Introduction
Esophageal cancer (EC) is the ninth most common
cancer worldwide and the sixth leading cause of death due to cancer
(1,2). The main pathological subtypes include
squamous cell carcinoma (SCC) and adenocarcinoma (AC) (1–4). In
contrast to Western countries, where esophageal adenocarcinoma
(EAC) is predominant, esophageal squamous cell carcinoma (ESCC) is
predominant in some Asian countries, including Japan and China
(2–4). EC remains a highly lethal malignant
tumor, with poor prognosis, despite advances in diagnosis and
treatment in recent decades. Approximately half of patients with EC
have distant metastases at the time of initial diagnosis and more
than one-third develop distant metastases following surgery or
radiotherapy (3,5). Although chemotherapy is standard
treatment for patients with EC with distant organ metastases, the
prognosis is dismal, with a five-year overall survival (OS) of less
than 5% (6,7). Most distant metastases of EC involve
the distant lymph nodes, liver, and lungs (8–10).
Bone is a frequent site of metastasis from breast,
prostate, and lung cancers (11–13), and
bone metastasis (BM) typically indicates a poor prognosis. BM's
incidence and prevalence has been increasing since a large portion
of the population is elderly. Patients with BMs should be treated
with a multidisciplinary approach, using modalities such as
radiotherapy, surgery and various medical treatments that include
chemotherapy, hormone therapy, and bone-modifying agents (BMAs)
(11). BMs frequently cause
skeletal-related events (SREs), such as pathological fracture,
spinal cord compression, and hypercalcemia, which may require
radiotherapy or surgery, and reduce physical function and quality
of life (14). EC generally
metastasizes to the skeletal system late in the course of the
disease, so patients with EC with BMs are relatively uncommon.
Several studies have reported BM incidence rates ranging from
5.2–7.7% in all-stage patients with EC and 15.3–23.6% in patients
with metastases (8–10,15).
However, there is little information regarding the
clinicopathological features and prognostic factors of patients
with EC with BMs. Therefore, we aimed to investigate these features
and factors retrospectively in patients with EC with BMs who were
treated at our institution.
Materials and methods
Study design and patients
We retrospectively and anonymously reviewed the
medical records of 58 patients with EC, including five patients
with esophagogastric junction (EGJ) cancers, who were diagnosed
with BMs and treated at the Osaka International Cancer Institute
between January 2007 and December 2016. The inclusion criteria
were: 1) histological diagnosis of SCC or AC with the esophagus or
EGJ recognized as the primary tumor; 2) BM diagnosis based on
clinical signs and symptoms as well as radiographic imaging
studies, such as computed tomography (CT), magnetic resonance
imaging (MRI), and fluorodeoxyglucose-positron emission tomography
(FDG-PET)/CT. The exclusion criteria was: 1) patients with other
synchronous malignancies. The Institutional Review Board of the
Osaka International Cancer Institute approved the study.
Data collection
Patient data, including age, sex, and Eastern
Cooperative Oncology Group performance status (ECOG PS), was
collected. Tumor characteristics, including location, histology,
differentiation, resection of primary site, visceral or brain
metastasis, and serum levels of SCC antigen (SCC-Ag),
carcinoembryonic antigen (CEA), C-reactive protein (CRP), lactate
dehydrogenase (LDH), albumin, and alkaline phosphatase (ALP), were
also noted. BM characteristics, including presence at initial
diagnosis, number, type (osteolytic, osteoblastic, mixed, and
intertrabecular), SRE, pathological fracture, spinal cord
compression, hypercalcemia, and treatment received (chemotherapy
before and after the BM diagnosis, BMA, radiotherapy, and surgery),
as well as follow-up period and outcome at last follow-up were
determined.
Statistical analysis
OS, defined as the time from the date of BM
diagnosis to the date of death from any cause or last follow-up
visit, was calculated using the Kaplan-Meier method. The impact of
prognostic factors on OS was first assessed using the log-rank test
in univariate analysis, and then multivariate analysis was
performed using the Cox proportional hazard model with variables
chosen using a forward conditional stepwise approach. Statistical
significance was defined as P<0.05. Statistical analyses
were performed using EZR software (Saitama Medical Center, Jichi
Medical University, Saitama, Japan), a graphical user interface for
R (The R Foundation for Statistical Computing, Vienna,
Austria).
Results
Patient and tumor-related
characteristics
Fifty-eight patients diagnosed with BMs from EC were
enrolled in this study. The median follow-up period for all
patients was three months (range, 0–54). Patient and tumor
characteristics are shown in Table
I. Fifty-three patients (91.4%) were male and five (8.6%) were
female. The median age was 67 years (range, 39–84). ECOG PS was 0–2
in 35 patients (60.3%) and 3–4 in 23 patients (39.7%). Tumor
location was esophagus in 53 patients (91.4%) and EGJ in five
patients (8.6%). Tumor histology of the primary lesion was SCC in
54 patients (93.1%) and AC in four patients (6.9%). Among the 32
patients whose tumor differentiation was evaluated, 12 (37.5%) had
poorly differentiated tumors and 20 (62.5%) had well to moderately
differentiated tumors. Twenty-four patients (41.4%) underwent
surgery for their primary tumor. Visceral or brain metastasis was
observed at the time of BM diagnosis in 38 patients (65.5%).
