Introduction
Breast cancer, a high-morbidity and mortality type
of cancer, is one of the leading causes of cancer-associated deaths
among women worldwide (1,2). According to the statistics of the
American Cancer Society, 252,710 new invasive breast cancer
patients and 40,610 diseased patients were estimated in 2017
(3). Human epidermal growth factor
receptor-2 (HER-2) is a member of HER protein family, which plays
an important role in the pathogenesis of breast cancer, regulates
cyclin E and is related to poor viability (4,5).
HER-2-positive cancer is one of the most aggressive subtypes of
breast cancer, which is often associated with metastasis, poor
prognosis and short survival time (6,7). At
present, the treatment strategies for HER-2-positive breast cancer
patients include local surgery, radiotherapy, systemic
chemotherapy, biotherapy, and endocrine therapy, as well as the
primary systemic therapy (also known as HER-2-targeted therapy and
neoadjuvant therapy) which has the highest breast-conserving
surgery rate (8,9). In the present study, a multi-angle
efficacy evaluation around a primary systemic therapy was carried
out, in order to provide clinical basic data for the breast cancer
treatment.
Tratuzumab is a monoclonal antibody used to target
HER-2 and inhibit its function. Tratuzumab can be used in early and
metastatic HER-2-positive breast cancer patients (10,11).
Studies have shown that trastuzumab not only has the function of
cutting off HER-2 signal transmission, but can also change the
immune microenvironment of tumors (10,11). The
genomic characteristics of tratuzumab can predict the survival of
HER-2-positive breast cancer patients (12). Carboplatin is a chemotherapeutic
agent widely used in malignant tumors, including breast cancer,
which can be used to treat various solid malignant tumors (13,14).
Denkert et al (15) have
reported that carboplatin can enhance the interaction of
chemotherapy with host immune response, having certain clinical
benefits for HER-2-positive early breast cancer patients. Docetaxel
is a taxane with antitumor activity, which can induce cell cycle to
stagnate at G2/M, produce cytotoxicity and cause apoptosis
(16,17). At present, docetaxel is considered
one of the drugs used in the first-line combination therapy of
HER-2-positive metastatic breast cancer patients (18). The aforementioned three drugs have
been applied to the treatment of HER-2-positive early and locally
advanced breast cancer patients as a therapeutic scheme with good
tolerance, high safety and no long-term toxicity or side-effects
(19).
Currently, there are few researches on the efficacy
evaluation of the combined treatment of the three drugs for
HER-2-breast cancer patients. In the present study, the efficacy,
safety, survival and changes of relevant indicators of these drugs
were observed and analyzed.
Patients and methods
General information
A total of 180 HER-2-positive breast cancer female
patients, admitted to The First People's Hospital of Yunnan
Province (Kunming, China) from January 2013 to June 2014, were
enrolled in this study. Eighty patients were selected as the
control group (CG) and were treated with carboplatin and docetaxel,
and 100 patients were selected as the research group (RG) and were
treated with trastuzumab carboplatin and docetaxel. The patients in
the CG were 22–78 years of age with an average age of 48.9±5.7
years, and the patients in the RG were 25–79 years of age with an
average age of 50.1±5.9 years. The study was approved by the Ethics
Committee of The First People's Hospital of Yunnan Province. Signed
informed consents were obtained from the patients and/or
guardians.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients
with HER-2-positive breast cancer, as diagnosed by a
histopathological examination (20);
patients who had not received any treatment method; patients with
no history of surgery; patients that accepted a 5-year follow-up
and were willing to cooperate for this research; and patients who
had complete clinicopathological data. The exclusion criteria were
as follows: Patients with contraindications or allergic history to
the treatment; patients with breast tissue inflammation; patients
with hyperthyroidism and other diseases that could affect the
results of the study; patients with other serious organ
dysfunction; patients with malignant tumors in the past; pregnant
women. Inclusion criteria were applicable to all subjects.
Treatment methods
Patients in both groups were treated with
conventional therapy. The patients in CG received intravenous drip
of carboplatin with AUC=5 mg/ml/min and docetaxel with AUC=75
mg/m2 (J55611 and J43650, respectively; Shanghai Jinsui
Biotechnology Co., Ltd.;) once every 3 weeks for 4–6 cycles of
treatment. The patients in RG received intravenous drip of
carboplatin, docetaxel and trastuzumab (TM-Tras-00002_1; Shanghai
TheraMabs Biotechnology Co., Ltd.), 8 mg/kg for the first time and
6 mg/kg for the second time, once every 3 weeks, for 17 cycles of
treatment.
