Introduction
Recent progress in the identification of
tumor-specific molecular alterations has contributed to novel
therapeutic approaches, and the development of molecular-targeting
anti-tumor drugs has improved patient survival. For example,
epidermal growth factor receptor (EGFR)-targeted therapy for
non-small cell lung cancer (NSCLC) cases harboring EGFR
oncogenic alterations is a promising strategy for improving the
clinical outcome of patients with NSCLC (1,2).
Human epidermal growth factor receptor 2 (HER2) is
part of the ErbB family of receptor tyrosine kinases. HER2 is
activated by homodimerization or heterodimerization with other
receptors in the ErbB family, particularly EGFR (3). HER2 has important roles in pathogenesis
of certain types of human cancer, and numerous studies have
reported the amplification and overexpression of HER2 in cancer,
particularly breast cancer (4–6). The
reported frequencies of HER2 overexpression and HER2
amplification in NSCLC range from 11–32 and 2–23%, respectively
(7–10). HER2 mutations have been
identified in 2–4% of all NSCLCs, and are usually mutually
exclusive with other driver mutations (11,12).
Several HER2 variants have been reported previously, the
majority of which are in-frame insertions in exon 20 of the kinase
domain, including A775insYVMA, G776VC, P780insGSP and G776LC
(12). Our previous study identified
two novel mutations in the HER2 transmembrane domain, which is
encoded by exon 17 (V659E and G660D), as rare HER2 variants in lung
adenocarcinoma, and the preliminary data suggested that these
mutations may be oncogenic (13). An
extracellular domain point mutation, S310F, in exon 8 has also been
reported to be oncogenic (14).
However, the benefit of HER2-targeted therapy against NSCLC
harboring HER2 alterations is far less well defined than the
known benefit against breast cancer and gastric cancer with
HER2 alterations (15).
Afatinib (BIBW 2992) is a pan-HER tyrosine kinase
inhibitor (TKI) that has been approved for the treatment of
patients with NSCLC harboring EGFR mutations. Recently, afatinib
has attracted attention as a HER2-targeting treatment agent.
Afatinib was reported to exhibit good clinical activity in patients
with lung adenocarcinoma carrying HER2 mutations (16,17). In
preclinical studies, afatinib inhibited the growth of
HER2-altered NSCLC cells (18).
Neratinib (HKI-272) is another pan-HER TKI that has
been reported to improve the overall survival of post-operative
patients with HER2-positive breast cancer previously treated with
trastuzumab-based adjuvant therapy (19). A phase II clinical trial
(PUMA-NER-4201) evaluating the usefulness of neratinib combined
with the mechanistic target of rapamycin kinase inhibitor
temsirolimus for patients with NSCLC harboring
HER2-mutations (insertions in exon 20) in currently ongoing;
patient accrual has been completed, and the final results of the
trial are being awaited (20,21). Thus far, the available data is
limited, and the benefits of neratinib treatment remain unclear,
particularly for cases with relatively rare mutations. Therefore,
the aim of the present study was to investigate the potential use
of neratinib against HER2-altered NSCLC, including cases with
relatively uncommon mutations.
Materials and methods
Cell lines and reagents
Four lung cancer cell lines (A549, Calu-3, NCI-H2170
and NCI-H1781) and one normal human bronchial epithelial cell line
(BEAS-2B) were used in the current study. Calu-3, H2170 and H1781
cells were received as gifts from Dr Adi F. Gazdar (University of
Texas Southwestern Medical Center at Dallas, Dallas, TX, USA)
(22,23). A549 was purchased from American Type
Culture Collection (Manassas, VA, USA). BEAS-2B was purchased from
European Collection of Authenticated Cell Cultures (Public Health
England, Porton Down, UK). All the cancer cell lines were cultured
in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS),
and the BEAS-2B cells were maintained in Dulbecco's modified
Eagle's medium supplemented with 10% FBS. They were cultured in a
humidified atmosphere containing 5% CO2 at 37°C.
Neratinib and erlotinib were purchased from Selleck Chemicals
(Houston, TX, USA).
Plasmid constructs and
transfection
Human cDNAs encoding full-length HER2 (wild-type and
its variants, A775insYVMA, G776VC, G776LC, P780insGSP, V659E, G660D
and S310F) were inserted into the pIDT-SMART (C-TSC) vector,
pCMViRTSC (24). Transient
transfection of the BEAS-2B cells with the mammalian expression
vectors was performed using Lipofectamine® 3000 (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol.
