Introduction
According to the 2018 global cancer statistic
estimates of cancer incidence and mortality, lung cancer is the
leading cause of cancer-associated mortality worldwide, with 2.1
million new cases (11.6% of the total cases) and 1.8 million deaths
(18.4% of the total cancer-associated mortalities) each year
(1). Of these deaths, 85% are the
result of non-small cell lung cancer (NSCLC), as two-thirds of
patients with NSCLC are diagnosed with an advanced disease stage,
where curative surgery is not an option (2). Despite advances in diagnosis and
treatment, the 5-year relative survival rate remains at 19% for
overall lung cancer and 23% for NSCLC (3). Systemic chemotherapy remains the
primary means of treatment for patients with advanced NSCLC.
Progression-free survival (PFS) of patients with NSCLC undergoing
platinum-based doublet chemotherapy ranges between 3.6 and 4.8
months, and OS ranges between 7.9 and 10.3 months (4,5). Thus,
the unsatisfactory PFS and OS times have necessitated the
development and use of alternative treatment options, particularly
for patients with unresectable NSCLC. In recent years, there have
been significant developments and refinements in several novel,
minimally invasive techniques, such as percutaneous image-guided
ablation therapy for patients who cannot undergo surgery (6).
The potential of various thermal ablation
technologies, including radiofrequency ablation (RFA), microwave
ablation (MWA), cryoablation (CA) and irreversible electroporation
for the treatment of NSCLC has been demonstrated (7). RFA and MWA are thermal-based ablative
techniques, where thermal ablation of the tumor is achieved by
radiofrequency waves in RFA and microwaves in MWA (8). In recent years, MWA has been
increasingly used for the treatment of pulmonary tumors. MWA offers
many theoretical advantages over RFA, including enhanced
thermocoagulation of tumor cells as a result of improved energy
deposition in an aerated lung, and increased heating near blood
vessels, which allows for increased intratumoral temperatures with
larger ablation zones (≤2 cm from the probe tip) in a shorter
period of time compared with RFA (9). In comparison to RFA, MWA has been
reported to be effective for lesions near vascular structures with
a decreased heat sink effect (10–12).
Furthermore, MWA also offers benefits of decreased treatment times
and pain between treatments over RFA (9,10).
Contrary to RFA and MWA, CA is a relatively novel
ablation technique, which uses pressurized argon gas to create a
temperature as low as −140°C to destroy tumor cells (13). The advantages of CA over other
ablative techniques include good visualization under computed
tomography (CT) or magnetic resonance imaging guidance,
preservation of the collagenous architecture and lower
intraprocedural pain (14). CA also
has the advantage of having the probe placed inside the tumor, thus
preventing probe displacement during treatment, which can occur
with expandable RFA electrodes frequently used in lung ablation
(15). Several studies have
demonstrated the efficacy of pulmonary CA in cases of primary
(16,17) and recurrent (18) lung cancer, as well as pulmonary
metastasis (19,20). Furthermore, CA is more cost-effective
compared with the other ablative techniques (21). However, although a number of studies
have compared MWA with RFA for the treatment of primary and
secondary neoplasms of the lung, comparisons between MWA and CA
have not been performed. Therefore, the aim of the present study
was to compare the effectiveness of CA and MWA in the treatment of
patients with advanced stage NSCLC.
Patients and methods
Ethics approval and consent to
participate
The study protocol was developed in accordance with
the Declaration of Helsinki (22)
and was approved by the Institutional Review Board of the
Affiliated Hospital of North Sichuan Medical College (Nanchong,
China) and Xuzhou City Center Hospital (Xuzhou, China). Informed
consent to undergo the procedure and to provide clinical follow-up
data was obtained from all patients.
Patients
The present study was a retrospective analysis of
patients with stage IIIB or IV NSCLC who had undergone MWA or CA at
the Interventional Radiology department of the Affiliated Hospital
of North Sichuan medical College (Nanchong, China) or Xuzhou
Central Hospital (Xuzhou, China) between March 2011 and September
2016. All patients were histologically or cytologically diagnosed
with stage IIIB or IV NSCLC according to the 8th edition of the
Tumor-Node-Metastasis (TNM) classification (23) and had an Eastern Co-operative
Oncology Group performance status of 0 or 1 (24). Patients whose tumors were considered
to be surgically inoperable and unresponsive to standard
chemotherapy or radiotherapy were included in the present study. In
addition, according to the criteria used to perform ablation
therapy (23–25), only patients with ≤3 lesions per
hemithorax and with the largest lesion diameter ≤5.0 cm were
treated with MWA or CA. The exclusion criteria were as follows: i)
Age <18 years; ii) uncontrolled malignant pleural effusion; iii)
symptomatic brain metastases; iv) life expectancy ≤3.0 months; v)
history of current extra pulmonary malignancies or previous
malignancies within the last 5 years; and vi) inadequate
hematologic, hepatic or renal function.
Pre-ablation assessment
The decision to perform lung ablation (MWA or CA)
was made by an interventional radiologist following consultation
with the patient and subsequent referral to a physician. Patients
attended the tumor ablation clinic for a pre-procedural visit ~1
month prior to percutaneous ablation procedures. Following the
completion of medical history and physical examinations,
suggestions for performing relevant imaging studies were reviewed
with the patient. The indications, risks and benefits of the
procedures were discussed. Pre-ablation complete blood cell counts,
platelet counts and prothrombin time and international normalized
ratio were routinely obtained. Patients receiving anticoagulant and
anti-platelet medications were instructed to stop taking them 2–7
days prior to ablation. Patients fasted for 12 h prior to arriving
at the computed tomography (CT) suite on the day of the
procedure.
CA procedure
An argon-based CA delivery system (AccuTarget
MediPharma Co. Ltd.) was used with 14–18-gauge cryoprobes. The
number, type and configuration of the needles were based on the
necessity to maintain a distance of ≤15 mm between adjacent CA
needles and ≤10 mm from the tumor margin, while avoiding or
displacing adjacent normal anatomical structures. CA was performed
using a three-cycle freeze-thaw phase protocol. The times for each
phase were recorded and varied depending on the size of the tumor
(target times: Freeze, 3 min; thaw, 3 min; freeze, 8 min; thaw, 5
min; freeze, 8 min; followed by active thawing). For lesions ≤3.0
cm in diameter, one cryoprobe was inserted, whereas two cryoprobes
were used for lesions >3.0 cm. Each procedure was monitored
using non-contrast CT imaging at 3–5 min intervals to visualize the
growing ablation zone, with the goal of achieving a circumferential
margin of 0.5 cm beyond the tumor. Ablation time and power were
recorded during all procedures. All procedures were performed under
local anesthesia. Cardiac status and vital signs were continuously
monitored throughout the ablation procedure.
All patients underwent an immediate post-ablation
contrast-enhanced CT scan and were admitted for overnight
observation. To prevent renal failure, which is induced by
myoglobinuria, a prophylactic regimen consisting of three ampules
of sodium bicarbonate (50.0 mEq per ampule) in 5.0% dextrose in
water was administered at 150.0 ml/h for 24 h if the
post-procedural serum myoglobin levels increased >1,000
mg/l.
MWA procedure
The MWA procedure was performed under CT guidance
(Philips MX16; Koninklijke Philips N.V.). The MWA instrument used
was a KY-2000 microwave multi-function therapeutic instrument
(Kangyou Medical Co., Ltd.). A microwave antenna, 14–20 gauge
depending on tumor size and location, was inserted into the lesion.