Efficacy evaluation
Clinical efficacy was evaluated according to the
efficacy evaluation criteria of solid tumors (RECIST version 1.1)
(21): Complete remission (CR) was
considered when the lesion completely disappeared and the duration
was ≥4 weeks. Partial remission (PR) was considered when the
reduction of the lesion's longest diameter was ≥30% and the
duration was ≥4 weeks. The occurrence of new lesions and increase
of lesion length ≥20% was considered as progressive disease (PD).
Stable disease (SD) was considered when the long diameter of
lesions decreased or increased, and the disease could be
characterized between PR and PD. According to the efficacy of both
groups, CR and PR were defined as effective and the total effective
rate was calculated as (CR + PR)/(total no. of cases) ×100%.
Pathological efficacy was evaluated with reference
to the Miller-Payne grading system (22) and was defined according to the lesion
density or the reduction percentage of the reaction volume:
G1 was 0%, G2 was <33%, G3 was
33–66%, G4 was 67–99% and G5 was 100%.
G1 to G5 describe the condition of tumor
cells in lesions from no improvement to necrosis or disappearance.
According to the efficacy of both groups, G3,
G4 and G5 were defined as effective and the
total effective rate was calculated as (G3 +
G4 + G5)/(total no. of cases) ×100%.
Observational indicators
Clinical efficacy, pathological efficacy, adverse
reactions, inflammatory factors interleukin-6 (IL-6) and tumor
necrosis factor-α (TNF-α), cellular immune indexes T-lymphocyte
subsets (CD4+, CD8+,
CD4+/CD8+), and the oxidative stress indexes
superoxide dismutase (SOD) and myeloperoxidase (MPO) before and
after treatment, as well as the 5-year disease-free survival (DFS)
and overall survival (OS) of patients, were observed and compared
between the two groups.
Detection methods
Before and after treatment, 3 ml of elbow venous
blood were collected from the patients of both groups, and placed
into anticoagulant-free and EDTA-K2 blood collection vessels. The
peripheral blood T-lymphocyte subsets were detected by flow
cytometry. A total of 10 µl of fluorescein isothiocyanate
(FITC)-labeled fluorescent monoclonal antibody (CD4-FITC/CD8-ECD)
(1:200; RM25013 and 737659, respectively; China Shanghai Haoran
Biotechnology Co., Ltd.) were added to each test tube, and 100 µl
of venous blood from the EDTA-K2 blood collection vessel were added
and mixed evenly. The samples were left in the dark at room
temperature for 20 min. Next, 500 µl of red blood cell lysis buffer
were added to lyse erythrocytes, and the samples were left in the
dark at room temperature for 15 min; 500 µl of PBS buffer were
added and mixed well, and the mixture was left at room temperature
for 10 min in the dark. Samples were detected by a flow cytometer
(NovoCyte™; ACEA Biosciences, Inc.), and the CD4+,
CD8+ and CD4+/CD8+ data were
analyzed using CellQuest software (Becton, Dickinson and Co.).
The venous blood in the anticoagulant-free blood
collection vessel was placed on a centrifuge and was centrifuged at
1,500 × g at 4°C for 10 min. The separated upper serum was stored
in a refrigerator at −20°C for later use. The concentrations of
serum IL-6, TNF-α, SOD and MPO were detected by ELISA (23), according to the manufacturer's
instructions of IL-6, TNF-α, SOD and MPO detection kits (Shanghai
Fanke Biotechnology Co., Ltd.). Blank wells (without any reagent),
standard wells and sample wells to be tested were set up. A total
of 50 liters of standard substance were added to the standard
substance well, 50 liters of sample were added to the sample wells,
and 50 liters of streptavidin-HRP were added to each well. The
plate was sealed and the temperature was kept at 37°C for 60 min.
Liquid was discarded, and the plate was washed by washing liquid,
and then spin-dried. This procedure was repeated 5 times. Developer
A (50 liters) and developer B (50 liters) were added to each well
and mixed well, and the wells were placed stably in the dark at
37°C for 10 min. Next, 50 µl of stop solution were added to each
well. BioTek full-automatic microplate reader (800TS; Shanghai
BioExcellence Co., Ltd.) was used. The blank wells were zeroed and
the absorbance value (OD value) of each well was sequentially
measured at 450 nm wavelength. The concentrations of IL-6, TNF-α,
SOD, and MPO were calculated.