Elevated levels of serum SCC-Ag (>1.5 ng/ml), CEA (>5 ng/ml),
CRP (>0.3 mg/dl), LDH (>250 U/l), and ALP (>350 U/l) at
the time of BM diagnosis were observed in 68.8, 27.5, 75.4, 27.8,
and 30.9% of patients, respectively. Decreased serum albumin level
(≤3.7 g/dl) was detected in 65.5% of the patients.
 | Table I.Patient and tumor-related
characteristics and univariate analysis of prognostic factors for
OS. |
Table I.
Patient and tumor-related
characteristics and univariate analysis of prognostic factors for
OS.
| Factors | Number (%) | 1-year OS, % | P-value |
|---|
| Age, years |
|
| 0.701 |
|
<65 | 22 (37.9) | 21.9 |
|
|
≥65 | 36 (62.1) | 28.3 |
|
| Sex |
|
| 0.719 |
|
Male | 53 (91.4) | 25.2 |
|
|
Female | 5 (8.6) | 25.0 |
|
| ECOG PS |
|
| <0.001 |
|
0–2 | 35 (60.3) | 34.7 |
|
|
3–4 | 23 (39.7) | 7.6 |
|
| Location |
|
| 0.373 |
|
Esophagus | 53 (91.4) | 28.6 |
|
|
EGJ | 5 (8.6) | 0 |
|
| Histology |
|
| 0.272 |
|
SCC | 54 (93.1) | 27.9 |
|
| AC | 4 (6.9) | 0 |
|
|
Differentiation |
|
| 0.536 |
|
Poorly | 12 (37.5) | 41.9 |
|
|
Moderately/Well | 20 (62.5) | 23.8 |
|
| Resection of
primary site |
|
| 0.224 |
|
Yes | 24 (41.4) | 37.3 |
|
| No | 34 (58.6) | 16.1 |
|
| Visceral or brain
metastasis |
|
| 0.018 |
|
Present | 38 (65.5) | 17.2 |
|
|
Absent | 20 (34.5) | 41.9 |
|
| SCC, ng/ml |
|
| 0.389 |
|
≤1.5 | 15 (31.3) | 39.6 |
|
|
>1.5 | 33 (68.8) | 25.5 |
|
| CEA, ng/ml |
|
| 0.012 |
| ≤5 | 29 (72.5) | 37.2 |
|
|
>5 | 11 (27.5) | 18.2 |
|
| CRP, mg/dl |
|
| 0.011 |
|
≤0.3 | 14 (24.6) | 42.3 |
|
|
>0.3 | 43 (75.4) | 20.5 |
|
| LDH, U/l |
|
| 0.213 |
|
≤250 | 39 (72.2) | 28.5 |
|
|
>250 | 15 (27.8) | 9.3 |
|
| Albumin, g/dl |
|
| 0.018 |
|
≤3.7 | 36 (65.5) | 15.2 |
|
|
>3.7 | 19 (34.5) | 43.8 |
|
| ALP, U/l |
|
| 0.878 |
|
≤350 | 38 (69.1) | 24.7 |
|
|
>350 | 17 (30.9) | 25.9 |
|
Bone metastasis-related
characteristics
The median interval from the diagnosis of EC to BM
detection was seven months (range, 0–80). BM characteristics are
shown in Table II. Fourteen
patients (24.1%) had BMs at initial presentation. A solitary BM was
found in 20 patients (34.5%) and multiple BMs were detected in 38
patients (65.5%). Frequent metastatic sites included the thoracic
vertebrae (31 patients, 53.4%), lumbar vertebrae (18 patients,
31.0%) and pelvic bones (16 patients, 27.6%). The bone metastatic
lesions were osteolytic in 52 patients (89.7%), mixed in two
(3.4%), and intertrabecular in four (6.9%). No patient showed an
osteoblastic type of BM. Chemotherapy was administered before the
diagnosis of BM in 38 patients (65.5%), and 32 patients (55.2%)
received palliative chemotherapy after the BM diagnosis. BMAs, such
as zoledronic acid and denosumab, were administered to 19 patients
(32.8%).
 | Table II.BM-related characteristics and
univariate analysis of prognostic factors for OS. |
Table II.