For lesions <3.0 cm in diameter, one antenna was inserted,
whereas two antennas were inserted for lesions >3.0 cm. The
lesion was ablated by maintaining an output power of 50–80 W, with
the aim of obtaining and ablative margin of 0.5 cm. If the tumor
was not ablated in one session, based on tumor size, location and
geometry, multiple sequential ablations were performed to achieve
complete necrosis. All procedures were performed with conscious
sedation and local anesthesia. Throughout the session, cardiac
status and vital signs were continuously monitored. At the end of
every procedure, a CT scan was performed to prevent complications,
and the patients were transferred to the in-patient ward for 24-h
observation.
Intraprocedural pain assessment
Pain experienced by the patient during the MWA and
CA procedures was compared. Patients reported on the experienced
pain using the visual analogue score (VAS) criteria, where the
minimum score of 0 indicates no pain experienced and the maximum
score of 10 indicates severe extreme pain (26).
Follow-up
Patients were followed up post-ablation as
outpatients, with CT scans performed at 1, 3 and 6 months and
subsequently every 6 months.
Outcome measures
The primary outcome measures of the present study
were technical success, clinical effectiveness, safety and OS.
Technical success was defined as the correct placement of the
ablation device into the target lesion and completion of the
planned ablation protocol, with no detectable enhancement observed
in the CT scans performed in the first 30 days following ablation.
Clinical effectiveness was defined as local disease control. The
areas of hypoattenuation that were not enhanced in the CT scan were
considered to represent the ablation zone. Irregular focal
enhancement of the lesion >15 Hounsfield units (HU) compared
with the initial post-ablation non-enhanced lesion was considered
as a sign of local tumor progression. A circumferential rim of
enhancement ≤0.5 cm around the ablation zone at 6 months
post-ablation was considered to indicate benign peritumoral
enhancement. Survival was assessed as PFS and OS. PFS was
calculated from the start of the ablation treatment to disease
progression, including progression in ablative sites, distant
metastasis or death. OS was calculated from the start of treatment
to death or the last follow-up. The safety was defined according to
the frequency of procedural and procedure-related complications.
These were evaluated using the common terminology criteria for
adverse events (AEs) (version 4.0) model (27).
Survival analysis of sub-groups
Tumor size has been reported as a prognostic marker
of disease progression in a number of previous studies (2,16,25,28–32).
Therefore, the survival function of patients treated with MWA and
CA were analyzed according to tumor size. In the present study,
tumors size ranged from 0.8–5.0 cm (mean ± standard deviation;
2.9±1.17 cm). Therefore, 3.0 cm was used as the threshold.
Statistical analysis
All statistical analyses were performed using SPSS
version 23.0 (IBM Corp.) and GraphPad Prism version 5.0 (GraphPad
Software, Inc.). The PFS and OS times were assessed using
Kaplan-Meier analysis. The comparisons of survival functions were
performed using a log-rank test. The median survival estimates were
reported with 95% confidence intervals (CIs). The associations
between AEs and clinicopathological characteristics were evaluated
using χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The present retrospective study included data from
101 patients with stage IIIB or IV primary NSCLC. The patients who
had undergone MWA were denoted as the MWA group, and those treated
with CA as the CA group. The MWA group comprised 56 patients (34
male and 22 female; mean age, 59.1 years; age range, 29–77 years),
whereas the CA group comprised 45 patients (26 male and 19 female;
mean age, 57.7 years; age range, 32–78 years). Of the 56 patients
in the MWA group, 32 (57.1%) had stage IIIB NSCLC and 24 (42.9%)
had stage IV NSCLC; 43 patients (76.8%) had adenocarcinoma, 10
(17.8%) had squamous cell carcinoma and 3 (5.4%) had large cell
carcinoma. In the CA group, 27 patients (57.1%) had stage IIIB
NSCLC and 18 (42.9%) had stage IV NSCLC; 33 patients (73.3%) had
adenocarcinoma and 12 (26.6%) had squamous cell carcinoma. The
baseline patient characteristics did not differ significantly
(Table I).
 | Table I.Baseline patient clinicopathological
characteristics. |
Table I.
Baseline patient clinicopathological
characteristics.
| Variable | CA (n=45), n
(%) | MWA (n=56), n
(%) | P-value |
|---|
| Sex |
|
| 0.76 |
|
Male | 26 (57.8) | 34 (60.7) |
|
|
Female | 19 (42.2) | 22 (39.3) |
|
| Age, years |
|
| 0.91 |
|
<60 | 27 (60.0) | 33 (58.9) |
|
|
≥60 | 18 (40.0) | 23 (41.1) |
|
| Pathology |
|
| 0.52 |
|
ADC | 33 (73.3) | 43 (76.8) |
|
|
Non-ADCa | 12 (26.7) | 13 (23.2) |
|
| Stageb |
|
| 0.77 |
|
IIIB | 27 (60.0) | 32 (57.1) |
|
| IV | 18 (40.0) | 24 (42.9) |
|
| Tumor site
(side) |
|
| 0.84 |
| Right
lung | 29 (64.4) | 35 (62.5) |
|
| Left
lung | 16 (35.6) | 21 (37.5) |
|
| Tumor site
(lobe) |
|
| 0.69 |
| Upper
and middle | 28 (62.2) | 37 (66.1) |
|
|
Lower | 17 (37.8) | 19 (33.9) |
|
| Tumor size, cm |
|
| 0.95 |
|
≤3.0 | 26 (57.8) | 32 (57.1) |
|
|
>3.0 | 19 (42.2) | 24 (42.9) |
|
| Tumor location |
|
| 0.43 |
|
Central | 12 (26.7) | 19 (33.9) |
|
|
Peripheral | 33 (73.3) | 37 (66.1) |
|
| Tumor distance from
pleura, cm |
|
| 0.83 |
| ≤1 | 17 (37.8) | 20 (35.7) |
|
|
>1 | 28 (62.2) | 36 (64.3) |
|
| Tumor distance from
vessel, mm |
|
| 0.78 |
| ≤3 | 14 (31.1) | 16 (28.6) |
|
|
>3 | 31 (68.9) | 40 (71.4) |
|
| Metastatic
site |
|
|
|
| Lymph
node | 27 (60.0) | 30 (58.9) | 0.55 |
|
Intra-pulmonary | 13 (28.9) | 17 (30.3) | 1.00 |
|
Distant | 30 (66.7) | 32 (57.1) | 0.41 |
| Metastases, n |
|
| 0.38 |
| 1 | 24 (53.3) | 25 (44.6) |
|
| ≥2 | 21 (46.7) | 31 (55.4) |
|
In the MWA group, 35 primary tumors were located in
the right lung and 21 in the left lung; 37 tumors were located in
the upper and middle lobes, whereas 19 were in the lower lobes. The
mean diameter of the primary tumors in the MWA group was 2.9 cm
(range, 0.8–5.0 cm), and 24 tumors (42.9%) were >3.0 cm. In the
CA group, 29 primary tumors were located in the right lung and 28
in the upper and middle lobes. The mean diameter of the primary
tumors was 2.6 cm (range, 0.9–5.0 cm), and 19 tumors (42.2%) were
>3.0 cm.
Effectiveness
In the MWA group, a total of 56 MWA sessions were
performed, with 80 antennas used for 56 primary tumor sites. Among
these, 32 patients were treated using one antenna, whereas 24
patients were treated using two antennas. The median ablation time
was 7 min (range, 5–10 min). Initial technical success (no
detectable enhancement in the initial post-ablation CT scan) was
achieved in 52 (92.86%) ablations. Re-ablation within a 6-month
period was performed in 4 patients (7.14%), which resulted in 100%
secondary technical success.
In the CA group, a total of 45 CA sessions were
performed, with 64 cryoprobes used for 45 primary tumor sites.
Among these, 26 patients were treated with one cryoprobe, whereas
19 were treated with two cryoprobes. Initial technical success was
achieved in 42 ablations (93.33%). Re-ablation within a 6-month
period was performed in 3 patients (6.67%) with 100% secondary
technical success.
