Introduction
Inverted papilloma (IP) is a benign epithelial
neoplasm in the sinonasal tract, accounting for 0.5–4.0% of all
nasal tumors (1). IP is
embryonically derived from the Schneiderian membrane, an ectodermal
sinonasal-derived respiratory mucosa. There are three
characteristic attributes of IP: Destructive or bone remodeling
capacity, high rates of recurrence, and possible association with
malignancy (1–3). Although the pathogenesis of IP is yet
to be elucidated, certain studies have indicated human
papillomavirus as a potential pathogenic factor, but its role is
still unclear (4,5). Histologically, IP may be associated
with a varying degree of dysplasia, atypia, carcinoma in
situ, and frank squamous cell carcinoma (SCC) (2). The incidence of sinonasal SCC
associated with IP ranges from 2–27% in the literature (6). SCC in IP frequently occurs
metachronously, arising in the same site where the IP had
previously been resected, or synchronously, diagnosed in the same
initial lesion (7). In a
meta-analysis of 63 case series representing >2,000 patients,
Mirza et al (8) reported a
7.1% incidence of synchronous cancer and a 3.6% incidence of
asynchronous SCC (8). Certain
symptoms, including nasal obstruction, epitaxis and rhinorrhea are
associated with the occurrence of IP and IP-related SCC (9); however, the lack of specificity of
these symptoms makes the identification of IP and SCC-associated IP
is often problematic. Therefore, complete surgical excision and
long-term follow-up are recommended treatment options for these
patients.
Due to the rarity of carcinomas associated with IP,
there are few reports in the literature regarding its
characteristics and subsequent survival rate (7,10).
Hence, the purpose of the present study is to review the clinical
characteristics, treatment outcomes, overall survival (OS), and
disease-specific survival (DSS). In addition, recurrence and
prognostic factors associated with this rare malignancy were also
analyzed.
Materials and methods
Patient population
A retrospective chart review was performed on 408
patients, who were diagnosed with IP or carcinoma associated with
IP in the nasal cavity and paranasal sinuses. Out of 408 patients,
21 cases (5.1%) of SCC associated with IP were treated at the
Department of Otorhinolaryngology of the Affiliated Eye Ear Nose
and Throat Hospital (AEENTH), Fudan University, between March 2007
and March 2017. The present study was approved by the institutional
review board of AEENTH, Fudan University (China). Informed consent
was obtained from all the patients.
Treatments and follow-up
All patients underwent surgical intervention, which
was performed by Dr Dehui Wang, the surgical interventions included
transnasal endoscopic resection and open surgical resection.
Patient demographics, the distribution of the sex, the mean age and
age range of the patients, Tumor-Node-Metastasis (TNM) staging
(11), surgical approach, the need
for an adjunct method, and relapse were analyzed. The time of
follow-up was from the initial diagnosis at the AEENTH to the date
of death or last contact.
Statistical analysis
The DSS and OS rates were calculated by the
Kaplan-Meier method. The significance of differences in prognostic
factors was analyzed by log-rank tests. The recurrence factors were
analyzed by Fisher's exact probability. P<0.05 were considered
to indicate a statistically significant difference. The SPSS 19.0
statistical software (SPSS, Inc.) was used for all statistical
analyses.
Results
Demographic data
The characteristics of patients included in this
series are shown in Table I. A total
of 21 patients were identified, comprising of 18 (85.7%) males and
3 (14.3%) females; the mean age was 59.2 years (range, 35–81
years). There were 7 cases of right-side lesions and 14 cases of
left-side lesions. The origin site was the maxillary sinus in 11
cases, and the nasal cavity and other sinuses in 10 cases. The
invading sites outside the nasal cavity included the orbital cavity
(orbital wall, 9 cases; intraorbit, 2 cases), infratemporal fossa
(n=4), pterygopalatine fossa (n=3), alveolar bone (n=2), and facial
subcutaneous tissue (n=1). The main symptoms of SCC associated with
IP presented nasal obstruction and epistaxis; other symptoms
included cheek pain, decreased vision and epiphora. According to
the American Joint Committee on Cancer (AJCC) TNM Classification
system (7th edition, 2010) (11),
the tumor grades were as follows: T1, 2 cases (9.5%); T2, 1 case
(4.8%); T3, 10 cases (47.6%); and T4, 8 cases (38.1%). The
malignancy was synchronous in 13 patients (61.9%) and metachronous
in 7 patients (38.1%).
 | Table I.Features of squamous cell carcinoma
associated with inverted papilloma. |
Table I.