Western blot analysis and
immunohistochemistry
Total cell lysates were extracted using a mixture of
radioimmunoprecipitation assay lysis buffer, phosphatase inhibitor
cocktails 2 and 3 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and Complete Mini protease inhibitor cocktail (Roche Diagnostics,
Basel, Switzerland). Western blot analysis was performed using the
conventional method with the following primary antibodies:
Anti-EGFR (4267S; 1:1,000), phospho-(p-) EGFR (Tyr1068) (3777S;
1:1,000), HER2 (4290S; 1:1,000), p-HER2 (Tyr1221⁄1222) (2243S;
1:1,000), Akt (9272S; 1:1,000), p-Akt (Ser473) (4060S; 1:1,000),
p44⁄p42 mitogen-activated protein kinase (MAPK) (9102S; 1:1,000),
p-p44⁄p42 MAPK (4370S; 1:1,000), cleaved poly (ADP-ribose)
polymerase (PARP; Asp214) (5625S; 1:1,000) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). β-actin (used as
loading control) (MAB1501R; 1:1,000) was purchased from EMD
Millipore (Billerica, MA, USA). The secondary antibodies were
horseradish peroxidase-conjugated anti-mouse IgG (sc-2031; 1:2,500)
or anti-rabbit IgG (sc-2030; 1:2,500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA).
To detect specific signals, the membranes were
examined using the ECL Prime Western Blotting Detection System (GE
Healthcare Life Sciences, Little Chalfont, UK) and a LAS-3000
imager (Fujifilm, Tokyo, Japan). Immunohistochemical staining with
anti-p-HER2 (1:300 Y1221/1222; Cell Signaling Technology, Inc.) was
conducted. The detailed protocol for the immunohistochemical
staining has been described previously (25).
Cell growth inhibition assay
Cells were cultured with or without the appropriate
drugs for 72 h and the sensitivities of the cells to the drugs were
determined via a modified MTS assay using Cell Titer 96 Aqueous One
Solution Reagent (Promega Corporation, Madison, WI, USA), as
described previously (26). The
anti-proliferative activity of each drug is presented as the
IC50, which is the concentration of the drug required to
inhibit cell proliferation by 50%.
Cell cycle analysis
The effects of neratinib on the cell cycle
distribution were assessed using a propidium iodide staining-based
assay (CycleTEST PLUS DNA reagent kit; BD Biosciences, Franklin
Lakes, NJ, USA) and a BD Accuri C6 flow cytometer (BD Biosciences).
Doublets, cell debris and fixation artifacts were gated out, and
cell cycle analysis was performed.
Xenograft model
NOD/SCID female mice [n=18, body weight (mean ±
standard)=19.9±0.6 g, 6-weeks-old] were purchased from Charles
River Laboratories, Inc. (Wilmington, MA, USA). All the mice were
provided with sterilized food and water, and housed in a barrier
facility under a 12:12-h light/dark cycle. Each cell line
(5×106 cells) was suspended in 200 µl RPMI-1640 medium
mixed with Matrigel Basement Membrane Matrix (Corning Incorporated,
Corning, NY, USA) and subcutaneously injected into the backs of the
mice. The tumor volume was calculated using the empirical formula,
V=1/2 × [(shortest diameter)2 × (longest diameter)].
When the tumor volume exceeded ~50 mm3, the mice were
orally administered with vehicle alone or neratinib (40 mg⁄kg, 6
days a week). Neratinib was prepared in 0.5 w/v (%) methyl
cellulose. The tumor volume was measured three times a week using
calipers. After 4 weeks of treatment, or when humane endpoints were
reached, mice were euthanized by cervical dislocation.
All animal experiments were performed in accordance
with protocols approved by the Animal Care and Use Committee of
Okayama University (Okayama, Japan; permission no. OKU-2018215) and
were conducted in accordance with recent legislation of the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals.
Statistical analysis
Statistical analysis was performed using EZR
software (Saitama Medical Center, Jichi Medical University,
Saitama, Japan), which is a graphical user interface for R (The R
Foundation for Statistical Computing, Vienna, Austria). EZR is a
modified version of R commander software (version 1.6–3) with
additional statistical functions frequently used in biostatistics
(27). Data from two groups were
compared using t-test. All tests were two-sided. P<0.05 was
considered to indicate a statistically significant difference.
Results
HER2 mutations activate HER2
signaling, which is inhibited by neratinib
To examine the effect of HER2 alterations on
the signal transduction pathways, normal bronchial epithelial cells
(BEAS-2B) were transiently transfected with vectors containing
wild-type HER2 or one of seven HER2 mutations: Four kinase
domain mutations (A775insYVMA, G776VC, G776LC, and P780insGSP), two
transmembrane domain mutations (V659E and G660D) and one
extracellular domain mutation (S310F).
The sensitivity of BEAS-2B cells ectopically
expressing wild-type or mutant HER2 to erlotinib (an
EGFR-TKI) or neratinib (a pan-HER-TKI) was examined. At 48 h after
transfection, the cells were cultured in the presence or absence of
erlotinib or neratinib for 6 h. Erlotinib had minimal effect on the
phosphorylation of HER2 and EGFR, whereas neratinib strongly
inhibited the phosphorylation of HER2 and EGFR compared with
untreated cells (Fig. 1). These
results suggest that the HER2 alterations were activating
mutations and that neratinib treatment had an inhibitory effect on
HER2 activation. Furthermore, the activation of EGFR via
cross-phosphorylation of HER2 was not suppressed by erlotinib
treatment.