BM-related characteristics and
univariate analysis of prognostic factors for OS.
| Factors | Number (%) | 1-year OS, % | P-value |
|---|
| BM at initial
diagnosis |
|
|
|
|
Yes | 14 (24.1) | 30.8 | 0.270 |
| No | 44 (75.9) | 23.5 |
|
| Number of BM |
|
|
|
|
Solitary | 20 (34.5) | 21.6 | 0.932 |
|
Multiple | 38 (65.5) | 27.2 |
|
| Type of BM |
|
|
|
|
Osteolytic | 52 (89.7) | 25.5 | 0.745 |
|
Mixed/intertrabecular | 6 (10.3) | 22.2 |
|
| Chemotherapy before
BM |
|
|
|
|
Yes | 38 (65.5) | 22.0 | 0.240 |
| No | 20 (34.5) | 30.6 |
|
| Chemotherapy after
BM |
|
|
|
|
Yes | 32 (55.2) | 37.7 | <0.001 |
| No | 26 (44.8) | 6.5 |
|
| Use of BMA |
|
|
|
|
Yes | 19 (32.8) | 38.3 | 0.147 |
| No | 39 (67.2) | 18.8 |
|
| SRE |
|
|
|
|
Present | 53 (91.4) | 25.1 | 0.836 |
|
Absent | 5 (8.6) | 30 (6-month) |
|
| Radiotherapy for
BM |
|
|
|
|
Yes | 48 (82.8) | 23.6 | 0.978 |
| No | 10 (17.2) | 40.0 |
|
| Orthopedic surgery
for BM |
|
|
|
|
Yes | 4 (6.9) | 25.0 | 0.956 |
| No | 54 (93.1) | 25.7 |
|
| Pathological
fracture |
|
|
|
|
Present | 13 (22.4) | 30.8 | 0.559 |
|
Absent | 45 (77.6) | 22.5 |
|
| Spinal cord
compression |
|
|
|
|
Present | 6 (10.3) | 31.2 | 0.429 |
|
Absent | 52 (89.7) | 24.8 |
|
| Hypercalcemia |
|
|
|
|
Present | 15 (30.0) | 10.0 | 0.086 |
|
Absent | 35 (70.0) | 28.9 |
|
Skeletal-related events
SREs occurred in 53 patients (91.4%), including
radiation therapy (48 patients, 82.8%), surgery (four patients,
6.9%), pathological fracture (13 patients, 22.4%), spinal cord
compression (six patients, 10.3%), and hypercalcemia (15 patients,
30.0%). SREs occurred at the time of the BM diagnosis in 44
patients. In the remaining nine patients, the median time from
identification of BM to SRE was two months (range, 1–13). The
association between type of BMs and interval from BM diagnosis to
SREs was shown in Table III.
 | Table III.Association between type of BMs and
interval from BM diagnosis to SREs. |
Table III.
Association between type of BMs and
interval from BM diagnosis to SREs.
|
|
| Timing of SRE
occurrence |
|
|---|
|
|
|
|
|
|---|
| Type of BM | Incidence rate of
SRE, n (%) | At BM diagnosis, n
(%) | After BM diagnosis,
n (%) | Median interval
from BM to SRE, months |
|---|