Features of squamous cell carcinoma
associated with inverted papilloma.
| Patients/sex/age | Lateral/origin
site | Extent site | Main symptom | TNM stage | Surgical
procedure | Surgical margin | Type | Adjuvant therapy | Recurrence | Follow up
(months) | Outcome |
|---|
| 1/M/58 | R/MS | NC, ES, IF, AB | Cheek pain | 4 | ETR + CLO | Positive | Synchronous | IT+CRT | 1 | 20 | AWD/brain
metastasis |
| 2/M/59 | L/NC | / | Nasal
obstruction | 1 | ETR | Free | Synchronous | No | No | 20 | NED |
| 3/M/49 | L/NC | MS, ES, OW | Nasal
obstruction | 3 | ETR + CLO | Free | Metachronous | No | 1 | 22 | NED |
| 4/M/70 | L/NC | ES | Nasal
obstruction | 2 | ETR | Free | Synchronous | CRT | No | 19 | DOC/cerebral
infarction |
| 5/M/64 | R/MS | NC, ES, SS, PF, IF,
AB | Nasal
obstruction | 4 | ETR | Positive | Synchronous | RT + CRT | 2 | 12 | DOD |
| 6/F/68 | L/NS, ES | OW | Epistaxis | 3 | LR | Free | Synchronous | RT + CRT | 4 | 123 | DOD/liver
metastasis |
| 7/M/67 | L/MS | PF, IF, ES, OW | Nasal
obstruction | 4 | ETR | Free | Metachronous | RT | No | 61 | NED |
| 8/M/68 | L/MS | NC, ABFST | Cheek pain | 3 | ETR | Positive | Metachronous | RT | No | 59 | NED |
| 9/F/49 | R/MS | NC, ES | Nasal
obstruction | 3 | ETR + PME | Free | Synchronous | No | No | 28 | Unknown |
| 10/M/66 | R/MS | / | Nasal
obstruction | 1 | ETR | Free | Metachronous | No | No | 103 | NED |
| 11/M/35 | L/MS | ES, OW | Nasal
obstruction | 3 | ETR | Free | Synchronous | RT + CRT | 2 | 75 | AWD |
| 12/M/53 | L/NC | MS, ES, SS, PF,
IO | Decreased
vision | 4 | ETR | Positive | Synchronous | RT + CRT | 1 | 3 | DOD |