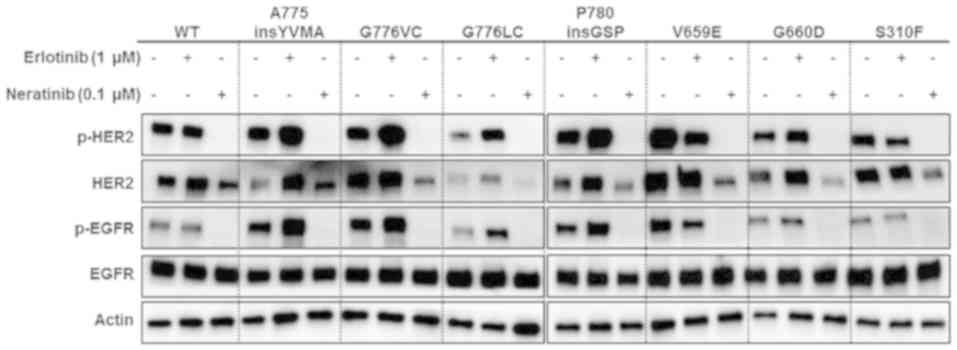 | Figure 1.Overexpression of wild-type or mutant
HER2 activates HER2 signaling, and neratinib inhibits this
signaling pathway. BEAS-2B cells were transiently transfected with
WT HER2, A775insYVMA, G776VC, G776LC, P780insGSP, G660D, V659E or
S310F mutants, or vector control. At 48 h post-transfection, cells
were treated with 1.0 µM erlotinib or 0.1 µM neratinib for 6 h.
Cells were cultured with media supplemented with fetal bovine
serum. Lysates were subjected to western blot analysis using the
indicated antibodies. HER2, human epidermal growth factor receptor
2; WT, wild-type; p-, phosphorylated; EGFR, epidermal growth factor
receptor. |
Neratinib inhibits the growth of
HER2-amplified and HER2-mutant lung cancer cells
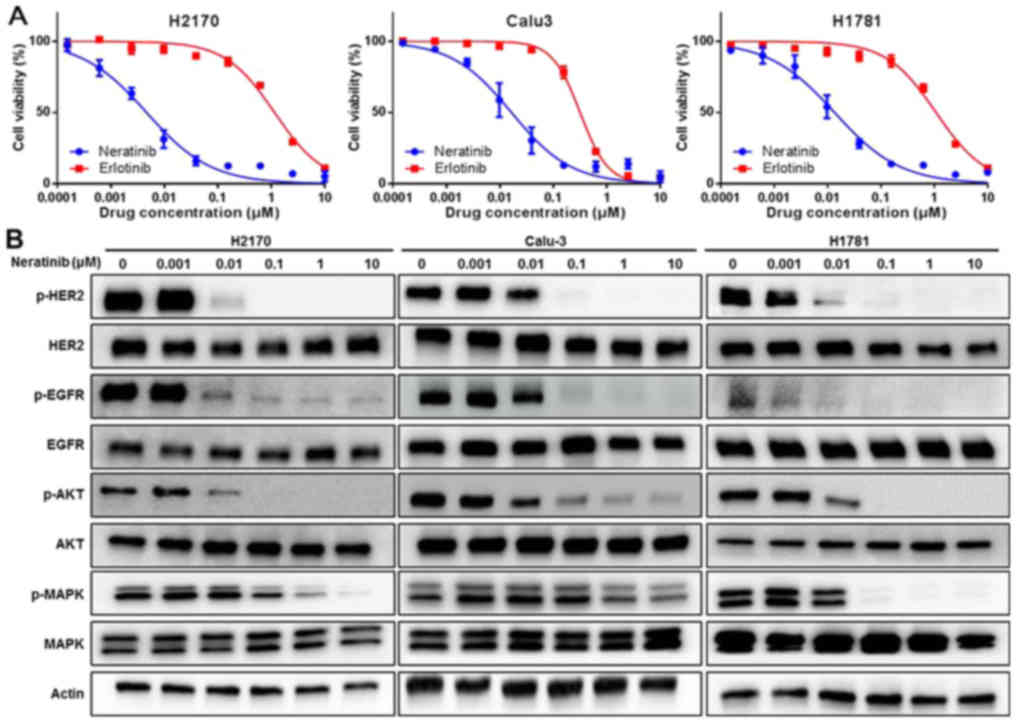
The anti-tumor activity of neratinib (a
pan-HER-TKIs) and erlotinib (an EGFR-TKI) against HER2-driven NSCLC
cell lines was subsequently examined (Table I; Fig.
