Introduction
Thyroid cancer is the most common malignancy of the
thyroid, with overall incidence rate of 15.04 per 100,000
individuals reported in 2015 (1).
Among the types of thyroid cancer, differentiated thyroid cancer
(DTC) accounts for 90–95% of all cases (2). The major types of DTC are papillary
thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC)
(3). Multiple treatments are applied
for DTC, including surgical treatment, thyroid-stimulating hormone
suppression, radioiodine, molecular-targeted radiation and
biological therapy (4,5). The development of individualized,
dynamic clinical treatment has greatly improved the survival rate
of patients with DTC, with the 10-year survival rate reaching 90%
in U.S over the past 40 years, according to the American Joint
Committee on Cancer (AJCC) Cancer Staging Manual (8th edition)
(6). However, a number of patients
with DTC still exhibit poor survival due to the lack of effective
treatment (7).
A high percentage of mortality from DTC is
associated with distant metastasis (DM), which is identified at
diagnosis in 2–5% of patients with DTC (8,9). The
lung parenchyma is the most common site of DM, followed by the
bone, brain, liver, kidney and skin (10,11).
Therefore, the detection of DM in patients with DTC at an early
stage is important for further improving the survival rate.
According to previous studies, the presence of
extrathyroidal extension (ETE) in patients with DTC, especially
gross ETE, may be used to evaluate postoperative therapeutic
strategies and is associated with the survival of patients with DM
(12,13). In addition, the survival rates of
patients with PTC and FTC have been reported to be significantly
associated with DM (14,15). Lymph node metastasis (LNM) status and
tumor size are also associated with DM in patients with PTC
(16,17). Previous studies have also identified
ETE and LNM status as independent risk factors for DM in patients
with DTC (18–20). However, the combined effect of these
factors, especially that of ETE with the other factors, remains
uncertain. Therefore, the present study aimed to investigate the
synergistic effect between ETE and tumor size, histologic subtype
or LNM status on the incidence of DM in patients with DTC, which
may help clinicians to better assess the prognosis of patients and
select appropriate treatment strategies.
Materials and methods
Data collection
The retrospective protocol of this study was
approved by the Ethics Committee of Zhongnan Hospital of Wuhan
University (Wuhan, China). Patient data were obtained from the
Surveillance, Epidemiology, and End Results (SEER) Program of the
National Cancer Institute (https://seer.cancer.gov/about/overview.html). The
study cohort included patients with a diagnosis of DTC (PTC or FTC)
according to the International Classification of Oncological
Diseases, 3rd edition (21), as the
index (first) diagnosed malignancy. Patients with Hürthle cell
thyroid carcinoma, poorly differentiated thyroid carcinoma,
anaplastic (undifferentiated) carcinoma, medullary thyroid cancer,
thyroid lymphoma, thyroid cancer arising from a thyroglossal duct
cyst and thyroid cancer in malignant struma ovarii were excluded
from the study. Patients with incomplete follow-up or missing data
were also excluded. Data were extracted using the SEER* Stat
software (version 8.3.2; Surveillance Research Program, National
Cancer Institute; www.seer.cancer.gov/seerstat) and imported into a
Microsoft Excel spreadsheet (Microsoft Corporation). Information on
clinicopathological characteristics, primary tumor and treatment
strategies were obtained from 96,788 patients with DTC between
January 2004 and December 2013. Informed consent was obtained from
each patient following a full explanation of the purpose and nature
of the cancer incidence and survival data used, and patient records
were anonymized and de-identified prior to analysis.
Patients were categorized by sex (male or female),
ethnicity (white, black, other or unknown) and age at diagnosis
(<55 or ≥55 years) (22). In
addition, patients were categorized by pathological features such
as tumor size (≤10 or >10 mm) (22), LNM status (N0 or N1 stage), DM (M0 or
M1 phase), multifocality, histologic subtype (PTC or FTC) and
presence of ETE. Patients were also grouped according to treatment,
including radiation therapy (none or refused, radiation
beam/radioactive implants or radioisotopes/radiation beam plus
isotopes/implants) and surgery (none, lobectomy,
subtotal/near-total thyroidectomy or total thyroidectomy).