At 6 months post-ablation, local disease control was
evaluated in terms of recurrence at the ablation site (residual
disease). In the MWA group, 15 patients (26.78%) exhibited disease
progression at the ablative sites, whereas in the CA group, 11
patients (24.44%) exhibited disease progression at the ablative
sites. The difference was not statistically significant
(P>0.05). An example case of local disease control following CA
based on tumor size reduction without evidence of enhancement
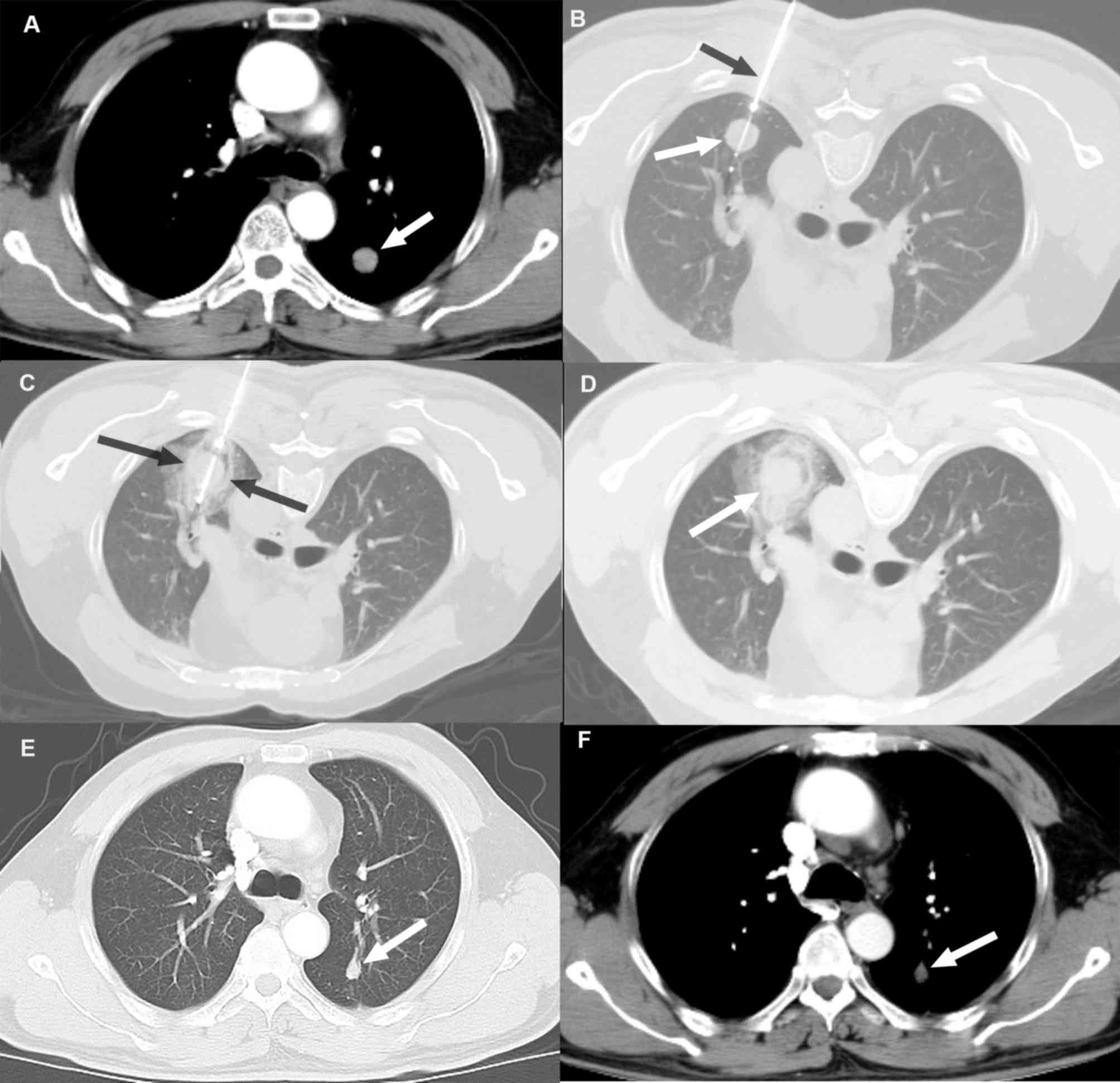
compared with the pre-ablation image is presented in Fig. 1.
Regarding disease progression at distant sites from
the ablation site, 41 patients (73.21%) in the MWA group developed
metastases in lobes other than the ablative site or distant sites,
and 7 patients (12.50%) presented with metastases at both the
ablative and a distant site during the 3-year follow-up. In the CA
group, 34 patients (75.55%) developed metastases in lobes other
than the ablative site or distant sites, and 6 patients (13.33%)
exhibited metastases at both the ablative and a distant site after
3 years of follow-up. The difference in disease progression rate
was not statistically significant between the two groups
(P>0.05).
OS analysis
The mean duration of follow-up in patients was
24.10±17.3 months, with a median duration of 19.5 months (range,
4.3–46.4 months). During the follow-up period, the cumulative OS
rate at 1, 2 and 3 years was 78.57, 51.78 and 35.71%, respectively,
for patients treated with MWA and 73.33, 40.00 and 22.22%,
respectively, for patients treated with CA. The median OS was 27.5
months (95% CI, 22.8–31.2 months) in the MWA group and 18.0 months
(95% CI, 12.5–23.5 months) in the CA group; however, the difference
was not significant (P=0.07; Fig.
2A). The cumulative PFS rates at 1, 2 and 3 years were 41.07,
17.85 and 7.14%, respectively, in patients treated with MWA, and
35.55, 11.11 and 4.44%, respectively, in patients treated with CA.
The median PFS time of the MWA group was 11.0 months (95% CI,
9.5–12.4 months) and did not significantly differ from the CA group
(10.0 months; 95% CI, 7.5–12.4 months; P=0.36; Fig. 2B).