| Osteolytic | 49/52 (94.2) | 41/49 (83.7) | 8/49 (16.3) | 4 (range,
1–13) |
| Mixed | 1/2 (50) | 1/1 (100) | 0/1 (0) | – |
|
Intertrabecular | 3/4 (75) | 2/3 (66.7) | 1/3 (33.3) | 1 |
| Total | 53/58 (91.4) | 44/53 (83) | 9/53 (17) | 2 (range,
1–13) |
Predictive factors of OS
The six-month, one-year, and two-year OS rates after
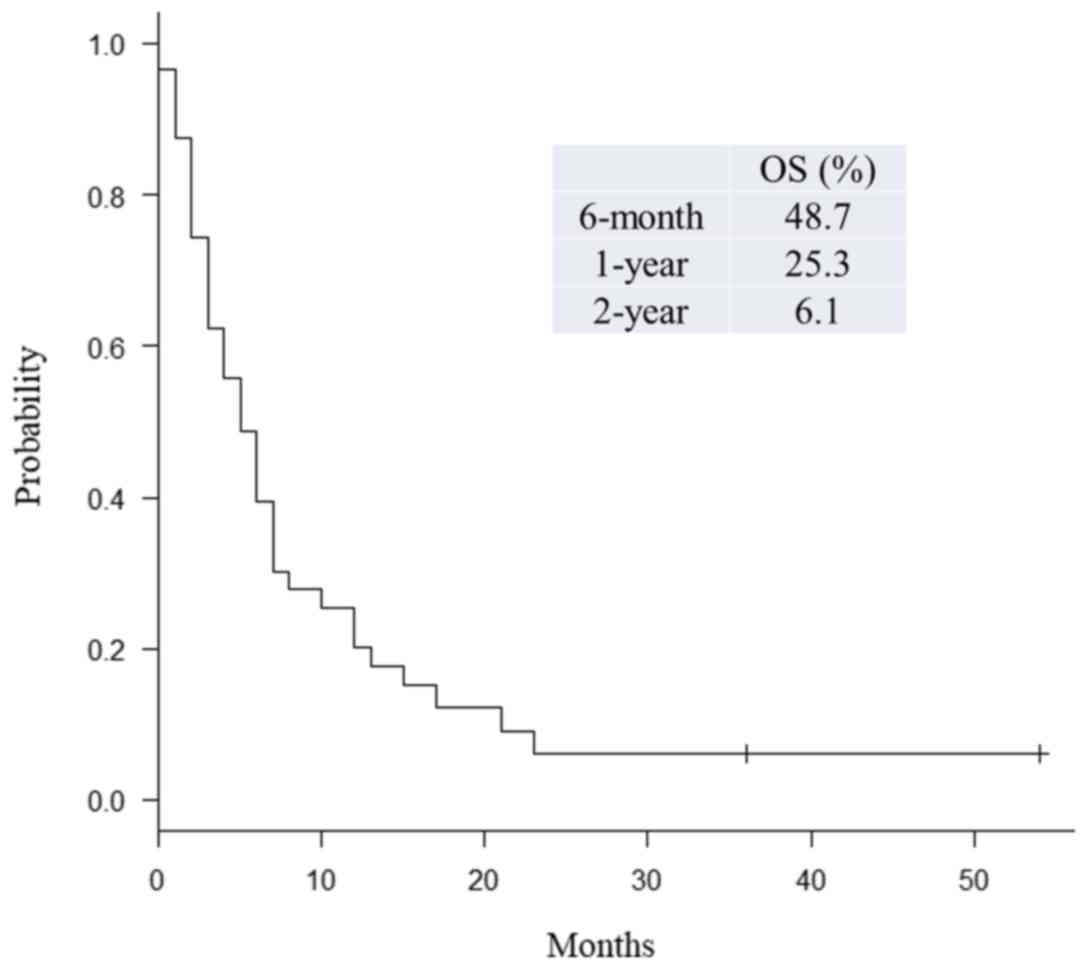
BM diagnosis were 48.7, 25.3, and 6.1%, respectively (Fig. 1). The median OS following BM
diagnosis was five months (range, 0–54). In univariate analyzes,
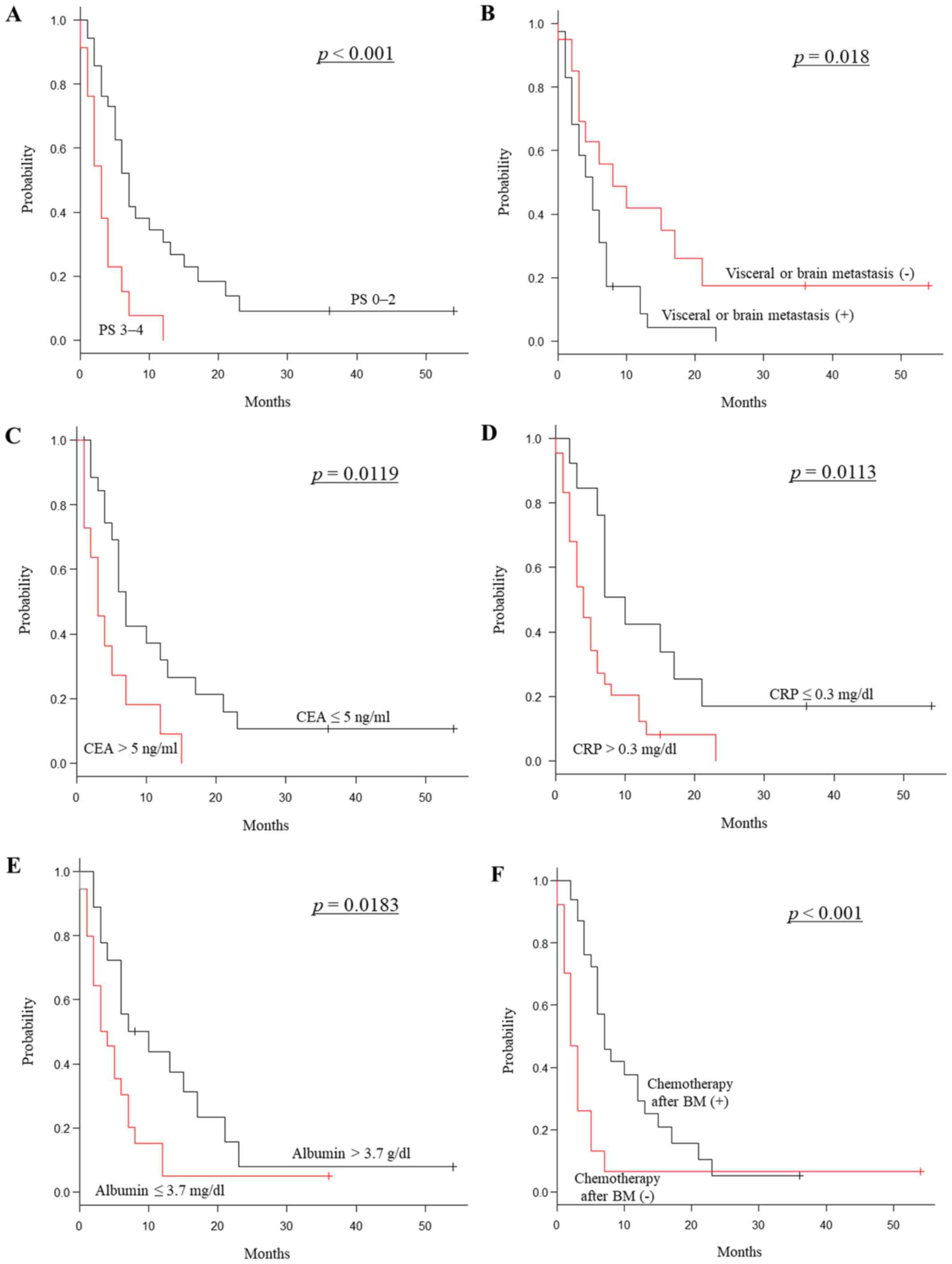
ECOG PS (P<0.001), visceral or brain metastasis
(P=0.018), serum levels of CEA (P=0.012), CRP
(P=0.011), albumin (P=0.018), and receipt of
chemotherapy following BM diagnosis (P<0.001) were
significant prognostic factors (Tables
I, II, Fig. 2A-F). The prognosis of EGJ cancer was
not significantly different from other types of EC
(P=0.373). Tumor histology was also not a significant impact
on OS (P=0.272). Multivariate analyzes showed that elevated
serum CEA level (hazard ratio (HR) 2.400; 95% confidence interval
(CI) 1.020–5.649; P=0.045) and no chemotherapy following the
diagnosis of BM (HR 2.621; 95% CI 1.015–6.769; P=0.046) were
significant independent prognostic factors for poor OS (Table IV).
 | Table IV.Multivariate analysis of prognostic
factors for OS. |
Table IV.
Multivariate analysis of prognostic
factors for OS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | P-value | HR | 95% CI | P-value |
|---|
| ECOG PS |
|
|
|
|
|
0–2 | <0.001 | 1 |
|
|
|
3–4 |
| 2.034 | 0.698–5.922 | 0.193 |
| Visceral or brain
metastasis |
|
|
|
|
|
Present | 0.018 | 1.444 | 0.529–3.938 | 0.473 |
|
Absent |
| 1 |
|
|
| CEA, ng/ml |
|
|
|
|
| ≤5 | 0.012 | 1 |
|
|
|
>5 |
| 2.400 | 1.020–5.649 | 0.045 |
| CRP, mg/dl |
|
|
|
|
|
≤0.3 | 0.011 | 1 |
|
|
|
>0.3 |
| 2.230 | 0.692–7.183 | 0.179 |
| Albumin, g/dl |
|
|
|
|
|
≤3.7 | 0.018 | 1.052 | 0.386–2.868 | 0.921 |
|
>3.7 |
| 1 |
|
|
| Chemotherapy after
BM |
|
|
|
|
|
Yes | <0.001 | 1 |
|
|
| No |
| 2.621 | 1.015–6.769 | 0.046 |
Smokers tend to have higher CEA levels and the
majority of patients with EC must have been heavy smokers (data not
shown). However, the cutoff line of 5.0 ng/ml was chosen in