2B). The cell lines in this panel consisted of two
HER2-amplified NSCLC cell lines (H2170 and Calu-3) and one
HER2-mutant NSCLC cell line (H1781). The detailed
HER2 genetic profiles of these three cell lines are
presented in Table I based on the
results of a previous study (18).
The proliferation of the two HER2-amplified lung cancer cell
lines, H2170 and Calu-3, was inhibited by neratinib, with
IC50 values of 4.7 and 16.5 nM, respectively. Neratinib
also exerted a strong cytotoxic effect against the H1781 cells,
with an IC50 of 13.6 nM. By contrast, the H2170 and
H1781 cell lines were resistant to erlotinib treatment, with
IC50 values of 1150 and 1080 nM, respectively. Calu-3
cells were partially sensitive to erlotinib, with an
IC50 of 316 nM. These results were consistent with those
of a previous report (Table I;
Fig. 2A) (28).
 | Table I.Characteristics and IC50
values for pan-HER tyrosine kinase inhibitor and EGFR tyrosine
kinase inhibitor in non-small cell lung cancer cell lines. |
Table I.
Characteristics and IC50
values for pan-HER tyrosine kinase inhibitor and EGFR tyrosine
kinase inhibitor in non-small cell lung cancer cell lines.
|
|
| Gene copy
number | Mutation
status |
| IC50
(nM) |
|---|
|
|
|
|
|
|
|
|---|
| Cell line | Histologic
subtype | EGFR | HER2 | EGFR | HER2 | Genetic
alteration | Neratinib | Erlotinib |
|---|
| H2170 | SQ | 2 | 95 | WT | WT | HER2
amplification |
4.7 | 1,150 |
| Calu-3 | AD | 4 | 111 | WT | WT | HER2
amplification | 16.5 | 316 |
| H1781 | AD | 2 | 3 | WT | G776VC | HER2
mutation | 13.6 | 1,080 |
Subsequently, the effect of neratinib on signal
transduction pathways in HER2-amplified or
HER2-mutant lung cancer cells was assessed. After 6 h of
treatment with neratinib, the cells were lysed and then subjected
to western blot analysis. As demonstrated in Fig. 2B, neratinib potently inhibited the
phosphorylation of HER2 and EGFR when administered at
concentrations as low as 0.01 µM, and downstream signals, including
phosphorylation of Akt and MAPK, were also inhibited by neratinib
in the HER2-amplified and the HER2-mutant lung cancer
cells. Taken together, these results suggest that neratinib has
strong anti-tumor activity against HER2-amplified and
HER2-mutant lung cancer cells in vitro.
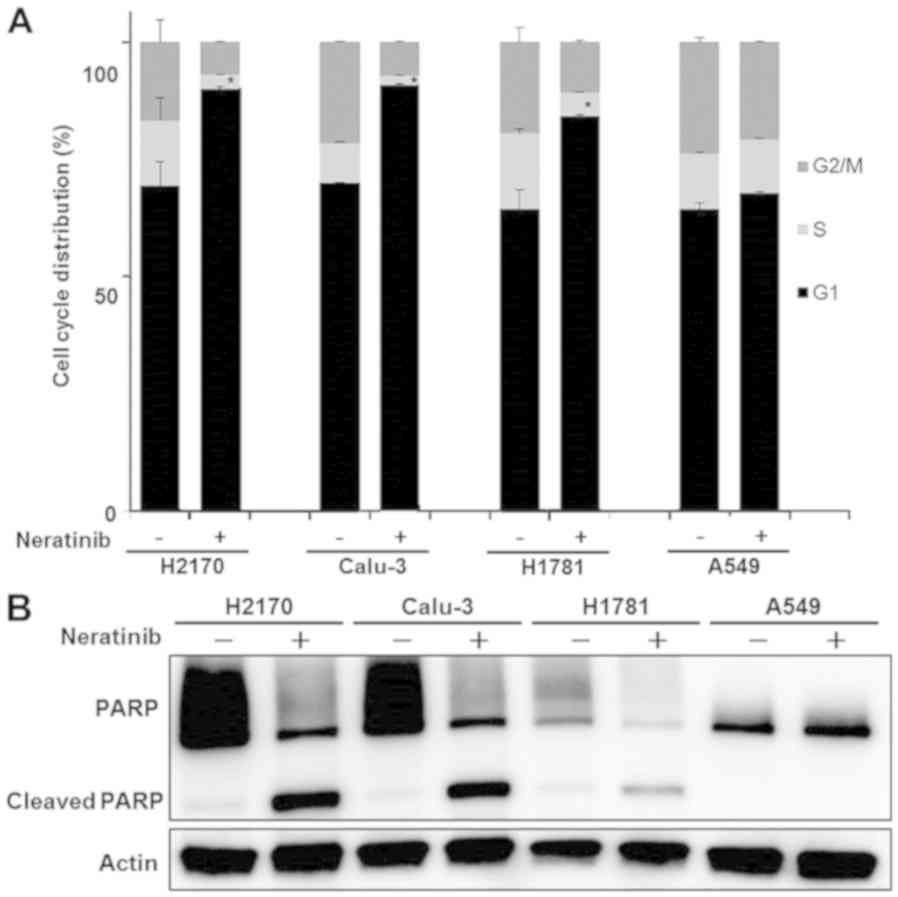
Neratinib induces cell cycle arrest
and apoptosis in HER2-dependent cells
The effect of neratinib on the cell cycle and
apoptosis in HER2-driven cells (H2170, Calu-3 and H1781) and
KRAS mutant cells (A549) was also examined to determine the
mechanism of growth inhibition. Cells were treated with 0.1 µM
neratinib for 48 h and then analyzed using flow cytometry. To
assess the cell cycle distribution, the sub-G1 fraction was
excluded and the percentage of cells in each cycle phase was
measured (Fig. 3A). Neratinib
treatment caused an increase in the number of H2170, Calu-3 and
H1781 cells in G1 phase compared with the distribution in untreated
cells; a similar increase was not detected in A549 cells.