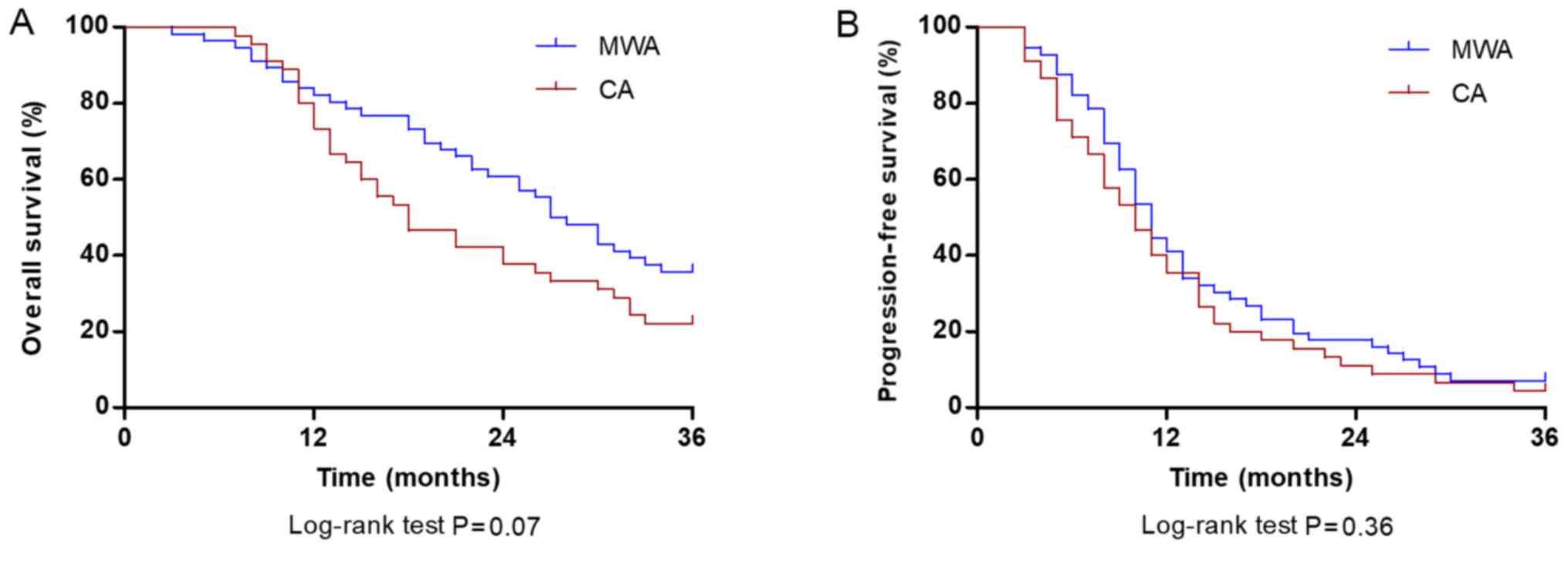 | Figure 2.Comparison of OS and PFS rate at 36
months between patients with stage IIIB/IV non-small cell lung
carcinoma in the CA and MWA groups. (A) OS rates at 1, 2 and 3
years were 78.57, 51.78 and 35.71% in patients treated with MWA
(blue line) and 73.33, 40.00 and 22.22% in patients treated with CA
(red line), respectively (P=0.07). (B) PFS rates at 1, 2 and 3
years were 41.07, 17.85 and 7.14% in patients treated with MWA
(blue line), and 35.55, 11.11 and 4.44% in patients treated with CA
(red line), respectively (P=0.36). OS, overall survival; PFS,
progression-free survival; CA, cryoablation; MWA, microwave
ablation. |
Survival analysis of the
subgroups
For patients with tumor diameter ≤3.0 cm, the median
OS in the MWA group was 30.0 months, which was not significantly
different compared with the CA group (26.5 months; P=0.39; Fig. 3A). The median PFS of patients with
tumors ≤3.0 cm in the MWA group was 11.0 months, which was not
significantly different compared with the CA group (13.0 months;
P=0.79; Fig. 3B).
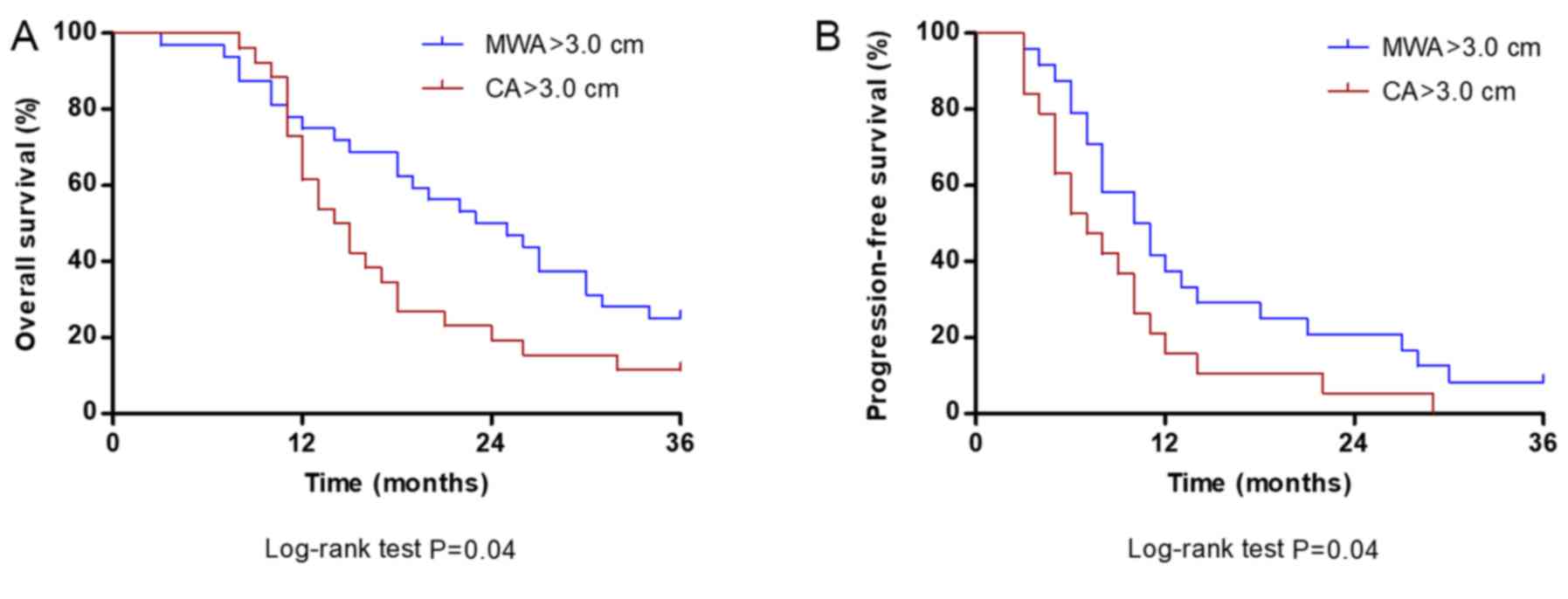
For tumors >3.0 cm, the median OS (24.5 months)
and PFS (10.5 months) times in the MWA group were significantly
longer compared with the median OS (14.5 months) and PFS (7.0
months) in the CA group (both P=0.04; Fig. 4A and B, respectively).
Intraprocedural pain
The intra-procedural VAS scores in the MWA group
(6.01±2.06) were significantly higher compared with the CA group
(2.43±1.39; P=0.001; data not shown).
AEs
Complications associated with the MWA and CA
procedures are presented in Table
II. No intraprocedural deaths occurred and no
mortality-associated AEs were observed. The differences in the
incidence rates of complications observed between the two groups
were not statistically significant (P>0.05). The most common
procedural complication observed in the two groups was
pneumothorax, which occurred in 23 patients (41.1%) treated with
MWA and 17 patients (37.8%) treated with CA. The majority of cases
of pneumothorax were clinically insignificant. However, 7 cases
(12.5% of procedures) in the MWA group and 5 cases (11.1% of
procedures) in the CA group required the use of a chest tube
drainage for pneumothorax. The second most commonly observed
complication was intrapulmonary hemorrhage, which occurred in 19
patients (33.9%) in the MWA group and 11 patients (24.4%) in the CA
group. Therefore, the association between the clinicopathological
characteristics of the patients with the two most common
complications was determined (Tables
III and IV).
 | Table II.Adverse events associated with the
procedures. |
Table II.
Adverse events associated with the
procedures.
| Adverse
eventsa | CA (n=45), n
(%) | MWA (n=56), n
(%) | P-value |
|---|
| Pneumothorax | 17 (37.8) | 23 (41.1) | 0.74 |
| Grade 1,
asymptomatic | 12 (26.7) | 16 (28.6) |
|
| Grade 2,
symptomatic requiring chest tube | 5 (11.1) | 7 (12.5) |
|
| Intra-pulmonary
hemorrhage | 11 (24.4) | 19 (33.9) | 0.30 |
| Grade 1, mild
symptoms; intervention not indicated | 11 (24.4) | 16 (28.6) |
|
| Grade 2, moderate
symptoms; medical intervention indicated | 0 (0.0) | 3 (5.4) |
|
| Pleural
effusion | 8 (17.8) | 14 (25.0) | 0.38 |
| Grade 1,
asymptomatic; clinical or diagnostic observations only | 8 (17.8) | 14 (25.0) |
|
| Hemoptysis | 7 (15.6) | 10 (17.9) |
|
| Grade 1, mild,
<100 ml, intervention not required | 7 (15.6) | 10 (17.9) | 0.80 |
| Infection | 5 (11.1) | 7 (12.5) | 0.83 |
| Post-ablation
syndrome | 1 (2.2) | 2 (3.6) | 0.69 |
| Burn | 0 (0.0) | 2 (3.6) | 0.20 |
| Complication
requiring admission (mean length of stay, 1–2 days) | 7 (15.6) | 12 (21.4) | 0.54 |
 | Table III.Association between the occurrence of
pneumothorax and intra-pulmonary hemorrhage with CA (n=45)
procedure and clinical characteristics. |
Table III.