Statistical analysis
Data were imported to SPSS (version 21.0; IBM Corp.)
for statistical analysis. Patients with DTC were divided into DM
and non-DM groups. To assess the independent risk factors affecting
DM, clinicopathological characteristics were compared using the
χ2 test for univariate analysis; binary logistic
regression analysis was performed for the relevant variables (age,
sex, ethnicity, tumor size, N stage, histology subtype,
extrathyroidal extension).
The association between DM incidence and risk
factors, including tumor size, histologic subtype and ETE, was
assessed using logistic regression analysis with adjustment for
other cofounding variables. In addition, 95% confidence intervals
(CIs) were calculated precisely. Patients were divided into
different groups based on the combination of ETE with the three
other factors. The relative excess risk due to interaction (RERI),
attributable proportion (AP) due to interaction and synergy index
(SI) were calculated to evaluate the synergistic effect of these
factors on DM in patients with DTC, where RERI>0, AP>0 or
S>1 indicated a synergistic effect. No additive synergistic
effect was indicated by the 95% CIs of RERI and AP=0 and SI=1. The
Kaplan-Meier method and log-rank test were used to evaluate the
influence of the four factors on patient survival.
P-values were two-sided, and P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS version 21.0 (IBM
Corp.), R statistical software (R Core Development Team), and
GraphPad Prism version 6 (GraphPad Software, Inc.).
Results
General characteristics of the study
population
Of the 96,788 patients with DTC, 60,841 (62.9%) were
aged <55 years and 74,325 (76.8%) were female. Of all patients,
79,063 (82.7%) were white, 6,299 (6.6%) were black and 10,246
(10.7%) were of other ethnicities. In addition, 37,584 (39.9%)
patients exhibited multifocality, 5,718 (5.9%) had FTC, 31,525
(34.3%) exhibited tumors <10 mm, 15,339 (16.0%) exhibited ETE,
20,963 (21.9%) were diagnosed with LNM and 1,488 (1.5%) exhibited
DM. All demographic and clinicopathological characteristics are
presented in Table I.
 | Table I.Demographic and clinicopathological
characteristics of 96,788 patients with differentiated thyroid
carcinoma. |
Table I.
Demographic and clinicopathological
characteristics of 96,788 patients with differentiated thyroid
carcinoma.
|
Characteristics | N (%) |
|---|
| Age at diagnosis,
years |
|
| Mean
(range) | 49 (2–105) |
| <55
years | 60,841 (62.9) |
| ≥55
years | 35,947 (37.1) |
| Sex |
|
|
Female | 74,325 (76.8) |
|
Male | 22,463 (23.2) |
| Ethnicity |
|
|
White | 79,063 (82.7) |
|
Black | 6,299 (6.6) |
|
Other | 10,246 (10.7) |
| Tumor size
(mm) |
|
| Mean
(range) | 18.59 (0–988) |
| ≤10
mm | 31,525 (34.3) |
| >10
mm | 60,451 (65.7) |
| N stage |
|
| N0 | 74,693 (78.1) |
| N1 | 20,963 (21.9) |
| Distant
metastasis | 1,488 (1.5) |
| Multifocality | 37,584 (39.9) |
| Histologic
subtype |
|
|
PTC | 91,070 (94.1) |
|
FTC | 5,718 (5.9) |
| Extrathyroidal
extension | 15,339 (16.0) |
| Radiation |
|
| None or
refused | 48,030 (50.8) |
|
Radiation beam/radioactive
implants | 1,831 (1.9) |
|
Radioisotopes/radiation beam
plus isotopes/implants | 44,761 (47.3) |
| Surgery |
|
|
Lobectomy | 13,723 (14.7) |
|
Subtotal/near-total
thyroidectomy | 3,572 (3.8) |
| Total
thyroidectomy | 76,075 (81.5) |
Factors associated with DM in DTC
The results of the univariate analysis (Table II) revealed that patients with DTC
with large tumor size (>10 mm; P<0.001), ETE (P<0.001) or
LNM (P<0.001) were more likely to develop DM. In addition,
compared with patients with PTC, those with FTC exhibited an
increased risk of DM (P<0.001). DM in patients with DTC was also
associated with age, sex, ethnicity and multifocality (all
P<0.001).