accordance with previous studies (16,17).
There were 10 patients who had SCC histologically but elevated
serum CEA levels ranging from 5.2 to 55 ng/ml. The prognosis of
those patients was also significantly worse than that of the
patients with SCC who had no elevation of CEA level (26 patients,
P=0.014).
Among 35 patients with EC with BMs whose ECOG PS was
0–2 at BM diagnosis, 26 patients (74.3%) received chemotherapy
after BM diagnosis. On the other hand, among 23 patients with ECOG
PS of 3–4, only six patients (26.1%) received chemotherapy after BM
diagnosis. The patients with ECOG PS of 0–2 who underwent
chemotherapy after BM diagnosis tended to show better prognosis
than those who did not. However, there was no statistically
significant difference between the two groups (P=0.183).
Discussion
BMs are generally categorized, based on morphology,
as osteolytic, osteoblastic, mixed, or intertrabecular. In
osteolytic lesions, factors secreted by tumor cells induce
osteoclast recruitment and activation, leading to increased
osteolysis (12). This frequently
decreases bone integrity and causes severe bone pain, an increased
risk of fracture, and the release of minerals from the bone matrix,
which results in hypercalcemia (14). For these reasons, osteolytic BMs are
associated with a higher probability of SREs, which frequently
cause morbidity and deterioration of PS. In turn, poor PS may
prevent a patient from receiving further available treatment.
Metastases from lung, kidney, and thyroid cancers are predominantly
osteolytic. In this study of patients with EC with BMs, multiple
osteolytic BMs commonly occurred in the axial skeleton, and SREs
occurred in nearly all (91.4%) patients. Therefore, physicians
caring for patients with EC should consider BM in the differential
diagnosis when a patient complains of spontaneous somatic axial
pain. Additionally, when routine follow-up CT reveals osteolytic
changes in a vertebral body, BM should be suspected. Serum ALP,
LDH, and tumor markers, including SCC-Ag and CEA, are not always
elevated when BMs are present. Since MRI and FDG-PET/CT are able to
easily detect BMs from EC (18,19),
using these modalities can allow early and accurate diagnosis to
prevent SREs and preserve PS.