Association between the occurrence of
pneumothorax and intra-pulmonary hemorrhage with CA (n=45)
procedure and clinical characteristics.
|
| Pneumothorax |
| Intra-pulmonary
hemorrhage |
|
|---|
|
|
|
|
|
|
|---|
| Variable | Yes (n=17,
37.8%) | No (n=28,
62.2%) | P-value | Yes (n=11,
24.4%) | No (n=34,
75.6%) | P-value |
|---|
| Sex |
|
| 0.76 |
|
| 0.65 |
|
Male | 9 (20.0) | 17 (37.8) |
| 7 (15.5) | 19 (42.2) |
|
|
Female | 8 (17.8) | 11 (24.4) |
| 4 (8.9) | 15 (33.4) |
|
| Age, years |
|
| 0.45 |
|
| 0.78 |
|
<60 | 9 (20.0) | 18 (40.0) |
| 6 (13.3) | 21 (46.7) |
|
| 60 | 8 (17.8) | 10 (22.2) |
| 5 (11.1) | 13 (28.9) |
|
| Stagea |
|
| 0.98 |
|
| 0.32 |
|
IIIB | 10 (22.2) | 17 (37.8) |
| 7 (15.6) | 20 (44.4) |
|
| IV | 7 (15.6) | 11 (24.4) |
| 4 (8.9) | 14 (31.1) |
|
| Nodule size,
cm |
|
| 0.03 |
|
| 0.80 |
|
>3 | 6 (13.3) | 20 (44.4) |
| 6 (13.3) | 20 (44.4) |
|
| ≤3 | 11 (24.5) | 8 (17.8) |
| 5 (11.1) | 14 (31.1) |
|
| Nodule location
(side) |
|
| 0.79 |
|
| 0.31 |
| Right
lung | 11 (24.5) | 18 (40.0) |
| 8 (17.8) | 21 (46.7) |
|
| Left
lung | 6 (13.3) | 10 (22.2) |
| 3 (6.7) | 13 (28.9) |
|
| Nodule location
(lobe) |
|
| 0.13 |
|
| 0.91 |
| Upper
and middle | 13 (28.9) | 15 (33.3) |
| 7 (15.6) | 21 (46.7) |
|
|
Lower | 4 (8.9) | 13 (28.9) |
| 4 (8.9) | 13 (28.9) |
|
| Tumor distance to
pleura, cm |
|
| 0.71 |
|
| 0.41 |
|
>1 | 10 (22.2) | 18 (40.0) |
| 8 (17.8) | 20 (44.4) |
|
| ≤1 | 7 (15.6) | 10 (22.2) |
| 3 (6.7) | 14 (31.1) |
|
| Tumor distance from
vessel, mm |
|
| 0.85 |
|
| 0.05 |
|
>3 | 12 (26.7) | 19 (42.2) |
| 5 (11.1) | 26 (57.8) |
|
| ≤3 | 5 (11.1) | 9 (20.0) |
| 6 (13.3) | 8 (17.8) |
|
| Nodule location
(region) |
|
| 0.03 |
|
| 0.02 |
| Central
(close to hilum) | 8 (17.8) | 4 (8.9) |
| 6 (13.3) | 6 (13.3) |
|
|
Peripheral | 9 (20.0) | 24 (53.3) |
| 5 (11.1) | 28 (62.2) |
|
| Number of
cryoprobes |
|
| 0.03 |
|
| 0.80 |
| 1 | 6 (13.3) | 20 (44.4) |
| 6 (13.3) | 20 (44.4) |
|
|
>1 | 11 (24.5) | 8 (17.8) |
| 5 (11.1) | 14 (31.1) |
|
 | Table IV.Association between occurrence of
pneumothorax and intra-pulmonary hemorrhage with MWA (n=56)
procedure and clinical characteristics. |
Table IV.
Association between occurrence of
pneumothorax and intra-pulmonary hemorrhage with MWA (n=56)
procedure and clinical characteristics.
|
| Pneumothorax |
| Intra-pulmonary
hemorrhage |
|
|---|
|
|
|
|
|
|
|---|
| Variable | Yes (n=23,
41.1%) | No (n=33,
58.9%) | P-value | Yes (n=19,
33.9%) | No (n=37,
66.1%) | P-value |
|---|
| Sex |
|
| 0.27 |
|
| 0.79 |
|
Male | 12 (21.4) | 22 (39.4) |
| 12 (21.4) | 22 (39.4) |
|
|
Female | 11 (19.6) | 11 (19.6) |
| 7 (12.5) | 15 (26.7) |
|
| Age, years |
|
| 0.16 |
|
| 0.645 |
|
<60 | 11 (19.6) | 22 (39.4) |
| 12 (21.4) | 21 (37.5) |
|
| 60 | 12 (21.4) | 11 (19.6) |
| 7 (12.5) | 16 (28.6) |
|
| Stagea |
|
| 0.24 |
|
| 0.62 |
|
IIIB | 11 (19.6) | 21 (37.5) |
| 10 (17.8) | 22 (39.4) |
|
| IV | 12 (21.4) | 12 (21.4) |
| 9 (16.1) | 15 (26.7) |
|
| Nodule size,
cm |
|
| 0.03 |
|
| 0.04 |
|
>3 | 9 (16.1) | 23 (41.1) |
| 7 (12.5) | 25 (44.6) |
|
| ≤3 | 14 (25.0) | 10 (17.8) |
| 12 (21.4) | 12 (21.4) |
|
| Nodule location
(side) |
|
| 0.18 |
|
| 0.09 |
| Right
lung | 12 (21.4) | 23 (41.1) |
| 9 (16.1) | 26 (46.4) |
|
| Left
lung | 11 (19.6) | 10 (17.9) |
| 10 (17.8) | 11 (19.6) |
|
| Nodule location
(lobe) |
|
| 0.07 |
|
| 0.13 |
| Upper
and middle | 12 (21.4) | 25 (44.7) |
| 10 (17.8) | 27 (48.2) |
|
|
Lower | 11 (19.6) | 8 (14.3) |
| 9 (16.1) | 10 (17.8) |
|
| Tumor distance from
pleura, cm |
|
| 0.11 |
|
| 0.47 |
|
>1 | 12 (21.4) | 24 (42.9) |
| 11 (19.6) | 25 (44.7) |
|
| ≤1 | 11 (19.6) | 9 (16.1) |
| 8 (14.3) | 12 (21.4) |
|
| Tumor distance from
vessel, mm |
|
| 0.14 |
|
| 0.13 |
|
>3 | 14 (25.0) | 26 (46.4) |
| 11 (19.6) | 29 (51.8) |
|
| ≤3 | 9 (16.1) | 7 (12.5) |
| 8 (14.3) | 8 (14.3) |
|
| Nodule location
(region) |
|
| 0.02 |
|
| 0.03 |
| Central
(close to hilum) | 12 (21.4) | 7 (12.5) |
| 10 (17.8) | 9 (16.1) |
|
|
Peripheral | 11 (19.6) | 26 (46.4) |
| 9 (16.1) | 28 (50.0) |
|
| Number of
antennas |
|
| 0.03 |
|
| 0.04 |
| 1 | 9 (16.1) | 23 (41.1) |
| 7 (12.5) | 25 (44.7) |
|
|
>1 | 14 (25.0) | 10 (17.8) |
| 12 (21.4) | 12 (21.4) |
|
For the MWA and CA procedures, the following were
all associated with the occurrence of pneumothorax and
intrapulmonary hemorrhage: i) Central tumors for which needles had
to traverse a large distance through the lung field; ii) the use of
more than one applicator (antennas in MWA and cryoprobes in CA) to
ablate a nodule; and iii) tumor size, which was directly associated
with the number of applicators required for ablation. However,
nodule distance ≤1 cm from the pleura was not associated with the
occurrence of pneumothorax in the MWA and CA groups. In the MWA
group, 14 of the 23 patients with post-procedural pneumothorax were
treated with two antennas. Additionally, 5 of 7 patients requiring
chest tube drainage were treated with two antennas for tumor
ablation. Similarly, in the CA group, of the 17 patients who
developed post-procedural pneumothorax, 11 patients were treated
with two cryoprobes. Furthermore, 3 of the 5 patients who developed
pneumothorax and required chest tube drainage in the CA group were
treated with two cryoprobes.