Subsequently, western blotting was performed to evaluated cell
apoptosis, using cleaved PARP antibody as an apoptosis marker.
Neratinib induced apoptosis in the H2170, Calu-3 and H1781 cells;
however, an increase in cleaved PARP was not detected in A549 cells
following neratinib treatment (Fig.
3B). These results suggest that neratinib induces
anti-proliferative effects via G1 arrest and apoptotic cell death
in HER2-altered cells (H2170, Calu-3 and H1781 cells), whereas
HER2-independent NSCLC cells were not sensitive to neratinib.
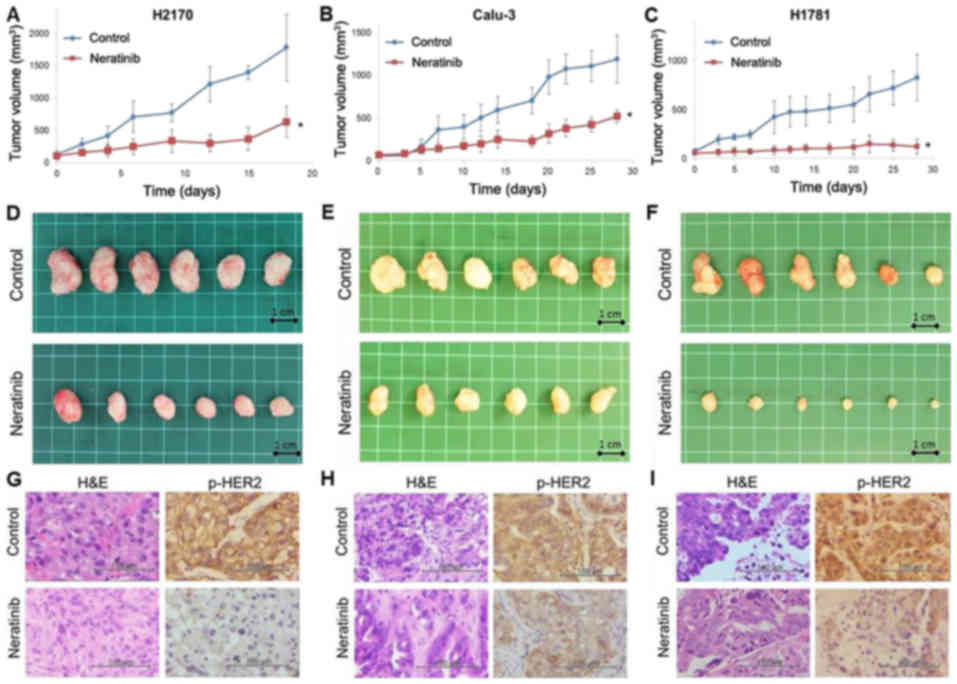
Anti-tumor effect of neratinib in a mouse xenograft
model of HER2-altered lung cancer. Based on the in vitro
data, the anti-tumor effect of neratinib was investigated using
mouse xenograft models of HER2-driven lung cancer. Two
HER2-amplified (H2170 and Calu-3) cell lines and one
HER2-mutant (H1781) cell line were used for this experiment.
The dose of neratinib was selected based on the results of previous
reports (29,30). Once the xenograft tumor volume reached
~50 mm3, the mice were orally treated with the vehicle
alone or neratinib (40 mg⁄kg, 6 days a week). As demonstrated in
Fig. 4, neratinib treatment
significantly inhibited the tumor growth of the H2170, Calu-3 and
H1781 ×enografts compared with the vehicle control (P<0.001). In
the H2170 ×enograft group treated with the vehicle control, the
mice were sacrificed on day 18, as the tumor volumes had reached
the humane endpoint (~2,000 mm3). The levels of p-HER2
in the xenografts were examined using immunohistochemistry. The
level of p-HER2 was suppressed in tumors from mice treated with
neratinib compared those that received the vehicle control
(Fig. 4G-I).