Currently, palliative radiotherapy for painful BM is
a well-established treatment. However, some patients with fracture,
spinal cord compression, or spinal instability due to BM require
surgery, if their life expectancy is not too short. Conversely,
patients with a short life expectancy should receive radiotherapy
and/or supportive care. Therefore, accurate survival data regarding
patients with BMs is necessary so appropriate treatment
recommendations can be made. In breast cancer, patients with BMs
have significantly better survival than those who have metastases
to other sites (20). On the other
hand, OS is significantly worse in patients with EC with BMs
compared to those with metastases to other sites (9,21). In
the current study, the median OS from the time of BM diagnosis in
patients with EC was five months, which is consistent with previous
reports of 2–4 months (9,22). These results suggest that the
prognosis of patients with EC with BMs is usually poor and that
palliative radiotherapy is a standard treatment for those patients.
However, for example, surgical treatment is widely considered more
effective for pathologic proximal femur fractures than radiotherapy
because they are mainly treated with surgery to stabilize the
fractured bones to improve quality of life via pain relief and
restoration of function and mobility. Operative methods are divided
into internal fixation and prosthesis replacement. Only palliative
surgery such as internal fixation may be appropriate for patients
with EC with BMs. On the other hand, despite relatively common
perioperative complications, salvage using endoprostheses is
associated with fewer failures for the treatment of pathologic
proximal fractures compared with internal fixation (23–25).
Araki et al (26) reported
that with regard to bone destruction, the involvement of the head,
neck, calcar, and intertrochanteric region, transverse destruction
>1/2, and soft-tissue tumor extension, were the factors that led
to the choice of prosthesis treatment.
Katagiri et al (22) identified six significant prognostic
factors for survival in patients with BMs: the primary lesion,
visceral or cerebral metastases, abnormal laboratory data, poor PS,
previous chemotherapy and multiple skeletal metastases. The study
included patients with BMs from various cancers. Wu et al
(9) demonstrated the relationship
between the patterns of distant metastasis and prognosis in
metastatic EC. The most common site of distant metastasis was the
liver, followed by distant lymph nodes, lungs, bone and brain. Site
and number of distant metastases were independent prognostic
factors for OS. OS was worst for BMs and greatest for distant lymph
node metastases. However, clinicopathological features and
prognostic factors of patients with EC with BMs remain unknown. To
the best of our knowledge, the present study is the first to report
clinicopathological features and prognostic factors in patients
with EC with BMs. Although univariate analysis showed that visceral
or brain metastasis, abnormal serum CRP and albumin levels, and PS
were significant prognostic factors, multivariate analysis found
that these factors were not significantly associated with OS. Only
elevated serum CEA level and no chemotherapy following BM diagnosis
were shown to be significant independent poor prognostic
factors.
Serum tumor markers play an important role in cancer
diagnosis and prognosis. CEA is relevant in several malignancies,
such as colorectal, lung, and breast cancers. In general, CEA is a
useful marker for EAC; however, it has been reported that CEA was
positive in only 11.4 to 39% of patients with ESCC (27,28). In
the present study, tumor histology was SCC in most patients
(93.1%), and SCC-Ag showed a higher positivity rate (68.8%) than
CEA (27.5%) at the time of BM diagnosis. Some studies have noted
that SCC-Ag was a better OS predictor in patients with EC (29–31),
while other studies have demonstrated CEA's efficacy as a
diagnostic and prognostic marker in patients with EC (17,32,33).
Until now, there has been no agreement on which biomarker is the
best predictor for prognosis in patients with EC with BMs. In the
current study, tumor histology was not associated with patients
with OS with EC. Multivariate analysis showed that serum CEA level
was an independent prognostic factor, but serum SCC-Ag level was
not. CEA is associated with adhesion of malignant tumors, which
might explain the correlation between CEA level and hematogenic
metastasis such as BM.
Chemotherapy improves survival compared to
supportive care alone in patients with metastatic EC, but the
improvement is modest and must be weighed against the side effects
of chemotherapy (34). First-line
chemotherapy usually includes platinum-based agents, such as
cisplatin and oxaliplatin, and a fluoropyrimidine, such as
fluorouracil and capecitabine (34–36). The
addition of a third drug, such as epirubicin or docetaxel, might be
considered for patients who are generally in good health (35,36).