| 13/M/55 | L/MS | NC, ES, IFOW | Epiphora | 4 | ETR | Free | Metachronous | RT + CRT | 1 | 17 | DOD |
| 14/M/45 | R/ES | FS, IO ACB | Epistaxis | 4 | ETR + EI | Positive | Metachronous | RT + CRT | 1 | 14 | DOD |
| 15/M/52 | L/MS | OW | Nasal
obstruction | 3 | ETR | Free | Metachronous | RT | No | 60 | NED |
| 16/F/57 | R/NC | MS | Nasal
obstruction | 3 | ETR | Free | Synchronous | RT | No | 87 | Unknown |
| 17/M/61 | L/NC | ES, MS | Nasal
obstruction | 3 | ETR | Free | Synchronous | CRT | No | 54 | NED |
| 18/M/68 | R/NC | ES, MS | Nasal
obstruction | 3 | ETR | Free | Synchronous | RT + CRT | No | 43 | DOC/Cerebral
infarction |
| 19/M56 | L/MS | NC, ES, SS, OW,
LS | Epiphora | 4 | ETR | Positive | Synchronous | RT | No | 12 | Unknown |
| 20/M/63 | L/NC, ES | FS, OW | Epistaxis | 4 | ETR | Free | Metachronous | RT | 4 | 60 | NED |
| 21/M/81 | L/MS | NC, ES | Epistaxis | 3 | ETR | Free | Synchronous | RT | No | 103 | NED |
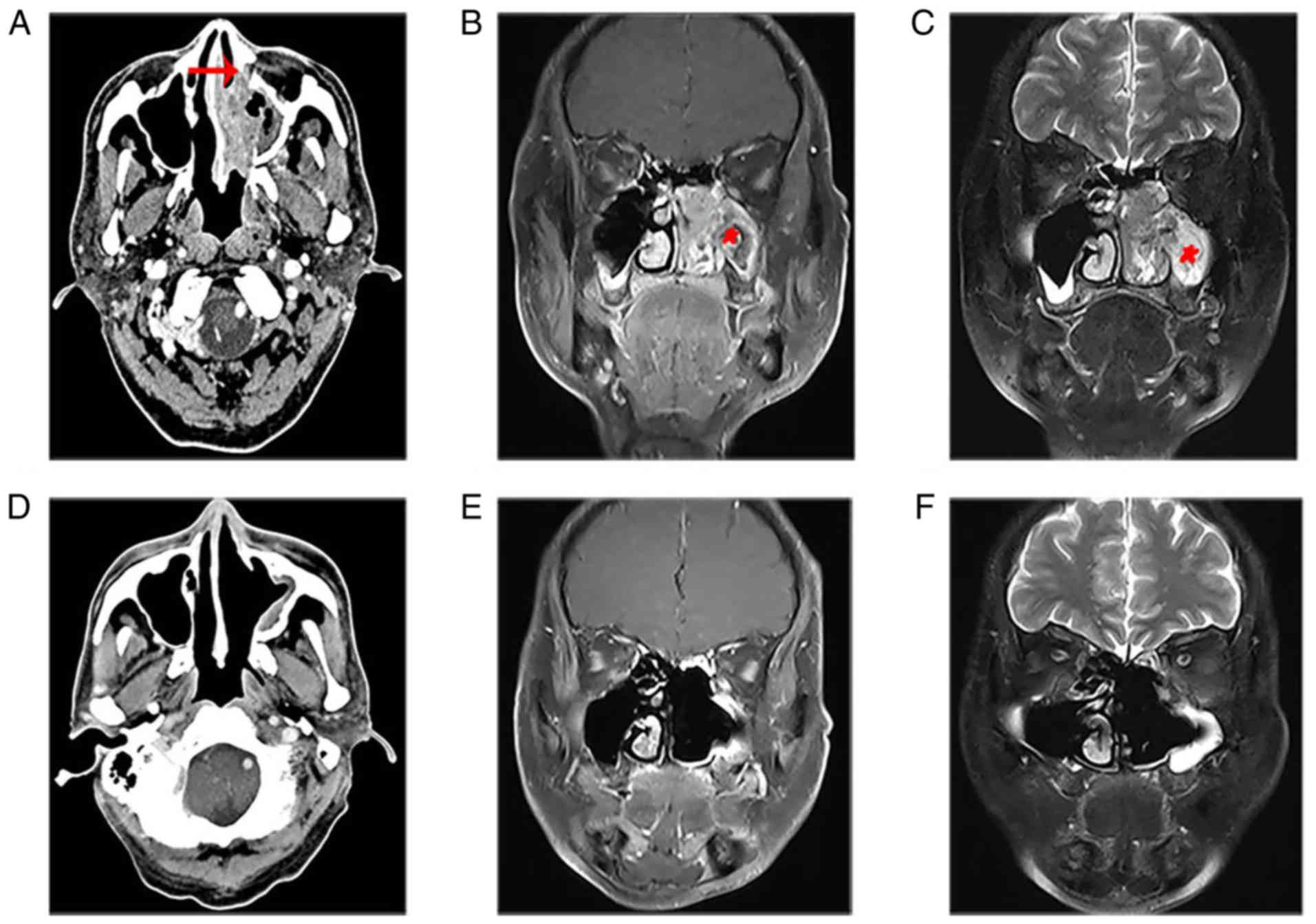
In the present study, patient 17 with stage T3
presented a large diffuse soft tissue mass in the left nasal
cavity, which invaded the middle and inferior nasal meatus,
maxillary sinus and ethmoid sinus. Enhanced CT showed partially
destroyed bone of the maxillary sinus (Fig. 1A). The MRI showed that the nasal mass
displayed characteristic CCP, which was lost in most of the mass of
the maxillary sinus (Fig. 1B and C).
The patients were treated with complete resection of the tumors
under nasal endoscopy. Following 54 months of follow-up, there was
no tendency of recurrence based on imaging examinations (Fig. 1D-F).