Intrapulmonary hemorrhage in the MWA group was not
associated with the distance between the tumor and a major vessel.
Of the 16 patients with tumors ≤3 mm from a major vessel (≥3 mm in
diameter), 8 patients developed intrapulmonary hemorrhage
post-ablation, and of these, 3 developed symptomatic hemorrhage
(hypotension); the patients stabilized following fluid
resuscitation. In the CA group, intrapulmonary hemorrhage was also
not associated with the distance between the tumor and a major
vessel. Of the 14 patients with tumors ≤3 mm from a major vessel, 6
patients developed intrapulmonary hemorrhage post-ablation,
although none exhibited symptomatic hemorrhage.
Discussion
MWA and CA have received increasing attention in
recent years for treating malignancies of the lung (6,28,32).
However, whether MWA or CA should be used in specific patients
remains unclear. In clinical practice, the decision should depend
on the safety and effectiveness of the particular ablation
technique on a case-by-case basis (33). However, to the best of our knowledge,
the relative rate of complications and the oncologic effectiveness
of these two ablative modalities have not previously been compared.
In the present study, the efficacy (progression and survival rates)
and safety (major complication rates) of CA and MWA in patients
with stage IIIB or IV NSCLC were compared.
Regarding technical success, MWA and CA displayed
complete tumor ablation in the majority of cases. For patients with
residual tumors, a total complete ablation was achieved following
second ablation in the two groups, and local recurrence rates were
similar between the groups.
The survival estimates obtained following MWA and CA
in the present study were similar to previous studies (2,34–36). A
limited number of studies on the long-term survival effects of CA
in patients with advanced lung cancer are available. Niu et
al (34) analyzed the efficacy
of CA in patients with stage IV lung cancer and reported that
median OS was 14 months. In another study by the same authors, the
1- and 2-year OS of patients with stage IIIB and IV lung cancer
treated with CA was 58 and 48%, respectively (35). Li et al (36) investigated the long-term effects of
CA in 253 patients with advanced lung cancer and reported that the
median survival time was 11.98 months. The survival estimates of
the CA group in the present study were similar to the
aforementioned studies, with median survival times of 18 months and
1-, 2- and 3-year survival rates of 78.57, 51.78 and 35.71%,
respectively. Similarly, a low number of studies have examined the
effectiveness of MWA solely in advanced stage primary lung
malignancy, with the majority of studies either including primary
tumors of various stages or combining primary tumors with
metastatic tumors; in addition, studies reporting the survival
effects of MWA in advanced stage lung cancer are primarily based on
patients treated with a combination of chemotherapy and MWA
(2,30,37,38).
However, the survival estimates of the MWA group in the present
study were similar to the results of previous studies. Wei et
al (2) reported median PFS and
OS times of 10.9 and 23.9 months, respectively, in patients with
advanced NSCLC treated with MWA in combination with chemotherapy.
In another study, treatment with MWA in combination with
chemotherapy resulted in median PFS and OS times of 8.7 and 21.3
months, respectively (37). Despite
treatment with a combination of chemotherapy and MWA,
aforementioned studies yielded lower survival rates compared with
the present study. The differences may be due to larger tumor sizes
in the previous studies [tumor size range, 1–9 cm in Wei et
al (2); and 1–11 cm in Wei et
al (37)]. To the best of our
knowledge, no previous studies have compared MWA with CA for the
treatment of lung carcinoma. In the present study, median PFS and
OS times were similar between the MWA and CA groups and did not
differ significantly.
There remains a lower probability of an ablation
technique successfully obtaining complete tumor necrosis in
patients with larger tumors. Larger tumors exhibit irregular tumor
shapes, increasing the difficulty of the use of ablation
applicators to optimize the entry route and completely kill the
tumor cells resulting in tumor residues (25). Studies have reported that both MWA
and CA have lower survival rates in patients with larger tumors
compared with smaller tumor sizes (16,25,28–30,39,40).
However, the threshold tumor size used to differentiate small and
large tumors varied across previous studies and depended on the
maximum size of the tumor observed in each study. In a study by
Pusceddu et al (28), 4 cm
was used as the threshold, where the tumor size ranged between 3
and 14 cm in size, with a mean (± standard deviation) size of 5±1.8
cm. Wei et al (2) used 3.5 cm
as the threshold, where the tumor sizes ranged between 1 and 7 cm.
Other studies enrolled patients with tumors ≤5 cm and thus used 3
cm as the threshold (28–30). Furthermore, previous studies did not
compare the survival functions of both the ablation technique (MWA
and CA) based on tumor size. In the present study, tumor size of
3.0 cm was used as the threshold, and survival function of both
techniques, MWA and CA, was compared for larger as well as smaller
tumors. PFS and OS were not significantly different between MWA and
CA groups in patients with tumors ≤3.0 cm; however, treatment with
MWA resulted in significantly improved PFS and OS compared with CA
in patients with tumors >3.0 cm, which may be due to the ability
of MWA to form a larger ablation zone.
The most frequently observed complications for the
CA and MWA procedures in the present study were pneumothorax and
intrapulmonary hemorrhage. The incidence of pneumothorax between
the two groups was not significantly different. The incidence of
pneumothorax in the CA group (37.7%) was similar to the 38%
incidence rate observed by Mcdevitt et al (39) and 37% in Zemlyak et al
(41), and within the previously
reported range of 12–62% (39–44). The
pneumothorax rate in the MWA group (41.1%) was similar to that of
39.1% observed by Wei et al (2) and within the reported range of 13–63%
(2,25,28,29,37).
No significant differences were observed in the
rates of intrapulmonary hemorrhage between the CA and MWA groups in
the present study. The rate of intrapulmonary hemorrhage in the CA
group was 24.4%, similar to the 24% reported by Chou et al
(45). The rate of intra-pulmonary
hemorrhage in the MWA group was 33.9% in the present study, which
was higher compared with the 25% incidence rate reported by Yang
et al (46). The lower rate
observed in the previously published study may be due to the low
number of tumors treated (n=11), which was lower than the number of
tumors located centrally (n=19) and tumors treated with two
antennas (n=24) in the present study. Data regarding intrapulmonary
hemorrhage post-ablation therapy has not been commonly reported.
This may partly be due to the spontaneous resolution of pulmonary
hemorrhage or an inherited bias towards underreporting the
incidence of minor clinical complications (47). However, it is important to consider
intrapulmonary hemorrhage in the clinical setting, as large
pulmonary hemorrhages can be fatal, particularly in patients with
co-morbidities, and their management during ablation therapy may be
difficult.