Second-line chemotherapy with docetaxel, paclitaxel, or irinotecan
might be considered for patients with stable PS (37–39). In
the present study, the patients with good PS who received
chemotherapy after BM diagnosis tended to show better prognosis
than those who did not, but there was no significant difference
between the two groups. One reason may be that a patient with good
PS who did not undergo chemotherapy after BM diagnosis had been
alive for 54 months. This patient had a solitary rib BM and no
visceral or brain metastasis. The BM was treated with radiotherapy
and no other metastatic lesion had occurred. ECOG PS and
chemotherapy after BM diagnosis ware significant prognostic factors
for OS in univariate analyses. However, only receipt of
chemotherapy but not good PS was associated with better OS in
multivariate analyses. The prognosis of patients with EC with BMs
who did not receive chemotherapy due to poor PS, advanced age,
comorbidity, or patient refusal was dismal. Our results indicate
that chemotherapy should be considered, whenever possible, at the
time of BM diagnosis in patients with EC.
In conclusion, the prognosis of BM from EC was
extremely poor, with a median OS of five months following BM
diagnosis. Multiple osteolytic BMs occurred predominantly in the
axial skeleton with a high incidence of SREs. Univariate analysis
showed that PS, visceral or brain metastasis, receipt of
chemotherapy following the diagnosis of BM, and serum CEA, CRP, and
albumin levels were significant prognostic factors for OS.
Multivariate analysis demonstrated that no chemotherapy following
the diagnosis of BM and elevated CEA level were independent
prognostic factors for poor OS. In patients with EC with BMs, early
diagnosis and appropriate treatment could prevent SREs and maintain
quality of life and PS, allowing continuation of chemotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Japan Orthopaedics and Traumatology Research Foundation, Inc.
(grant no. 372) and JSPS KAKENHI (grant no. JP19K18481).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YI, SY, KS, HM, RI, and MY organized the study. YI,
TW, TT, HT and NN collected and analyzed the data. YI wrote the
manuscript. SY, KS, HM, RI and MY treated the patients presented in
this manuscript. SY, TW, TT, HT, KS, HM, RI, MY and NN revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the Osaka International Cancer Institute (Osaka,
Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagergren J, Smyth E, Cunningham D and
Lagergren P: Oesophageal cancer. Lancet. 390:2383–2396. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–2509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnold M, Soerjomataram I, Ferlay J and
Forman D: Global incidence of oesophageal cancer by histological
subtype in 2012. Gut. 64:381–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka T, Fujita H, Matono S, Nagano T,
Nishimura K, Murata K, Shirouzu K, Suzuki G, Hayabuchi N and Yamana
H: Outcomes of multimodality therapy for stage IVB esophageal
cancer with distant organ metastasis (M1-Org). Dis Esophagus.
23:646–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu SG, Xie WH, Zhang ZQ, Sun JY, Li FY,
Lin HX, Yong Bao and He ZY: Surgery combined with radiotherapy
improved survival in metastatic esophageal cancer in a surveillance
epidemiology and end results population-based study. Sci Rep.
6:282802016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ai D, Zhu H, Ren W, Chen Y, Liu Q, Deng J,
Ye J, Fan J and Zhao K: Patterns of distant organ metastases in
esophageal cancer: A population-based study. J Thorac Dis.
9:3023–3030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu SG, Zhang WW, He ZY, Sun JY, Chen YX
and Guo L: Sites of metastasis and overall survival in esophageal
cancer: A population-based study. Cancer Manag Res. 9:781–788.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu SG, Zhang WW, Sun JY, Li FY, Lin Q and
He ZY: Patterns of distant metastasis between histological types in
esophageal cancer. Front Oncol. 8:3022018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nielsen OS, Munro AJ and Tannock IF: Bone
metastases: Pathophysiology and management policy. J Clin Oncol.
9:509–524. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berenson JR, Rosen LS, Howell A, Porter L,
Coleman RE, Morley W, Dreicer R, Kuross SA, Lipton A and Seaman JJ:
Zoledronic acid reduces skeletal-related events in patients with
osteolytic metastases. Cancer. 91:1191–1200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goodner JT and Turnbull AD: Bone
metastases in cancer of the esophagus. Am J Roentgenol Radium Ther
Nucl Med. 111:365–367. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma Z, Wu X, Xu B, Jiang H, Tang P, Yue J,
Ma M, Chen C, Zhang H and Yu Z: Development of a novel biomarker
model for predicting preoperative lymph node metastatic extent in
esophageal squamous cell carcinoma1. Oncotarget.
8:105790–105799. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Huang X, Zhou L, Deng T, Ning T,
Liu R, Zhang L, Bai M, Zhang H, Li H and Ba Y: Clinical use of
tumor biomarkers in prediction for prognosis and chemotherapeutic
effect in esophageal squamous cell carcinoma. BMC Cancer.
19:5262019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rybak LD and Rosenthal DI: Radiological
imaging for the diagnosis of bone metastases. Q J Nucl Med.