Follow-up
Patients were followed up 4 times/year for 5 years
through telephone calls, visits and consulting pathological data.
DFS time was defined as the period from the first treatment of the
patient till the first relapse of the disease, and OS time was
defined as the period from the diagnosis of the disease till the
death of the patient or the last follow-up day.
Statistical analysis
GraphPad Prism 6 software (GraphPad Software, Inc.)
was used for the statistical analysis of the data, and the
production and analysis of the figures. Counting data were
expressed by the number of cases and percentage [n (%)], and
Chi-square test was used for their comparisons between groups.
Measurement data were expressed as the mean ± SD and their
comparison between two groups was conducted by the independent
samples t-test, whereas for the comparisons between multiple groups
ANOVA with LSD post hoc test were carried out. Kaplan-Meier method
was used to analyze the DFS and OS of the patients, and log-rank
test was used to evaluate the differences in survival time between
the two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinicopathological data
There was no significant difference between the two
groups in age, menstrual status, histological grade, hormone
receptor status, T staging, N staging, TNM staging, breast surgery,
adjuvant endocrine therapy, postoperative radiotherapy, tumor
diameter, or place of residence (P>0.05) (Table I).
 | Table I.Comparison of clinicopathological
characteristics of patients in the two groups [n (%), mean ±
SD]. |
Table I.
Comparison of clinicopathological
characteristics of patients in the two groups [n (%), mean ±
SD].
| Clinicopathological
characteristics | Control group
(n=80) | Research group
(n=100) | χ2/t
value | P-value |
|---|
| Age (years) |
|
| 0.180 | 0.671 |
|
<35 | 8 (10.00) | 12 (12.00) |
|
|
| ≥35 | 72 (90.00) | 88 (88.00) |
|
|
| Menstrual status |
|
| 0.422 | 0.650 |
| Before
menopause | 29 (36.25) | 41 (41.00) |
|
|
| After
menopause | 51 (63.75) | 59 (59.00) |
|
|
| Histological
classification |
|
| 0.321 | 0.852 |
| I | 2 (2.50) | 4 (4.00) |
|
|
| II | 23 (28.75) | 29 (29.00) |
|
|
| III | 55 (68.75) | 67 (67.00) |
|
|
| Hormone receptor
status |
|
| 1.608 | 0.205 |
|
Positive | 34 (42.50) | 52 (52.00) |
|
|
|
Negative | 46 (57.50) | 48 (48.00) |
|
|
| T staging |
|
| 0.302 | 0.583 |
| 1/2 | 71 (88.75) | 86 (86.00) |
|
|
|
3/4 | 9 (11.25) | 14 (14.00) |
|
|
| N staging |
|
| 0.384 | 0.825 |
| 0 | 35 (43.75) | 41 (41.00) |
|
|
| 1 | 34 (42.50) | 42 (42.00) |
|
|
| 2 | 11 (13.75) | 17 (17.00) |
|
|
| TNM staging |
|
| 1.381 | 0.501 |
| I | 2 (2.50) | 5 (5.00) |
|
|
| II | 60 (75.00) | 68 (68.00) |
|
|
|
III | 18 (22.50) | 27 (27.00) |
|
|
| Breast surgery |
|
| 0.497 | 0.481 |
|
Breast-conserving | 7 (8.75) | 12 (12.00) |
|
|
| Total
removal | 73 (91.25) | 88 (88.00) |
|
|
| Adjuvant endocrine
therapy |
|
| 0.360 | 0.549 |
|
Yes | 42 (52.50) | 48 (48.00) |
|
|
| No | 38 (47.50) | 52 (52.00) |
|
|
| Postoperative
radiotherapy |
|
| 0.120 | 0.729 |
|
Yes | 50 (62.50) | 65 (65.00) |
|
|
| No | 30 (37.50) | 35 (35.00) |
|
|
| Tumor diameter
(cm) | 4.37±1.35 | 4.49±1.47 | 0.564 | 0.573 |
| Place of
residence |
|
| 0.587 | 0.444 |
|
Countryside | 26 (32.50) | 38 (38.00) |
|
|
| Cities
and towns | 54 (67.50) | 62 (62.00) |
|
|
Comparison of clinical efficacy
Clinical efficacy results for the CG showed that CR,
PR, SD and PD cases were 15, 27, 20 and 18, respectively, with a
total effective rate of 52.50%. The clinical efficacy results for
the RG showed that CR, PR, SD and PD cases were 18, 58, 18 and 6,
respectively, with a total effective rate of 76.00%. The total
effective rate in the RG was significantly higher than that of the
CG (P<0.001) (Table II).
 | Table II.Comparison of clinical efficacy
between the two groups before and after treatment [n (%)]. |
Table II.