Previous studies have reported that the use of
multiple applicators and the length of the lung traversed by the
applicator(s) (central tumor/close to hilum) are associated with an
increased risk of pneumothorax and intra-parenchymal hemorrhage
(48–51). In agreement with these studies,
patients with centrally located tumors and patients treated with
two applicators exhibited a higher incidence of pneumothorax and
intrapulmonary hemorrhage in the CA and MWA groups in the present
study. As the number of applicators used was directly associated
with the size of the tumor, a larger tumor size was one of the risk
factors of developing pneumothorax or intrapulmonary hemorrhage.
Tumor distance ≤3 mm from a major vessel (≥3 mm in diameter) was
not determined to be a risk factor of intrapulmonary hemorrhage for
either of the treatment groups in the present study, in agreement
with Lyons et al (52).
Several studies have reported that heat-based ablation is
associated with the occurrence of pleural effusion as there is an
increase in the pleural temperature during this procedure, which
may induce pleural effusion, secondary to pleuritis, induced by
thermal injury (49,53,54).
Furthermore, for ablation techniques like RFA and MWA, nodule
distance ≤1 cm from the pleura has been reported to be a
significant risk factor for the development of pleural effusion
(49,51,54). In
the present study, although the difference between the rates of
pleural effusion between CA and MWA was not significantly
different, the rate of pleural effusion was higher in the MWA
group, particularly in patients with tumors closer to the pleura.
Furthermore, in the present study, a small number of patients with
tumors in contact with the pleura experienced skin burn in the MWA
group. Of note, patients in the CA group experienced significantly
less intraprocedural pain compared with those in the MWA group.
Several previous studies have reported CA to be less
painful compared with other ablation techniques (43,55,56).
Extreme cold acts as an anesthetic and may be the reason for less
intraprocedural pain during CA. Electrophysiologic experiments have
confirmed that the cold temperature blocks nerve conduction
(57,58). In addition, vasoconstriction of blood
vessels from cooling may minimize the resulting edema and reduce
the release of pain-inducing substances from damaged tissue
(56).
The present study had certain limitations that
should be considered. The study was designed retrospectively,
contained data from a single center and had a relatively small
cohort in both groups. Biopsies were not routinely performed during
follow-up. Therefore, the present study lacks histopathological
proof of treatment success. In addition, as the evaluation of local
tumor progression was based only on CT images, evaluation of the
viability of parts of the tumor was difficult and CT resolution was
insufficient to allow the detection of microscopic relapses or
lymphatic involvement. However, all imaging modalities have
difficulties in detecting microscopic relapse, demonstrating a
limitation of non-invasive approaches in general (36).
In conclusion, the present study demonstrated that
the CA and MWA procedures were comparably safe for treating
patients with advanced stage NSCLC. MWA exhibited improved
treatment outcomes with significantly higher survival rates
compared with CA in patients with large tumors. However, both CA
and MWA were comparably effective treatment modalities with similar
survival benefits in patients with advanced NSCLC with small
tumors. In addition, treatment with CA had the advantage of
decreased intra-procedural pain compared with MWA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SKD, YYH and HFY conceived and designed the study.
SKD, YYH, BL, XXY and HFY analyzed and interpreted the data. SKD,
YYH, BL and RHX collected the data. XXY and RHX performed
statistical analysis. SKD wrote the manuscript. SKD, YYH and HFY
critically revised the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Review Board of Affiliated Hospital of North Sichuan Medical
College (Nanchong, China) and Xuzhou City Center Hospital (Xuzhou,
China), and written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei Z, Ye X, Yang X, Huang G, Li W, Wang J
and Han X: Microwave ablation plus chemotherapy improved
progression-free survival of advanced non-small cell lung cancer
compared to chemotherapy alone. Med Oncol. 32:4642015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Facts & Figures 2019. American
Cancer Society's (ACS) publication; January. 2019, https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
|
|
4
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palussière J, Catena V and Buy X:
Percutaneous thermal ablation of lung tumors-Radiofrequency,
microwave and cryotherapy: Where are we going? Diagn Interv
Imaging. 98:619–625. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Baere T, Tselikas L, Catena V, Buy X,
Deschamps F and Palussière J: Percutaneous thermal ablation of
primary lung cancer. Diagn Interv Imaging. 97:1019–1024. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baisi A, De Simone M, Raveglia F and
Cioffi U: Thermal ablation in the treatment of lung cancer: Present
and future. Eur J Cardiothorac Surg. 43:683–686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simon CJ, Dupuy DE and Mayo-Smith WW:
Microwave ablation: Principles and applications. Radiographics. 25
(Suppl 1):S69–S83. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chi J, Ding M, Shi Y, Wang T, Cui D, Tang
X, Li P and Zhai B: Comparison study of computed tomography-guided
radiofrequency and microwave ablation for pulmonary tumors: A
retrospective, case-controlled observational study. Thorac Cancer.
9:1241–1248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ierardi AM, Floridi C, Fontana F, Chini C,
Giorlando F, Piacentino F, Brunese L, Pinotti G, Bacuzzi A and
Carrafiello G: Microwave ablation of liver metastases to overcome
the limitations of radiofrequency ablation. Radiol Med.
118:949–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Tilborg AA, Scheffer HJ, de Jong MC,
Vroomen LG, Nielsen K, van Kuijk C, van den Tol PM and Meijerink
MR: MWA versus RFA for perivascular and peribiliary CRLM: A
retrospective patient- and lesion-based analysis of two historical
cohorts. Cardiovasc Intervent Radiol. 39:1438–1446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niu L, Xu K and Mu F: Cryosurgery for lung
cancer. J Thorac Dis. 4:408–419. 2012.PubMed/NCBI
|
|
14
|
Sonntag PD, Hinshaw JL, Lubner MG, Brace
CL and Lee FT Jr: Thermal ablation of lung tumors. Surg Oncol Clin
N Am. 20369–387. (ix)2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Baere T, Tselikas L, Gravel G and
Deschamps F: Lung ablation: Best practice/results/response
assessment/role alongside other ablative therapies. Clin Radiol.
72:657–664. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamauchi Y, Izumi Y, Hashimoto K, Yashiro
H, Inoue M, Nakatsuka S, Goto T, Anraku M, Ohtsuka T, Kohno M, et
al: Percutaneous cryoablation for the treatment of medically
inoperable stage I non-small cell lung cancer. PLoS One.
7:e332232012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moore W, Talati R, Bhattacharji P and
Bilfinger T: Five-year survival after cryoablation of stage I
non-small cell lung cancer in medically inoperable patients. J Vasc
Interv Radiol. 26:312–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goto T, Izumi Y, Nakatsuka S and Nomori H:
Percutaneous cryoablation as a salvage therapy for local recurrence
of lung cancer. Ann Thorac Surg. 94:e31–e33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawamura M, Izumi Y, Tsukada N, Asakura K,
Sugiura H, Yashiro H, Nakano K, Nakatsuka S, Kuribayashi S and
Kobayashi K: Percutaneous cryoablation of small pulmonary malignant
tumors under computed tomographic guidance with local anesthesia
for nonsurgical candidates. J Thorac Cardiovasc Surg.
131:1007–1013. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Baere T, Tselikas L, Woodrum D, Abtin
F, Littrup P, Deschamps F, Suh R, Aoun HD and Callstrom M:
Evaluating cryoablation of metastatic lung tumors in
patients-safety and efficacy: The ECLIPSE trial-interim analysis at
1 year. J Thorac Oncol. 10:1468–1474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Welch BT, Brinjikji W, Schmit GD,
Callstrom MR, Kurup AN, Cloft HJ, Woodrum DA, Nichols FC and Atwell
TD: A national analysis of the complications, cost, and mortality
of percutaneous lung ablation. J Vasc Interv Radiol. 26:787–791.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang X, Ye X, Zheng A, Huang G, Ni X, Wang
j, Han X, Li W and Wei Z: Percutaneous microwave ablation of stage
I medically inoperable non-small cell lung cancer: Clinical
evaluation of 47 cases. J Surg Oncol. 110:758–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Common terminology criteria for adverse
events (version 4.0). https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdfAugust
19–2018
|
|
27
|
Wewes ME and Lowe NK: A critical review of
visual analogue scales in the measurement of clinical phenomena.