Discussion
The findings of the current study demonstrated the
in vivo and in vitro anti-tumor efficacy of neratinib
against lung cancer cells harboring HER2 alterations. Neratinib is
an irreversible human EGFR-TKI that also binds to the tyrosine
kinase domains of HER2 and HER4. In patients with breast cancer,
neratinib treatment has improved overall survival among
post-operative patients with HER2-positive breast cancer previously
treated with trastuzumab, and neratinib has been approved for
extended adjuvant treatment in patients with early-stage
HER2-positive breast cancer (19). By
contrast, only a few reports have discussed the efficacy of
neratinib against NSCLC. Furthermore, recent advances in clinical
tumor sequencing have identified a large number of variants in
oncogenic driver genes with unknown significance, including
EGFR, anaplastic lymphoma kinase and HER2 variants.
Heterogeneity among the functions and/or drug sensitivities of
tumors with these mutations has been reported. Notably, suitable
TKI selection for patients with individual oncogene variants,
including relatively rare mutations, has been proposed (31). Thus, further preclinical and clinical
investigations of patients with tumors harboring uncommon
HER2 gene variants are warranted.
In the current study, neratinib inhibited the growth
of cells harboring HER2 mutations in the transmembrane
domain, extracellular domain and kinase domain. Although neratinib
has been previously reported to inhibit the growth of cells
harboring HER2 mutations in the kinase domain, to the best
of our knowledge, there are no reports of neratinib inhibiting the
growth of cells with HER2 mutations in the transmembrane and
extracellular domains (32). Genomic
and functional analyses have suggested that mutations in the
transmembrane domain of HER2 encoded by exon 17 (V659E and
G660D) are oncogenic in lung adenocarcinoma. Additionally, although
the variant rate is low, mutations in the extracellular domain
(such as S310F) are also considered to be oncogenic alterations in
lung adenocarcinoma (14). For cells
harboring mutations in the extracellular domain, anti-HER2
monoclonal antibodies, such as trastuzumab, can target this region
of the receptor and prevent homo-dimerization and receptor
activation (33). However, because
the kinase domain is constitutively activated in tumors harboring
kinase domain mutations, the anti-proliferative effects of
monoclonal antibodies may be limited even if dimerization is
inhibited (34). The effect of
trastuzumab and other antibodies may also be limited in tumors
harboring mutations in the transmembrane domain, as HER2
dimerization is thought to be stable even if trastuzumab or other
antibodies bind to the extracellular domain (35). On the other hand, neratinib targets
the kinase domain of HER2 and inhibits the phosphorylation and
activity of HER receptors, and therefore may have a therapeutic
advantage over trastuzumab and other monoclonal antibodies, as
neratinib can exert anti-tumor effects regardless of the domain in
which the mutations exist, as demonstrated in the present report.
Although HER2 mutations in the extracellular domain are rare
(36), neratinib may be a useful
therapeutic option in patients with such mutations.
The findings of the present study demonstrated the
efficacy of neratinib against NSCLC cell lines harboring several
HER2 variants. The anti-tumor activity of neratinib was also
evaluated in a mouse xenograft model using lung cancer cells with
HER2 amplification or HER2 mutation. To the best of
our knowledge, this is the first report of in vivo
experiments using neratinib for mouse xenograft models of lung
cancer cells harboring HER2 amplification or HER2
mutations.
In conclusion, the anti-tumor effect of neratinib
against lung cancers harboring HER2 alterations was
demonstrated in vitro and in vivo. The findings
suggest that neratinib has potential as a promising therapeutic
option for the treatment of HER2-altered NSCLC.
Acknowledgements
The abstract was presented at the Annual Meeting of
the American Associated for Cancer Research Apr 14–18 2018 in
Chicago, IL, USA and published as abstract no. 4777 in Cancer Res
78 (Suppl 13), 2018. The authors would like thank Ms. Fumiko Isobe
(Department of Thoracic, Breast and Endocrinological Surgery,
Okayama University Graduate School of Medicine, Dentistry and
Pharmaceutical Sciences, Okayama, Japan) for her technical
assistance.
Funding
This study was supported by a Management Expenses
Grant.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YO, KSh, KSu, JS, HY and SToy conceived and designed
the study. YO, KSh, and MS performed majority of the in
vitro experiments. MS performed cell transfection. YO, YT, STom
and KSh performed the statistical analysis. JS, HY, TY, HT, HS, EK
and KN participated in the animal experiments and helped perform
the analysis with constructive discussion. MS, STom, KSu, KSh and
SToy supervised the study.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with protocols approved by the Animal Care and Use Committee of
Okayama University (Okayama, Japan; permission no. OKU-2018215) and
were conducted in accordance with recent legislation of the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HER2
|
human epidermal growth factor receptor
2
|
|
NSCLC
|
non-small cell lung cancer
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oxnard GR, Binder A and Janne PA: New
targetable oncogenes in non-small-cell lung cancer. J Clin Oncol.
