Online.
48:98–116. 2011. View Article : Google Scholar
|
