Res Nurs Health. 13:227–236. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pusceddu C, Melis L, Sotgia B, Guerzoni D,
Porcu A and Fancellu A: Usefulness of percutaneous microwave
ablation for large non-small cell lung cancer: A preliminary
report. Oncol Lett. 18:659–666. 2019.PubMed/NCBI
|
|
29
|
Wolf FJ, Grand DJ, Machan JT, Dipetrillo
TA, Mayo-Smith WW and Dupuy DE: Microwave ablation of lung
malignancies: Effectiveness, CT findings, and safety in 50
patients. Radiology. 247:871–879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li C, Wang J, Shao JB, Zhu LM, Sun ZG and
Zhang N: Microwave ablation combined with chemotherapy improved
progression free survival of IV stage lung adenocarcinoma patients
compared with chemotherapy alone. Thorac Cancer. 10:1628–1635.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang X, Tian J, Zhao L, Wu B, Kacher DS,
Ma X, Liu S, Ren C and Xiao YY: CT-guided conformal cryoablation
for peripheral NSCLC: Initial experience. Eur J Radiol.
8:3354–3362. 2012. View Article : Google Scholar
|
|
32
|
Mahnken AH, König AM and Figiel JH:
Current technique and application of percutaneous cryotherapy.
Rofo. 190:836–846. 2018.(In English, German). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee
FT Jr and Brace CL: Percutaneous tumor ablation tools: Microwave,
radiofrequency, or cryoablation-what should you use and why?
Radiographics. 34:1344–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niu L, Chen J, Yao F, Zhou L, Zhang C, Wen
W, Bi X, Hu Y, Piao X, Jiang F, et al: Percutaneous cryoablation
for stage IV lung cancer: A retrospective analysis. Cryobiology.
67:151–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niu L, Xu K, He W, GuoZ Q, Zhang SP, He TS
and Zuo JS: Percutaneous Cryoablation for patients with advanced
non-small cell lung cancer. Technol Cancer Res T. 6:451–452.
2007.
|
|
36
|
Li Y, Feng H and Nie Z: The long-term
effects and risk factors analysis in 253 cases advanced non-small
cell lung cancer treated with percutaneous cryosurgery. Chin Clin
Oncol. 15:346–349. 2010.
|
|
37
|
Wei Z, Ye X, Yang X, Zheng A, Huang G, Li
W, Ni X, Wang J and Han X: Microwave ablation in combination with
chemotherapy for the treatment of advanced non-small cell lung
cancer. Cardiovasc Intervent Radiol. 38:135–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Zhao M, Wang J, Fan W, Li W, Pan T
and Wu P: Percutaneous CT-guided radiofrequency ablation as
supplemental therapy after systemic chemotherapy for selected
advanced non-small cell lung cancer. AJR Am J Roentgenol.
201:1362–1367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McDevitt JL, Mouli SK, Nemcek AA,
Lewandowski RJ, Salem R and Sato KT: Percutaneous cryoablation for
the treatment of primary and metastatic lung tumors: Identification
of risk factors for recurrence and major complications. J Vasc
Interv Radiol. 27:1371–1379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yashiro H, Nakatsuka S, Inoue M, Kawamura
M, Tsukada N, Asakura K, Yamauchi Y, Hashimoto K and Kuribayashi S:
Factors affecting local progression after percutaneous cryoablation
of lung tumors. J Vasc Interv Radiol. 24:813–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zemlyak A, Moore WH and Bilfinger TV:
Comparison of survival after sublobar resections and ablative
therapies for stage I non-small cell lung cancer. J Am Coll Surg.
211:68–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pusceddu C, Sotgia B, Fele RM and Melis L:
CT-guided thin needles percutaneous cryoablation (PCA) in patients
with primary and secondary lung tumors: A preliminary experience.
Eur J Radiol. 82:e246–e253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inoue M, Nakatsuka S, Yashiro H, Ito N,
Izumi Y, Yamauchi Y, Hashimoto K, Asakura K, Tsukada N, Kawamura M,
et al: Percutaneous cryoablation of lung tumors: Feasibility and
safety. J Vasc Interv Radiol. 23:295–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Littrup PJ, Duan Y, Zhang Y, Feng
H and Nie Z: Thoracic masses treated with percutaneous cryotherapy:
Initial experience with more than 200 procedures. Radiology.
235:289–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chou HP, Chen CK, Shen SH, Sheu MH, Wu MH,
Wu YC and Chang CY: Percutaneous cryoablation for inoperable
malignant lung tumors: Midterm results. Cryobiology. 70:60–65.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang X, Ye X, Zhang L, Geng D, Du Z, Yu G,
Ren H, Wang J, Huang G, Wei Z, et al: Microwave ablation for lung
cancer patients with a single lung: Clinical evaluation of 11
cases. Thorac Cancer. 9:548–554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Steinke K, King J, Glenn D and Morris DL:
Pulmonary hemorrhage during percutaneous radiofrequency ablation: A
more frequent complication than assumed? Interact Cardiovasc Thorac
Surg. 2:462–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nour-Eldin NE, Naguib NN, Mack M,
Abskharon JE and Vogl TJ: Pulmonary hemorrhage complicating
radiofrequency ablation, from mild hemoptysis to life-threatening
pattern. Eur Radiol. 21:197–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hiraki T, Tajiri N, Mimura H, Yasui K,
Gobara H, Mukai T, Hase S, Fujiwara H, Iguchi T, Sano Y, et al:
Pneumothorax, pleural effusion, and chest tube placement after
radiofrequency ablation of lung tumors: Incidence and risk factors.
Radiology. 241:275–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Welch BT, Brinjikji W, Schmit GD,
Callstrom MR, Kurup AN, Cloft HJ, Woodrum DA, Nichols FC and Atwell
TD: A national analysis of the complications, cost, and mortality
of percutaneous lung ablation. J Vasc Interv Radiol. 26:787–791.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zheng A, Wang X, Yang X, Wang W, Huang G,
Gai Y and Ye X: Major complications after lung microwave ablation:
A single-center experience on 204 sessions. Ann Thorac Surg.
98:243–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lyons GR, Askin G and Pua BB: Clinical
outcomes after pulmonary cryoablation with the use of a triple
freeze protocol. J Vasc Interv Radiol. 29:714–721. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ye X, Fan W, Wang H, Wang J, Wang Z, Gu S,
Feng W, Zhuang Y, Liu B, Li X, et al: Expert consensus workshop
report: Guidelines for thermal ablation of primary and metastatic
lung tumors (2018 edition). J Cancer Res Ther. 14:730–744. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hiraki T, Gobara H, Fujiwara H, Ishii H,
Tomita K, Uka M, Makimoto S and Kanazawa S: Lung cancer ablation:
Complications. Semin Intervent Radiol. 30:169–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Colak E, Tatlı S, ShynP B, Tuncalı K and
Silverman SG: CT-guided percutaneous cryoablation of central lung
tumors. Diagn Interv Radiol. 20:316–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Allaf ME, Varkarakis IM, Bhayani SB,
Inagaki T, Kavoussi LR and Solomon SB: Pain control requirements
for percutaneous ablation of renal tumors: Cryoablation versus
radiofrequency ablation-initial observations. Radiology.
237:366–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li CL: Effect of cooling on neuromuscular
transmission in the rat. Am J Physiol. 194:200–206. 1958.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Douglas WW and Malcolm LL: The effect of
localized cooling on conduction in cat nerves. J Physiol.
130:53–71. 1955. View Article : Google Scholar : PubMed/NCBI
|