Treatment and Outcomes
All patients underwent surgical treatment, 15
patients (66.7%) received endoscopic transnasal resection,
endoscopy combined with Caldwell-Luc surgery was administered in 4
patients (19.0%), and other surgical procedures included lateral
rhinotomy (n=1) and endoscopy combined with external incision
(n=1). In addition, patient 9 simultaneously underwent partial
right maxillary excision. Surgery prior to or after adjuvant
radiochemotherapy was performed in 7 patients (33.3%). Exclusive
radiation therapy was carried out in 7 patients (33.3%), exclusive
chemotherapy was performed in 2 patients (9.5%), and patient 1
underwent immunotherapy with programmed cell death-1 (PD-1)
antibody drugs and chemotherapy following surgical resection of the
tumor.
During a mean follow-up time of 47.4 months (range,
3–123 months), the 1-, 3-, and 5-year OS rates were 90.5, 75.4 and
68.5% (Fig. 2), and the 1-, 3-, and
5-year DSS was 90.5, 80.4 and 80.4%, respectively (Fig. 3). The tumors in 15 patients (71.4%)
were completely resected at the AEENTH as the initial surgery, and
residual tumors were found in 6 patients (28.6%). Nine patients
(42.9%) experienced a local recurrence, and the risk factors of T4
stage and invasive orbital cavity had a significant influence on
recurrence. Tumors with a positive surgical margin demonstrated a
higher rate of recurrence than those with a negative margin, but
there was no significant difference (P=0.33). In addition, the
recurrence comparison did not reveal significant differences
regarding the factors of sex, age ≥65 years or origin site
(Table II). Five patients (23.8%)
died due to tumor progression, and 2 patients (9.5%) died due to
cerebral infarction accident. Remission was confirmed upon physical
exam, and/or disappearance of the tumor was confirmed by imaging
studies in 9 patients (42.9%), whereas 2 patients (9.5%) remained
alive with the disease. Three patients were lost during
follow-up.
 | Table II.Prognostic factors for recurrence and
survival. |
Table II.
Prognostic factors for recurrence and
survival.
|
Characteristics | Patients | Recurrences, n
(%) | Recurrences,
P-value | 1-year DSS, % | 3-year DSS, % | Survival,
P-value |
|---|
| All patients | 21 | 9 (42.9) | / | 90.5 | 80.4 | / |
| Sex |
|
| >0.05 |
|
| 0.38 |
|
Male | 18 | 8 (44.4) |
| 88.9 | 77.0 |
|
|
Female | 3 | 1 (33.3) |
| 100 | 100 |
|
| Age, years |
|
| 0.15 |
|
| 0.12 |
|
≥65 | 7 | 1 (14.3) |
| 100 | 100 |
|
|
<65 | 14 | 8 (57.1) |
| 85.7 | 70.1 |
|
| T stage |
|
| 0.03 |
|
| <0.01 |
| T4 | 8 | 6 (75.0) |
| 75.0 | 45.0 |
|
| T1, T2,
T3 | 13 | 3 (23.1) |
| 100 | 100 |
|
| Invasive orbital
cavity |
|
| 0.03 |
|
| 0.22 |
|
Yes | 10 | 7 (70.0) |
| 90.0 | 67.5 |
|
| No | 11 | 2 (18.2) |
| 90.9 | 90.9 |
|
| Origin site |
|
| 0.57 |
|
| 0.92 |
|
Maxillary sinus | 11 | 4 (36.4) |
| 90.9 | 80.8 |
|
| Other
sites | 10 | 5 (50.0) |
| 90.0 | 80.0 |
|
| Surgical
margin |
|
| 0.33 |
|
| <0.01 |
|
Negative | 15 | 5 (37.5) |
| 93.3 | 93.3 |
|
|
Positive | 6 | 4 (60.0) |
| 66.7 | 44.4 |
|
Prognostic factors
Prognostic factors for the treatment outcomes of SCC
associated with IP are shown in Table
II. T4 tumors influenced survival; 3-year DSS of these patients
was 45.0%, in contrast to patients with a stage of T3 or less for
whom 3-year DSS was 100% (P<0.01; Fig. 4). Positive surgical margins adversely
affected patient outcome; these patients had a 44.4% 3-year
cumulative survival probability, whereas negative surgical margins
improved survival to 93.3% (P<0.01; Fig. 5). Although the 3-year DSS of patients
aged ≥65 years (100%) was higher than those aged <65 years
(70.1%), there was no significant difference between the two groups
(P=0.12). Males were not significantly different in terms of DSS
from females (P=0.38). The invasion of the orbital cavity showed
lower survival rates compared with non-invasion, but the survival
comparison did not reveal any significant differences (P=0.22).