 | Table II.Association between
clinicopathological factors and distant metastasis in
differentiated thyroid cancer. |
Table II.
Association between
clinicopathological factors and distant metastasis in
differentiated thyroid cancer.
| Characteristic | Group | M0 N (%)
(N=95,300) | M1 N (%)
(N=1,488) | P-value |
|---|
| Age, years |
| 49.31±15.267 | 60.54±18.613 | <0.001 |
|
| <55 | 60,384 (99.2) | 457 (0.8) |
|
|
| ≥55 | 34,916 (97.1) | 1,031 (2.9) |
|
| Sex | Female | 73,488 (98.9) | 837 (1.1) | <0.001 |
|
| Male | 21,812 (97.1) | 651 (2.9) |
|
| Ethnicity | White | 77,962 (98.6) | 1,101 (1.4) | <0.001 |
|
| Black | 6,160 (97.8) | 139 (2.2) |
|
|
| Other | 10,001 (97.6) | 245 (2.4) |
|
| Tumor size, mm |
| 18.33±19.18 | 38.34±28.03 | <0.001 |
|
| ≤10 mm | 31,387 (99.6) | 138 (0.4) |
|
|
| >10 mm | 59,413 (98.3) | 1,038 (1.7) |
|
| N stage | N0 | 74,134 (99.3) | 559 (0.7) | <0.001 |
|
| N1 | 20,210 (96.4) | 753 (3.6) |
|
| Multifocality | NO | 55,922 (98.9) | 626 (1.1) | <0.001 |
|
| YES | 37,045 (98.6) | 539 (1.4) |
|
| Histologic
subtype | PTC | 89,913 (98.7) | 1,157 (1.3) | <0.001 |
|
| FTC | 5,387 (94.2) | 331 (5.8) |
|
| Extrathyroidal
extension |
|
| No | 79,816 (99.4) | 511 (0.6) | <0.001 |
|
| Yes | 14,593 (95.1) | 746 (4.9) |
|
To further investigate the association between the
clinicopathological factors (ETE, histologic subtype, tumor size
and LNM status) and DM, multivariate analysis was performed with
adjustments for age, sex, and ethnicity. Similar results to
univariate analysis were obtained (Table III): Tumor size, LNM status,
histologic subtype and ETE were identified as independent risk
factors for DM [odds ratio (OR)=2.433; 95% CI, 1.910–3.098;
P<0.001; OR=3.998; 95% CI, 3.415–4.681; P<0.001; OR=6.266;
95% CI, 5.176–7.586; P<0.001; and OR=3.873; 95% CI, 3.338–4.494;
P<0.001, respectively).
 | Table III.Multivariate analysis of distant
metastases in differentiated thyroid cancer. |
Table III.
Multivariate analysis of distant
metastases in differentiated thyroid cancer.
| Characteristic | OR (95% CI) | P-value |
|---|
| Age | 3.449
(3.001–3.964) | <0.001 |
| Sex | 1.690
(1.479–1.932) | <0.001 |
| Ethnicity
(White) | Reference | <0.001 |
| Ethnicity
(Black) | 1.457
(1.119–1.897) | 0.005 |
| Ethnicity
(other) | 1.498
(1.255–1.787) | <0.001 |
| Size | 2.433
(1.910–3.098) | <0.001 |
| N stage | 3.998
(3.415–4.681) | <0.001 |
| Histologic
subtype | 6.266
(5.176–7.586) | <0.001 |
| Extrathyroidal
extension | 3.873
(3.338~4.494) | <0.001 |
Synergistic effects of ETE and
histologic subtype, tumor size or LNM status on DM
To comprehensively examine the synergistic effects
of ETE and the other clinicopathological factors on DM, all
patients were divided into three groups with two factors compared
in each group (ETE and histologic subtype, ETE and LNM status and
ETE and tumor size).