Comparison of clinical efficacy
between the two groups before and after treatment [n (%)].
| Group | n | CR | PR | SD | PD | Total efficacy |
|---|
| CG | 80 | 15 (18.75) | 27 (33.75) | 20 (25.00) | 18 (22.50) | 52.50% |
| RG | 100 | 18 (18.00) | 58 (58.00) | 18 (18.00) | 6 (6.00) | 76.00% |
| χ2
value | – | – | – | – | – | 10.870 |
| P-value | – | – | – | – | – | 0.001 |
Comparison of pathological
efficacy
Pathological efficacy results for the CG showed that
G1, G2, G3, G4 and
G5 cases were 20, 28, 18, 12 and 2, respectively, with a
total effective rate of 40.00%. The pathological efficacy results
for the RG showed that G1, G2, G3,
G4 and G5 cases were 13, 24, 33, 22 and 8,
respectively, with a total effective rate of 63.00%. The total
effective rate in the RG was significantly higher than that of the
CG (P<0.01) (Table III).
 | Table III.Comparison of pathological efficacy
between the two groups before and after treatment [n (%)]. |
Table III.
Comparison of pathological efficacy
between the two groups before and after treatment [n (%)].
| Group | n | G1 | G2 | G3 | G4 | G5 | Total efficacy |
|---|
| CG | 80 | 20 (25.00) | 28 (35.00) | 18 (22.50) | 12 (15.00) | 2 (2.50) | 40.00% |
| RG | 100 | 13 (13.00) | 24 (24.00) | 33 (33.00) | 22 (22.00) | 8 (8.00) | 63.00% |
| χ2
value | – | – | – | – | – | – | 9.434 |
| P-value | – | – | – | – | – | – | 0.002 |
Comparison of adverse reactions
After treatment, the adverse reactions in both
groups of patients included of III–IV degree myelosuppression,
III–IV degree gastrointestinal reaction, liver function damage,
cardiac toxicity, and peripheral neurotoxicity. The main adverse
reactions were III–IV degree myelosuppression, III–IV degree
gastrointestinal reaction, and peripheral neurotoxicity. The
incidence rate of adverse reactions in both groups of patients had
no significant difference (P>0.05) (Table IV).
 | Table IV.Comparison of the adverse reactions
in the two groups [n (%)]. |
Table IV.
Comparison of the adverse reactions
in the two groups [n (%)].
| Category | CG (n=80) | RG (n=100) | χ2
value | P-value |
|---|
| III–IV degree
myelosuppression | 20 (25.00) | 28 (28.00) | 0.205 | 0.651 |
| III–IV degree
gastrointestinal reaction | 9 (11.25) | 13 (13.00) | 0.127 | 0.722 |
| Liver function
damage | 6 (7.50) | 8 (8.00) | 0.010 | 0.920 |
| Cardiac
toxicity | 0 (0.00) | 1 (1.00) | 0.804 | 0.370 |
| Peripheral
neurotoxicity | 9 (11.25) | 12 (12.00) | 0.024 | 0.876 |
Changes of the levels of inflammatory
factors
There was no significant difference in the levels of
inflammatory factors between RG and CG before treatment
(P>0.05). After treatment, IL-6 and TNF-α levels decreased
significantly in both groups (P<0.001), and the levels in RG
were significantly lower than those in CG (P<0.001) (Fig. 1).
Changes of cellular immune
indexes
There was no significant difference in cellular
immune indexes between RG and CG before treatment (P>0.05).
After treatment, CD4+, CD4+/CD8+
levels increased significantly in both groups (P<0.001), and the
levels in RG were significantly higher than those in CG
(P<0.001). However, CD8+ decreased significantly in
both groups after treatment (P<0.001), and CD8+ level
was significantly lower in RG than that in CG (P<0.01) (Fig. 2).