Furthermore, there was no significant difference in the survival
rate between the maxillary sinus and other origin sites
(P=0.92).
Discussion
The current literature suggests that the incidence
of malignancy among patients with IP varies widely and ranges from
2–27% (6). Although adenocarcinomas,
mucoepidermoid carcinomas, nasal undifferentiated carcinomas, small
cell carcinomas, and NOS (not specifically specified) were also
associated with IP, major histological findings most often
confirmed SCC (12). The number of
males with SCC associated with IP was larger than the number of
women (13). The results of the
present study coincided with these results, and the rate of
association with malignancy was 5.1% (21/408), and all
IP-associated carcinoma was SCC. Males were the predominant group
of patients and accounted for 85.7% (18/21). There was a
significant association between male sex and malignancy. In recent
years, some studies have reported that sinonasal IP progression to
SCC was significantly associated with the presence of human
papillomavirus (HPV) infection (14,15).
However, Mohajeri et al compared HPV positivity among
sinonasal IP samples and those from patients with SCC by
immunohistochemistry and found that HPV was not supported as an
etiological driver of IP development or progression to SCC
(16). Thus, further research is
required to confirm the association between HPV infection and SCC
with IP.
Lesperance et al described an average
interval of 63 months (6 months to 13 years) between the onset of
IP and the development of metachronous cancer (17). Another study showed that one of six
cases (17%) developed metachronous cancer approximately 10 years
after undergoing IP surgery (7). In
this series, the average interval between benign IP and cancer
onset was 44 months (range, 8 months-10 years) in 7 patients.
Patient 15 underwent maxillary sinus fenestration due to the
invasion of the tumor, and the pathological findings suggested
benign IP. The patient remained disease-free for five years
following the first surgery, when the tumor recurred again in the
maxillary sinus and the patient underwent lateral rhinotomy
resection. Ten years following the first surgery, maxillary sinus
IP recurred for the second time and invaded the orbital floor, and
endoscopic transnasal resection was performed. However, the
pathological type changed from IP to SCC. Therefore, long-term
follow-up is required to monitor metachronous carcinoma despite
complete resection of benign IP, particularly IP presenting with
severe atypical hyperplasia of mucosal epithelium.
Computed tomography (CT) scans are performed in
patients with suspected IP, which can be helpful in predicting IP
attachment sites (18). Bone erosion
and orbital wall involvement on CT presentation are suggestive of
an aggressive tumor, in accord with SCC associated with IP.
However, orbital wall invasion can also be found in recurrent
benign IP tumors. Therefore, the sensitivity and specificity in the
diagnosis of SCC in IP are low (19). Magnetic resonance imaging (MRI)
features are more valuable for distinguishing IP from SCC in IP. IP
shows a distinctive gross mucosal morphology known as a convoluted
cerebriform pattern (CCP). CCP is defined as a mixture of linear or
curvilinear hypointensity and hyperintensity locally or diffusely
presented in the solid components of tumors on enhanced T1-weighted
or T2-weighted MRI. A partial loss of CCP was associated with SCC
in IP patients and can be used in preoperative analysis (20,21).
Sinonasal IP is different from other benign tumors
affecting paranasal sinuses notably, and its high recurrence rate
is between 10 and 20%. Risk factors for local recurrence of IP
remain controversial (22,23). Lisan et al reported that the
history of previous resection was the only factor associated with
recurrence when compared with those who underwent a first
resection, which may correspond to incomplete initial resection
(24). This was also supported by
another study, which demonstrated that recurrence was associated
with the thoroughness of the first surgical excision of the lesions
(25). The risk factors associated
with the recurrence of SCC in IP are seldom addressed in the
literature. In the present study, the risk factors of T4 stage and
invasive orbital cavity had a significant influence on local
recurrence. This seemed to indicate that the invasion of areas
outside the nasal cavity, such as the orbit, pterygopalatine fossa,
and infratemporal fossa, led to the incomplete resection of tumors
and recurrence. In addition, the recurrence rate of patients with
positive margins was higher (4/6, 66.7%) compared with those with
negative margins (5/15, 33.3%). However, there was no significant
difference between the two groups. The reason may be that the
number of patients in this study was not very large.