31:1097–1104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Youlden DR, Cramb SM and Baade PD: The
international epidemiology of lung cancer: Geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moasser MM: The oncogene HER2: Its
signaling and transforming functions and its role in human cancer
pathogenesis. Oncogene. 26:6469–6487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu D and Hung MC: Overexpression of ErbB2
in cancer and ErbB2-targeting strategies. Oncogene. 19:6115–6121.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan D, Deeb G, Wang J, Slocum HK, Winston
J, Wiseman S, Beck A, Sait S, Anderson T and Nwogu C: HER-2/neu
protein expression and gene alteration in stage I–IIIA
non-small-cell lung cancer: A study of 140 cases using a
combination of high throughput tissue microarray,
immunohistochemistry, and fluorescent in situ hybridization. Diagn
Mol Pathol. 12:201–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pellegrini C, Falleni M, Marchetti A,
Cassani B, Miozzo M, Buttitta F, Roncalli M, Coggi G and Bosari S:
HER-2/Neu alterations in non-small cell lung cancer: A
comprehensive evaluation by real time reverse transcription-PCR,
fluorescence in situ hybridization, and immunohistochemistry. Clin
Cancer Res. 9:3645–3652. 2003.PubMed/NCBI
|
|
9
|
Cappuzzo F, Varella-Garcia M, Shigematsu
H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V,
Gregorc V, Toschi L, et al: Increased HER2 gene copy number is
associated with response to gefitinib therapy in epidermal growth
factor receptor-positive non-small-cell lung cancer patients. J
Clin Oncol. 23:5007–5018. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swanton C, Futreal A and Eisen T:
Her2-targeted therapies in non-small cell lung cancer. Clin Cancer
Res. 12:4377s–4383s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stephens P, Hunter C, Bignell G, Edkins S,
Davies H, Teague J, Stevens C, O'Meara S, Smith R, Parker A, et al:
Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature.
431:525–526. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shigematsu H, Takahashi T, Nomura M,
Majmudar K, Suzuki M, Lee H, Wistuba II, Fong KM, Toyooka S,
Shimizu N, et al: Somatic mutations of the HER2 kinase domain in
lung adenocarcinomas. Cancer Res. 65:1642–1646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto H, Higasa K, Sakaguchi M, Shien
K, Soh J, Ichimura K, Furukawa M, Hashida S, Tsukuda K, Takigawa N,
et al: Novel germline mutation in the transmembrane domain of HER2
in familial lung adenocarcinomas. J Natl Cancer Inst.
106:djt3382014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greulich H, Kaplan B, Mertins P, Chen TH,
Tanaka KE, Yun CH, Zhang X, Lee SH, Cho J, Ambrogio L, et al:
Functional analysis of receptor tyrosine kinase mutations in lung
cancer identifies oncogenic extracellular domain mutations of
ERBB2. Proc Natl Acad Sci USA. 109:14476–14481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mar N, Vredenburgh JJ and Wasser JS:
Targeting HER2 in the treatment of non-small cell lung cancer. Lung
Cancer. 87:220–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De G, rève J, Teugels E, Geers C, Decoster
L, Galdermans D, De Mey J, Everaert H, Umelo I, In't Veld P and
Schallier D: Clinical activity of afatinib (BIBW 2992) in patients
with lung adenocarcinoma with mutations in the kinase domain of
HER2/neu. Lung Cancer. 76:123–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mazieres J, Peters S, Lepage B, Cortot AB,
Barlesi F, Beau-Faller M, Besse B, Blons H, Mansuet-Lupo A, Urban
T, et al: Lung cancer that harbors an HER2 mutation: Epidemiologic
characteristics and therapeutic perspectives. J Clin Oncol.