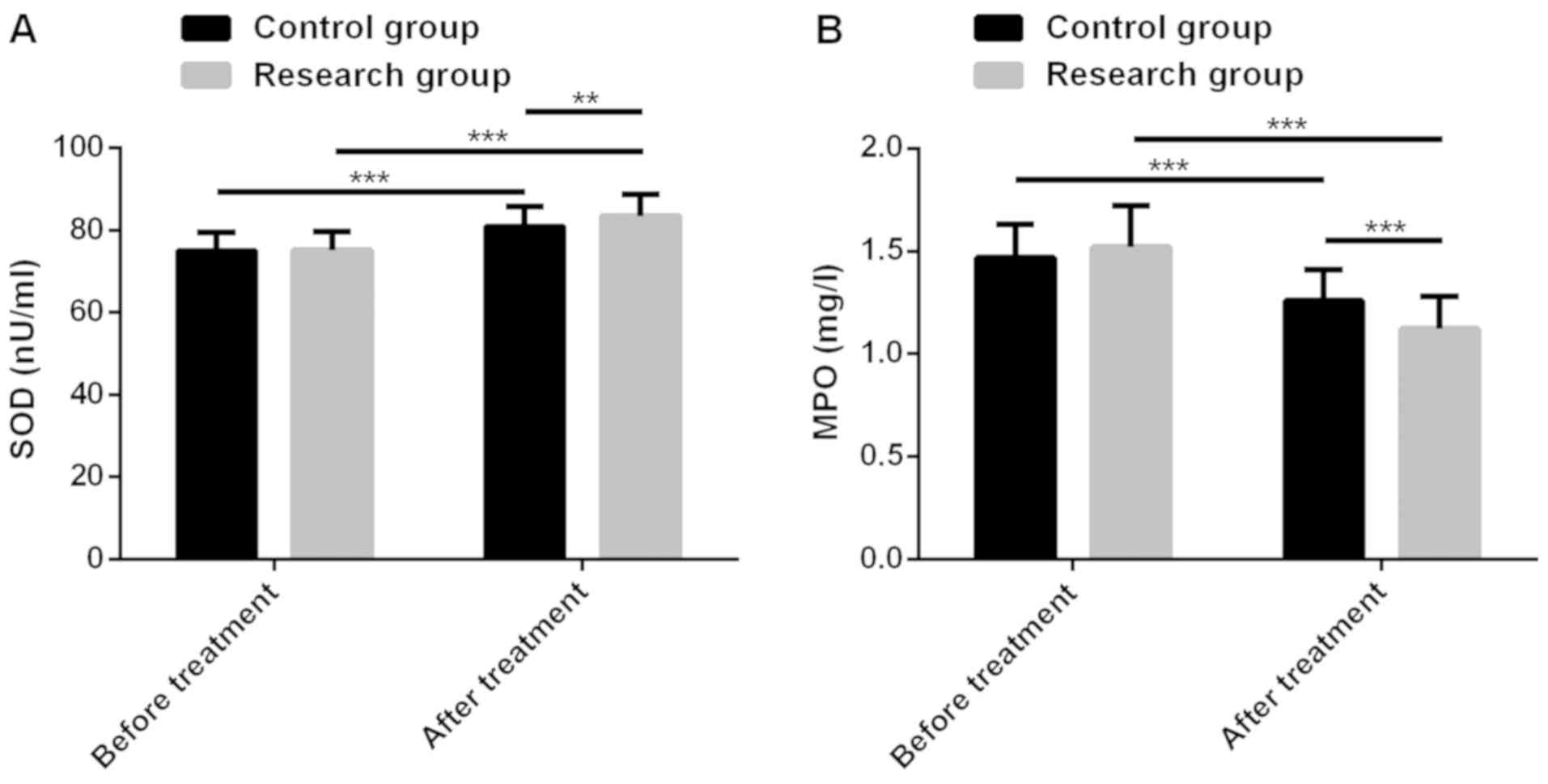
Changes of oxidative stress
indexes
There was no significant difference in oxidative
stress indexes between RG and CG before treatment (P>0.05).
After treatment, SOD of patients in both groups increased
significantly (P<0.001), and SOD level in RG was significantly
higher than that in CG (P<0.01). However, MPO in both groups
after treatment reduced significantly (P<0.001), and MPO level
in RG was significantly lower than that in CG (P<0.001)
(Fig. 3).
Survival analysis
A total of 180 patients were successfully followed
up for 5 years. The 5-year DFS and OS of RG were significantly
higher than those of CG (P<0.05) (Fig. 4).