The prognosis of SCC associated with IP varies
according to different reports. Lee et al (27) revealed that the median survival time
of SCC in IP was >10 years, and ~60% of patients become
long-term survivors (26). This
result seemed to be comparable with the survival rate of patients
with sinonasal SCC, which was reported to be between 50 and 60%. A
study by Yu et al (28)
compared the prognoses between the 21 patients with SCC in IP and
65 patients with SCC, and the 5-year DFS was not significantly
different, which was 61.5% in the SCC in the IP group and 52.8% in
the SCC group. However, some studies also suggested that SCC in IP
had a more favorable prognosis compared with SCC only (7,29,30).
Karligkiotis et al (31)
observed a 5-year DFS of SCC in IP of 71.2% during an average
follow-up period of 60 months. The same result was shown in a study
of 32 patients, indicating a median survival time of 62.2 months
with a 5-year OS of 72.5% (30). In
the present study, the 5-year DFS and OS were 80.4 and 68.5%,
respectively, which appeared to be a more favorable prognosis than
most published cases of primary sinonasal SCC.
The AJCC staging system is valuable for maxillary
sinus, nasal cavity and ethmoid sinus carcinomas. As SCC in IP
patients rarely present with lymph node or distant metastasis, T
staging regarding primary tumors is useful in predicting prognosis.
Karligkiotis et al (31)
reported that the 3-year DSS in the IP-associated malignancy was
100% for T1, 100% for T2, 77.8% for T3, and 40% for T4, indicating
that the advanced T classification (T3 or greater) was
significantly associated with poorer outcome. This was also
supported by Kim et al (13)
demonstrating that patients with a T stage of T2 or less had a
significantly better DFS and longer disease-free interval than
those with a T stage of T3 or more. In contrast, Choi et al
(7) demonstrated that tumor stage
was not associated with the clinical outcome of SCC in IP. In this
series, the prognosis of stage T4 was not satisfactory with a
3-year DSS of 45%, and the clinical outcomes of T1, T2 and T3 were
excellent, with a 3-year DSS of 100%. Furthermore, positive
surgical margins also adversely affected outcomes. In addition,
there were some limitations in this study: T4 and positive surgical
margins were risk factors for an adverse prognosis. However, there
may be a confounding factor between the two. Considering the small
number of patients available at the AEENTH, more patients with SCC
associated with IP should be enrolled, and confounding factors
should be excluded for future studies.
Of the 5 patients who died as a result of disease
progression, patient 6 with a stage of T3 died following 123
months, while 4 patients with a stage of T4 died during the first
few years. These T4 tumors often invaded the sphenoid sinus,
frontal sinus, infratemporal fossa and intraorbital region. The
invasion range of these tumors was large and could not be entirely
resected by surgery. Although radiotherapy and chemotherapy were
administered following the operation, the lesions recurred, and
patients died within a short time. This finding indicated that
these invading regions from the primary site strongly associate
with poor prognosis, but there were no statistically significant
conclusions drawn, due to the small number of patients in the
present study. In addition, among the other 4 patients with stage
T4, patient 1 lived with disease and incurred brain metastasis 20
months following subtotal resection of the tumor in the initial
operation, patient 7 and patient 20 lived without disease following
complete resection and postoperative radiotherapy, and patient 19
was lost during follow-up. Thus, local lesions are difficult to
control without entire resection in the treatment of T4 disease.
Various surgical methods, such as endoscopic transnasal resection,
the Caldwell-Luc approach and lateral rhinotomy, are used for
treatment, but the complete removal of lesions is considered the
key aspect not the removal method. In addition, some studies noted
that postoperative radiotherapy was recommended for patients with
advanced T3 or higher, and positive margins, critical areas (such
as orbit or anterior skull base), and unresectable disease affected
outcomes (32,33).