45:53–64. 2001.PubMed/NCBI
|
|
19
|
Kato H, Miyazaki T, Nakajima M, Takita J,
Kimura H, Faried A, Sohda M, Fukai Y, Masuda N, Fukuchi M, et al:
Comparison between whole-body positron emission tomography and bone
scintigraphy in evaluating bony metastases of esophageal
carcinomas. Anticancer Res. 25:4439–4444. 2005.PubMed/NCBI
|
|
20
|
Wu SG, Li H, Tang LY, Sun JY, Zhang WW, Li
FY, Chen YX and He ZY: The effect of distant metastases sites on
survival in de novo stage-IV breast cancer: A SEER database
analysis. Tumour Biol. 39:10104283177050822017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu M, Wang C, Gao L, Lv C and Cai X: A
nomogram to predict long-time survival for patients with M1
diseases of esophageal cancer. J Cancer. 9:3986–3990. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katagiri H, Okada R, Takagi T, Takahashi
M, Murata H, Harada H, Nishimura T, Asakura H and Ogawa H: New
prognostic factors and scoring system for patients with skeletal
metastasis. Cancer Med. 3:1359–1367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wedin R and Bauer HC: Surgical treatment
of skeletal metastatic lesions of the proximal femur:
Endoprosthesis or reconstruction nail? J Bone Joint Surg Br.
87:1653–1657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Forsberg JA, Wedin R and Bauer H: Which
implant is best after failed treatment for pathologic femur
fractures? Clin Orthop Relat Res. 471:735–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steensma M, Boland PJ, Morris CD,
Athanasian E and Healey JH: Endoprosthetic treatment is more
durable for pathologic proximal femur fractures. Clin Orthop Relat
Res. 470:920–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Araki N, Chuman H, Matsunobu T, Tanaka K,
Katagiri H, Kunisada T, Hiruma T, Hiraga H, Morioka H, Hatano H, et
al: Factors associated with the decision of operative procedure for
proximal femoral bone metastasis: Questionnaire survey to
institutions participating the Bone and Soft Tissue Tumor Study
Group of the Japan Clinical Oncology Group. J Orthop Sci.
22:938–945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimada H, Takeda A, Arima M, Okazumi S,
Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et
al: Serum p53 antibody is a useful tumor marker in superficial
esophageal squamous cell carcinoma. Cancer. 89:1677–1683. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Munck-Wikland E, Kuylenstierna R, Wahren
B, Lindholm J and Haglund S: Tumor markers carcinoembryonic
antigen, CA 50, and CA 19-9 and squamous cell carcinoma of the
esophagus. Pretreatment screening. Cancer. 62:2281–2286. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao X, Zhang L, Feng GR, Yang J, Wang RY,
Li J, Zheng XM and Han YJ: Preoperative Cyfra21-1 and SCC-Ag serum
titers predict survival in patients with stage II esophageal
squamous cell carcinoma. J Transl Med. 10:1972012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimada H, Nabeya Y, Okazumi S, Matsubara
H, Shiratori T, Gunji Y, Kobayashi S, Hayashi H and Ochiai T:
Prediction of survival with squamous cell carcinoma antigen in
patients with resectable esophageal squamous cell carcinoma.
Surgery. 133:486–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kosugi S, Nishimaki T, Kanda T, Nakagawa
S, Ohashi M and Hatakeyama K: Clinical significance of serum
carcinoembryonic antigen, carbohydrate antigen 19-9, and squamous
cell carcinoma antigen levels in esophageal cancer patients. World
J Surg. 28:680–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang HQ, Wang RB, Yan HJ, Zhao W, Zhu KL,
Jiang SM, Hu XG and Yu JM: Prognostic significance of CYFRA21-1,
CEA and hemoglobin in patients with esophageal squamous cancer
undergoing concurrent chemoradiotherapy. Asian Pac J Cancer Prev.
13:199–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yi Y, Li B, Wang Z, Sun H, Gong H and
Zhang Z: CYFRA21-1 and CEA are useful markers for predicting the
sensitivity to chemoradiotherapy of esophageal squamous cell
carcinoma. Biomarkers. 14:480–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. CD0040642010.PubMed/NCBI
|
|
35
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Phase III study of docetaxel and cisplatin plus
fluorouracil compared with cisplatin and fluorouracil as first-line
therapy for advanced gastric cancer: A report of the V325 Study
Group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Al-Batran SE, Hartmann JT, Probst S,
Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G,
Homann N, Wilhelm G, et al: Phase III trial in metastatic
gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus
either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft
Internistische Onkologie. J Clin Oncol. 26:1435–1442. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ford HE, Marshall A, Bridgewater JA,
Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S,
Middleton GW, et al: Docetaxel versus active symptom control for
refractory oesophagogastric adenocarcinoma (COUGAR-02): An
open-label, phase 3 randomised controlled trial. Lancet Oncol.
15:78–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hironaka S, Ueda S, Yasui H, Nishina T,
Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki
T, et al: Randomized, open-label, phase III study comparing
irinotecan with paclitaxel in patients with advanced gastric cancer
without severe peritoneal metastasis after failure of prior
combination chemotherapy using fluoropyrimidine plus platinum: WJOG
4007 trial. J Clin Oncol. 31:4438–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|