Discussion
Inflammatory response in tumor microenvironment is
related to poor prognosis in breast cancer, which may be related to
the involvement of inflammatory mediators in stimulating
proliferation, invasion and angiogenesis of breast cancer cells
(24). Thriveni et al
(25) have reported that TNF-α is
expressed in the plasma of patients with invasive cancer diseases
at high levels, as an inflammatory microenvironment marker for
patients with primary breast cancer. IL-6 is an inflammatory medium
related to the activity of cancer cells. The amplification or
overexpression of IL-6 has a certain predictive ability for the
recurrence of estrogen or progesterone receptor-positive breast
cancer in females (26,27). Co-expression of serum IL-6 and TNF-α
can be used as an effective tumor marker for tumor invasion and
breast cancer prognosis (28). In
the present study, the levels of inflammatory factors IL-6 and
TNF-α of patients in both groups were significantly reduced after
treatment, and the levels in the RG were significantly lower than
those in CG, indicating that trastuzumab, carboplatin and docetaxel
have better recovery effect for patients with inflammatory
imbalance. Seo et al (29)
have considered that the infiltration of T-lymphocyte subsets is
tied to the phenotype of breast cancer stem cells and
epithelial-mesenchymal transformation. T-lymphocyte subsets, as
indicators of immune function, have a certain predictive ability
for tumor progression and lymph node metastasis. CD4 and
CD4+/CD8+ have been reported to be negatively
correlated with breast cancer tumor progression whereas, a positive
correlation has been reported for CD8+ (30). In the present study, CD4+
and CD4+/CD8+ had higher expression levels in
RG after treatment, while CD8+ expression level was
lower in RG after treatment, indicating that trastuzumab,
carboplatin and docetaxel have better effect on relieving
immunosuppression in patients. Oxidative stress balance is involved
in the occurrence and development of breast cancer. Oxidation and
anti-oxidant preparations play an important part in the regulation
of breast cancer. Low level SOD has been linked to the occurrence
and development of breast cancer (31). Recently, studies have also reported
that SOD mimetics, which mimic SOD performance, have inhibitory
effects on the migration, invasion and angiogenesis of breast
cancer cells and can be used as drugs in redox therapy for breast
cancer (32). MPO is an endogenous
metabolic/oxidative lysosomal enzyme secreted by neutrophils and
monocytes, which can play a crucial role in tumor invasion by
activating carcinogens into genotoxic intermediates and then
enhancing xenogenic carcinogenicity (33). As reported, breast cancer patients
have higher MPO levels, which may also reflect the oxidative stress
imbalance of the disease (34). As
to the role of oxidative stress in breast cancer, Zapf et al
(35) have stated that breast cancer
patients have low levels of SOD and high levels of MPO, and simple
chemotherapy would aggravate the oxidative stress levels of both.
In the present study, the patients of the RG had higher level of
SOD and lower level of MPO after treatment, suggesting that
trastuzumab, carboplatin and docetaxel have more gratifying effects
on reversing the oxidative stress imbalance of the patients.
The results of the study revealed that the clinical
efficacy and pathological efficacy in the RG were significantly
higher than those in the CG, and there was no significant
difference in the incidence rate of adverse reactions between the
two groups. Finally, a 5-year follow-up of the patients in both
groups was conducted. Compared with the patients in the CG, the
patients in the RG had higher 5-year DFS and OS, indicating that
trastuzumab, carboplatin and docetaxel could significantly improve
the 5-year DFS and OS of patients.
The present study also confirmed the clinical
benefits of trastuzumab, carboplatin and docetaxel; however, there
is still room for improvement. First of all, further research could
be conducted on cell biology and the specific regulatory mechanism
on breast cancer cells. Secondly, a larger sample size should be
included in order to improve the accuracy of the research
results.
In conclusion, trastuzumab, carboplatin and
docetaxel can be potentially used in clinic for the HER-2 positive
breast cancer treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW and LX conceived and designed the study. DW and
LX were responsible for the collection, analysis and interpretation
of the data. DW drafted the manuscript. LX revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First People's Hospital of Yunnan Province (Kunming, China).
Signed informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asif HM, Sultana S, Ahmed S, Akhtar N and
Tariq M: HER-2 positive breast cancer-a mini-review. Asian Pac J
Cancer Prev. 17:1609–1615. 2016.PubMed/NCBI
|
|
2
|
Ghislain I, Zikos E, Coens C, Quinten C,
Balta V, Tryfonidis K, Piccart M, Zardavas D, Nagele E,
Bjelic-Radisic V, et al European Organisation for Research and
Treatment of Cancer (EORTC) Quality of Life Group and Breast Cancer
Group; EORTC Headquarters, : Health-related quality of life in
locally advanced and metastatic breast cancer: Methodological and
clinical issues in randomised controlled trials. Lancet Oncol.