SCC associated with IP has a high recurrence rate,
and the risk factors of T4 stage and invasive disease in the
orbital cavity have a significant influence on local recurrence. In
addition, SCC associated with IP has a favorable overall survival,
and the prognosis of patients with stage T4 was not as satisfactory
as those with a stage of T3 or less, and positive surgical margins
adversely affected outcome. Thus, early diagnosis and completely
resected lesions are required for a good prognosis for SCC
associated with IP. Regarding tumors with stage T4, more aggressive
surgical approaches combined with postoperative adjuvant therapy
seem to be effective.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National
Natural Science Foundation of China (grant no. 81870703).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW, WL and HL conceived and designed the study. WL,
HL, HZ, XS and LH acquired the data. WL, HL, HZ, XS and LH drafted
the manuscript. WL and HL performed the statistical analysis. DW
supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This article does not contain any studies with human
participants or animals performed by any of the authors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Myers EN, Fernau JL, Johnson JT, Tabet JC
and Barnes EL: Management of inverted papilloma. Laryngoscope.
100:481–490. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Outzen KE, Grøntveld A, Jørgensen K,
Clausen PP and Ladefoged C: Inverted papilloma: Incidence and late
results of surgical treatment. Rhinology. 34:114–118.
1996.PubMed/NCBI
|
|
3
|
Sham CL, Woo JK, van Hasselt CA and Tong
MC: Treatment results of sinonasal inverted papilloma: An 18-year
study. Am J Rhinol Allergy. 23:203–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Govindaraj S and Wang H: Does human
papilloma virus play a role in sinonasal inverted papilloma? Curr
Opin Otolaryngol Head Neck Surg. 22:47–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Justice JM, Davis KM, Saenz DA and Lanza
DC: Evidence that human papillomavirus causes inverted papilloma is
sparse. Int Forum Allergy Rhinol. 4:995–1001. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewis JS Jr, Westra WH, Thompson LD,
Barnes L, Cardesa A, Hunt JL, Williams MD, Slootweg PJ,
Triantafyllou A, Woolgar JA, et al: The sinonasal tract: Another
potential ‘hot spot’ for carcinomas with transcriptionally-active
human papillomavirus. Head Neck Pathol. 8:241–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi JW, Kim SG, Kim YM, Yoon YH, Kim AY
and Rha KS: Clinical and histologic features of inverted
papilloma-associated malignancy. Eur Arch Otorhinolaryngol.
269:2349–2354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mirza S, Bradley PJ, Acharya A, Stacey M
and Jones NS: Sinonasal inverted papillomas: Recurrence, and
synchronous and metachronous malignancy. J Laryngol Otol.
121:857–864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lawson W and Patel ZM: The evolution of
management for inverted papilloma: An analysis of 200 cases.
Otolaryngol Head Neck Surg. 140:330–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yasumatsu R, Nakashima T, Sato M, Nakano
T, Kogo R, Hashimoto K, Sawatsubashi M and Nakagawa T: Clinical
management of squamous cell carcinoma associated with sinonasal
inverted papilloma. Auris Nasus Larynx. 44:98–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Re M, Gioacchini FM, Bajraktari A,
Tomasetti M, Kaleci S, Rubini C, Bertini A, Magliulo G and Pasquini
E: Malignant transformation of sinonasal inverted papilloma and
related genetic alterations: A systematic review. Eur Arch
Otorhinolaryngol. 274:2991–3000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim K, Kim D, Koo Y, Kim CH, Choi EC, Lee
JG and Yoon JH: Sinonasal carcinoma associated with inverted
papilloma: A report of 16 cases. J Craniomaxillofac Surg.
40:e125–e129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Udager AM, McHugh JB, Goudsmit CM,
Weigelin HC, Lim MS, Elenitoba-Johnson KSJ, Betz BL, Carey TE and
Brown NA: Human papillomavirus (HPV) and somatic EGFR mutations are
essential, mutually exclusive oncogenic mechanisms for inverted
sinonasal papillomas and associated sinonasal squamous cell
carcinomas. Ann Oncol. 29:466–471. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahnane N, Ottini G, Turri-Zanoni M,
Furlan D, Battaglia P, Karligkiotis A, Albeni C, Cerutti R, Mura E,
Chiaravalli AM, et al: Comprehensive analysis of HPV infection,
EGFR exon 20 mutations and LINE1 hypomethylation as risk factors
for malignant transformation of sinonasal-inverted papilloma to
squamous cell carcinoma. Int J Cancer. 144:1313–1320. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohajeri S, Lai C, Purgina B, Almutairi D,
Baghai T, Dimitroulakos J and Kilty S: Human papillomavirus: An
unlikely etiologic factor in sinonasal inverted papilloma.