31:1997–2003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzawa K, Toyooka S, Sakaguchi M, Morita
M, Yamamoto H, Tomida S, Ohtsuka T, Watanabe M, Hashida S, Maki Y,
et al: Antitumor effect of afatinib, as a human epidermal growth
factor receptor 2-targeted therapy, in lung cancers harboring HER2
oncogene alterations. Cancer Sci. 107:45–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan A, Delaloge S, Holmes FA, Moy B,
Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, von Minckwitz
G, et al: Neratinib after trastuzumab-based adjuvant therapy in
patients with HER2-positive breast cancer (ExteNET): A multicentre,
randomised, double-blind, placebo-controlled, phase 3 trial. Lancet
Oncol. 17:367–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gandhi L, Besse B, Mazieres J, Waqar S,
Cortot A, Barlesi F, Quoix E, Otterson G, Ettinger D, Horn L, et
al: MA04.02 Neratinib ± temsirolimus in HER2-mutant lung cancers:
An international, randomized phase II study. J Thorac Oncol. 12
Suppl:S358–S359. 2017. View Article : Google Scholar
|
|
21
|
Mazieres J, Barlesi F, Filleron T, Besse
B, Monnet I, Beau-Faller M, Peters S, Dansin E, Früh M, Pless M, et
al: Lung cancer patients with HER2 mutations treated with
chemotherapy and HER2-targeted drugs: results from the European
EUHER2 cohort. Ann Oncol. 27:281–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gandhi J, Zhang J, Xie Y, Soh J,
Shigematsu H, Zhang W, Yamamoto H, Peyton M, Girard L, Lockwood WW,
et al: Alterations in genes of the EGFR signaling pathway and their
relationship to EGFR tyrosine kinase inhibitor sensitivity in lung
cancer cell lines. PLoS One. 4:e45762009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Girard L, Zöchbauer-Müller S, Virmani AK,
Gazdar AF and Minna JD: Genome-wide allelotyping of lung cancer
identifies new regions of allelic loss, differences between small
cell lung cancer and non-small cell lung cancer, and loci
clustering. Cancer Res. 60:4894–4906. 2000.PubMed/NCBI
|
|
24
|
Sakaguchi M, Watanabe M, Kinoshita R, Kaku
H, Ueki H, Futami J, Murata H, Inoue Y, Li SA, Huang P, et al:
Dramatic increase in expression of a transgene by insertion of
promoters downstream of the cargo gene. Mol Biotechnol. 56:621–630.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shien K, Toyooka S, Ichimura K, Soh J,
Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, et al:
Prognostic impact of cancer stem cell-related markers in non-small
cell lung cancer patients treated with induction chemoradiotherapy.
Lung Cancer. 77:162–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shien K, Toyooka S, Yamamoto H, Soh J,
Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H, et al:
Acquired resistance to EGFR inhibitors is associated with a
manifestation of stem cell-like properties in cancer cells. Cancer
Res. 73:3051–3061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engelman JA, Janne PA, Mermel C, Pearlberg
J, Mukohara T, Fleet C, Cichowski K, Johnson BE and Cantley LC:
ErbB-3 mediates phosphoinositide 3-kinase activity in
gefitinib-sensitive non-small cell lung cancer cell lines. Proc
Natl Acad Sc USA. 102:3788–3793. 2005. View Article : Google Scholar
|
|
29
|
Schwab CL, English DP, Black J, Bellone S,
Lopez S, Cocco E, Bonazzoli E, Bussi B, Predolini F, Ferrari F, et
al: Neratinib shows efficacy in the treatment of HER2 amplified
carcinosarcoma in vitro and in vivo. Gynecol Oncol. 139:112–117.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Menderes G, Bonazzoli E, Bellone S, Black
JD, Lopez S, Pettinella F, Masserdotti A, Zammataro L, Litkouhi B,
Ratner E, et al: Efficacy of neratinib in the treatment of
HER2/neu-amplified epithelial ovarian carcinoma in vitro and in
vivo. Med Oncol. 34:912017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kohsaka S, Nagano M, Ueno T, Suehara Y,
Hayashi T, Shimada N, Takahashi K, Suzuki K, Takamochi K, Takahashi
F and Mano H: A method of high-throughput functional evaluation of
EGFR gene variants of unknown significance in cancer. Sci Transl
Med. 9(pii): eaan65662017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Minami Y, Shimamura T, Shah K, LaFramboise
T, Glatt KA, Liniker E, Borgman CL, Haringsma HJ, Feng W, Weir BA,
et al: The major lung cancer-derived mutants of ERBB2 are oncogenic
and are associated with sensitivity to the irreversible EGFR/ERBB2
inhibitor HKI-272. Oncogene. 26:5023–5027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tai W, Mahato R and Cheng K: The role of
HER2 in cancer therapy and targeted drug delivery. J Control
Release. 146:264–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang SE, Narasanna A, Perez-Torres M,
Xiang B, Wu FY, Yang S, Carpenter G, Gazdar AF, Muthuswamy SK and
Arteaga CL: HER2 kinase domain mutation results in constitutive
phosphorylation and activation of HER2 and EGFR and resistance to
EGFR tyrosine kinase inhibitors. Cancer Cell. 10:25–38. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ou SI, Schrock AB, Bocharov EV, Klempner
SJ, Haddad CK, Steinecker G, Johnson M, Gitlitz BJ, Chung J,
Campregher PV, et al: HER2 transmembrane domain (TMD) mutations
(V659/G660) that stabilize homo- and heterodimerization are rare
oncogenic drivers in lung adenocarcinoma that respond to Afatinib.
J Thorac Oncol. 12:446–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eng J, Hsu M, Chaft JE, Kris MG, Arcila ME
and Li BT: Outcomes of chemotherapies and HER2 directed therapies
in advanced HER2-mutant lung cancers. Lung Cancer. 99:53–56. 2016.
View Article : Google Scholar : PubMed/NCBI
|