17:e294–e304. 2016.PubMed/NCBI
|
|
3
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017.PubMed/NCBI
|
|
4
|
Suzawa K, Toyooka S, Sakaguchi M, Morita
M, Yamamoto H, Tomida S, Ohtsuka T, Watanabe M, Hashida S, Maki Y,
et al: Antitumor effect of afatinib, as a human epidermal growth
factor receptor 2-targeted therapy, in lung cancers harboring HER2
oncogene alterations. Cancer Sci. 107:45–52. 2016.PubMed/NCBI
|
|
5
|
Luhtala S, Staff S, Tanner M and Isola J:
Cyclin E amplification, over-expression, and relapse-free survival
in HER-2-positive primary breast cancer. Tumour Biol. 37:9813–9823.
2016.PubMed/NCBI
|
|
6
|
Grosset AA, Poirier F, Gaboury L and
St-Pierre Y: Galectin-7 expression potentiates her-2-positive
phenotype in breast cancer. PLoS One. 11:e01667312016.PubMed/NCBI
|
|
7
|
Yang F, Lyu S, Dong S, Liu Y, Zhang X and
Wang O: Expression profile analysis of long noncoding RNA in
HER-2-enriched subtype breast cancer by next-generation sequencing
and bioinformatics. OncoTargets Ther. 9:761–772. 2016.
|
|
8
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast cancer version 2.2015. J Natl Compr Canc
Netw. 13:448–475. 2015.PubMed/NCBI
|
|
9
|
Ahmed S, Sami A and Xiang J: HER2-directed
therapy: Current treatment options for HER2-positive breast cancer.
Breast Cancer. 22:101–116. 2015.PubMed/NCBI
|
|
10
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–11663. 2015.PubMed/NCBI
|
|
11
|
Perez EA, Barrios C, Eiermann W, Toi M, Im
YH, Conte P, Martin M, Pienkowski T, Pivot X, Burris H III, et al:
Trastuzumab emtansine with or without pertuzumab versus trastuzumab
plus taxane for human epidermal growth factor receptor 2-positive,
advanced breast cancer: Primary results from the phase III MARIANNE
study. J Clin Oncol. 35:141–148. 2017.PubMed/NCBI
|
|
12
|
Hendricks WPD, Briones N, Halperin RF,
Facista S, Heaton PR, Mahadevan D and Kim S: Genomic signature of
trastuzumab neoadjuvant therapy predictive of patient survival in
HER2-positive breast cancer. Cancers (Basel).
11:E15662019.PubMed/NCBI
|
|
13
|
Ho GY, Woodward N and Coward JI: Cisplatin
versus carboplatin: Comparative review of therapeutic management in
solid malignancies. Crit Rev Oncol Hematol. 102:37–46.
2016.PubMed/NCBI
|
|
14
|
Theile D: Under-reported aspects of
platinum drug pharmacology. Molecules. 22:3822017.
|
|
15
|
Denkert C, Loibl S, Salat C, Sinn BV,
Schem C, Endris V, Klare P, Schmitt WD, Blohmer JU, Weichert W, et
al: Abstract S1-06: Increased tumor-associated lymphocytes predict
benefit from addition of carboplatin to neoadjuvant therapy for
triple-negative and HER2-positive early breast cancer in the
GeparSixto trial (GBG 66). Cancer Res. 73:(abstract nr S1-06).
2013.
|
|
16
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114.
1999.PubMed/NCBI
|
|
17
|
Lyseng-Williamson KA and Fenton C:
Docetaxel: A review of its use in metastatic breast cancer. Drugs.
65:2513–2531. 2005.PubMed/NCBI
|
|
18
|
Leung HW, Chan AL, Muo CH and Leung JH:
Cost-effectiveness of pertuzumab combined with trastuzumab and
docetaxel as a first-line treatment for HER-2 positive metastatic
breast cancer. Expert Rev Pharmacoecon Outcomes Res. 18:207–213.