Laryngoscope. 128:2443–2447. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lesperance MM and Esclamado RM: Squamous
cell carcinoma arising in inverted papilloma. Laryngoscope.
105:178–183. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yousuf K and Wright ED: Site of attachment
of inverted papilloma predicted by CT findings of osteitis. Am J
Rhinol. 21:32–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karkos PD, Fyrmpas G, Carrie SC and Swift
AC: Endoscopic versus open surgical interventions for inverted
nasal papilloma: A systematic review. Clin Otolaryngol. 31:499–503.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan CH, Tong CCL, Penta M, Patel VS,
Palmer JN, Adappa ND, Nayak JV, Hwang PH and Patel ZM: Imaging
predictors for malignant transformation of inverted papilloma.
Laryngoscope. 129:777–782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon TY, Kim HJ, Chung SK, Dhong HJ, Kim
HY, Yim YJ, Kim ST, Jeon P and Kim KH: Sinonasal inverted
papilloma: Value of convoluted cerebriform pattern on MR imaging.
AJNR Am J Neuroradiol. 29:1556–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim DY, Hong SL, Lee CH, Jin HR, Kang JM,
Lee BJ, Moon IJ, Chung SK, Rha KS, Cho SH, et al: Inverted
papilloma of the nasal cavity and paranasal sinuses: A Korean
multicenter study. Laryngoscope. 122:487–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Busquets JM and Hwang PH: Endoscopic
resection of sinonasal inverted papilloma: A meta-analysis.
Otolaryngol Head Neck Surg. 134:476–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lisan Q, Laccourreye O and Bonfils P:
Sinonasal inverted papilloma: Risk factors for local recurrence
after surgical resection. Ann Otol Rhinol Laryngol. 126:498–504.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao-Ting W, Peng L, Xiu-Qing W, Hai-Bo W,
Wen-Hui P, Bing L, Er-Peng Z and Guang-Gang S: Factors affecting
recurrence of sinonasal inverted papilloma. Eur Arch
Otorhinolaryngol. 270:1349–1353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanvetyanon T, Qin D, Padhya T, Kapoor R,
McCaffrey J and Trotti A: Survival outcomes of squamous cell
carcinoma arising from sinonasal inverted papilloma: Report of 6
cases with systematic review and pooled analysis. Am J Otolaryngol.
30:38–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee CH, Hur DG, Roh HJ, Rha KS, Jin HR,
Rhee CS and Min YG: Survival rates of sinonasal squamous cell
carcinoma with the new AJCC staging system. Arch Otolaryngol Head
Neck Surg. 133:131–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu MS, Lim WS, Lee BJ and Chung YS:
Squamous cell carcinoma associated with inverted papilloma of the
maxillary sinus: Our experience with 21 patients. Clin Otolaryngol.
42:1048–1052. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shipchandler TZ, Batra PS, Citardi MJ,
Bolger WE and Lanza DC: Outcomes for endoscopic resection of
sinonasal squamous cell carcinoma. Laryngoscope. 115:1983–1987.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu HX and Liu G: Malignant transformation
of sinonasal inverted papilloma: A retrospective analysis of 32
cases. Oncol Lett. 8:2637–2641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karligkiotis A, Lepera D, Volpi L,
Turri-Zanoni M, Battaglia P, Lombardi D, Accorona R, Bignami M,
Nicolai P and Castelnuovo P: Survival outcomes after endoscopic
resection for sinonasal squamous cell carcinoma arising on inverted
papilloma. Head Neck. 38:1604–1614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
von Buchwald C and Bradley PJ: Risks of
malignancy in inverted papilloma of the nose and paranasal sinuses.
Curr Opin Otolaryngol Head Neck Surg. 15:95–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wormald PJ, Ooi E, van Hasselt CA and Nair
S: Endoscopic removal of sinonasal inverted papilloma including
endoscopic medial maxillectomy. Laryngoscope. 113:867–873. 2003.
View Article : Google Scholar : PubMed/NCBI
|