2018.PubMed/NCBI
|
|
19
|
Echavarria I, Granja M, Bueno C,
Lopez-Tarruella S, Peinado P, Sotelo M, Jerez Y, Moreno F, Torres
G, Lobo M, et al: Multicenter analysis of neoadjuvant docetaxel,
carboplatin, and trastuzumab in HER2-positive breast cancer. Breast
Cancer Res Treat. 162:181–189. 2017.PubMed/NCBI
|
|
20
|
Kataja V and Castiglione M; ESMO
Guidelines Working Group, : Primary breast cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 (Suppl 4):10–14. 2009.PubMed/NCBI
|
|
21
|
Keil S, Barabasch A, Dirrichs T, Bruners
P, Hansen NL, Bieling HB, Brümmendorf TH and Kuhl CK: Target lesion
selection: An important factor causing variability of response
classification in the Response Evaluation Criteria for Solid Tumors
1.1. Invest Radiol. 49:509–517. 2014.PubMed/NCBI
|
|
22
|
Shintia C, Endang H and Diani K:
Assessment of pathological response to neoadjuvant chemotherapy in
locally advanced breast cancer using the Miller-Payne system and
TUNEL. Malays J Pathol. 38:25–32. 2016.PubMed/NCBI
|
|
23
|
Hornbeck PV: Enzyme-linked immunosorbent
assays. Curr Protoc Immunol. 110:2.1.1–23. 2015.
|
|
24
|
Cho YA, Sung MK, Yeon JY, Ro J and Kim J:
Prognostic role of interleukin-6, interleukin-8, and leptin levels
according to breast cancer subtype. Cancer Res Treat. 45:210–219.
2013.PubMed/NCBI
|
|
25
|
Thriveni K, Raju A, Ramaswamy G,
Shachidevi Krishnamurthy S and Kumar R: Tumor necrosis factor: An
inflammatory microenvironment marker in primary breast cancer
patients. IJMIO. 3:252018.
|
|
26
|
Drygin D, Ho CB, Omori M, Bliesath J,
Proffitt C, Rice R, Siddiqui-Jain A, O'Brien S, Padgett C, Lim JK,
et al: Protein kinase CK2 modulates IL-6 expression in inflammatory
breast cancer. Biochem Biophys Res Commun. 415:163–167.
2011.PubMed/NCBI
|
|
27
|
Muss HB, Bunn JY, Crocker A, Plaut K, Koh
J, Heintz N, Rincon M, Weaver DL, Tam D, Beatty B, et al: Cyclin
D-1, interleukin-6, HER-2/neu, transforming growth factor
receptor-II and prediction of relapse in women with early stage,
hormone receptor-positive breast cancer treated with tamoxifen.
Breast J. 13:337–345. 2007.PubMed/NCBI
|
|
28
|
Tripsianis G, Papadopoulou E,
Anagnostopoulos K, Botaitis S, Katotomichelakis M, Romanidis K,
Kontomanolis E, Tentes I and Kortsaris A: Coexpression of IL-6 and
TNF-α: Prognostic significance on breast cancer outcome. Neoplasma.
61:205–212. 2014.PubMed/NCBI
|
|
29
|
Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH,
Lee HE, Kim YJ, Kim JH and Park SY: Tumour-infiltrating
CD8+ lymphocytes as an independent predictive factor for
pathological complete response to primary systemic therapy in
breast cancer. Br J Cancer. 109:2705–2713. 2013.PubMed/NCBI
|
|
30
|
Wang B and Pan F: Analysis of T lymphocyte
subsets in peripheral blood of patients with breast cancer and its
clinical value. Int J Lab Med. 39:1230–1232, 1237. 2018.
|
|
31
|
Sahu A, Varma M and Kachhawa K: A
prognostic study of MDA, SOD and catalase in breast Cancer
patients. Int J Sci Res (Ahmedabad). 4:157–159. 2015.
|
|
32
|
Fernandes AS, Saraiva N and Oliveira NG:
Redox therapeutics in breast cancer: Role of SOD mimics.
Redox-Active Therapeutics. Batinic-Haberle I, Reboucas JS and
Spasojevic I: Springer. (Cham, Switzerland). 451–467. 2016.
|
|
33
|
Yang WJ, Wang MY, Pan FZ, Shi C and Cen H:
Association between MPO-463G>A polymorphism and cancer risk:
Evidence from 60 case-control studies. World J Surg Oncol.
15:1442017.PubMed/NCBI
|
|
34
|
Abusoglu S, Eryavuz D, Bal C, Nural C,
Ozcan E, Yildirimel M, Celik S and Unlu A: Assessment of serum
ischemia-modified albumin, prolidase and thiol-disulphide levels in
subjects with breast cancer. Rev Rom Med Lab. 27:25–33. 2019.
|
|
35
|
Zapf I, Moezzi M, Fekecs T, Nedvig K,
Lőrinczy D and Ferencz A: Influence of oxidative injury and
monitoring of blood plasma by DSC on breast cancer patients. J
Therm Anal Calorim. 123:2029–2035. 2016.
|