As presented in Table
IV, the probability of DM was the highest in patients with FTC
and ETE (OR=26.598; 95% CI, 19.703–35.906; P<0.001) compared
with the other subgroups. Additionally, the probability of DM was
higher in patients with FTC without ETE (OR=5.819; 95% CI,
4.598–7.363; P<0.001) and in patients with PTC and ETE
(OR=3.739; 95% CI, 3.182–4.393; P<0.001) compared with that in
patients with PTC without ETE (Table
IV).
 | Table IV.Measures for estimation of
synergistic effect between histologic subtype and ETE for the risk
of distant metastasis in differentiated thyroid cancer. |
Table IV.
Measures for estimation of
synergistic effect between histologic subtype and ETE for the risk
of distant metastasis in differentiated thyroid cancer.
| Subtype | ETE | M1 cases (%) | Total cases | OR (95% CI) | P-value |
|---|
| PTC | No | 368 (0.5) | 75,278 | Reference |
|
| FTC | No | 143 (2.8) | 5,049 | 5.819
(4.598–7.363) | <0.001 |
| PTC | Yes | 644 (4.3) | 14,807 | 3.739
(3.182–4.393) | <0.001 |
| FTC | Yes | 102
(19.2) | 532 | 26.598
(19.703–35.906) | <0.001 |
| RERI |
|
| 34.097
(23.068–45.126) |
|
| AP |
|
| 0.706
(0.638–0.775) |
|
| SI |
|
| 3.585
(2.82–4.556) |
|
Based on these results, the RERI of ETE and
histologic subtype was 34.097 (95% CI, 23.068–45.126), which
indicated that the additive synergistic effect of the FTC
histologic subtype and ETE contributed a 34.097 relative excess
risk on the incidence of DM. The AP was 0.706 (95% CI,
0.638–0.775), which suggested that 70.6% of DM cases exposed to the
two risk factors may be caused by the synergistic effect. The SI
was 3.585 (95% CI, 2.82–4.556), which suggested the existence of a
synergistic effect between the DTC histologic subtype and ETE on
the incidence of DM.
In the estimation of the synergistic effect between
LNM status and ETE, the probability of DM was the highest in
patients with ETE and N1 stage (OR=16.097; 95% CI, 13.415–19.317;
P<0.001) compared with the other subgroups (Table V). The probability of DM was higher
in patients with only LNM (OR=5.047; 95% CI, 4.062–6.270;
P<0.001) or ETE (OR=4.902; 95% CI, 3.967–6.059; P<0.001)
compared with that in patients without ETE and LNM. In addition, a
RERI of 6.425 was contributed by the additive synergistic effect of
ETE and LNM on the incidence of DM (95% CI, 4.543–8.307). In
addition, 41.0% of DMs exposed to the two risk factors were likely
caused by the additive synergistic effect (AP=0.410; 95% CI,
0.323–0.498). The SI was 1.781 (95% CI, 1.511–2.098), suggesting
the existence of a synergistic effect between LNM status and ETE on
the incidence of DM.
 | Table V.Measures for estimation of
synergistic effect between N stage and ETE for the risk of distant
metastasis in differentiated thyroid cancer. |
Table V.
Measures for estimation of
synergistic effect between N stage and ETE for the risk of distant
metastasis in differentiated thyroid cancer.
| N stage | ETE | M1 cases | Total cases | OR (95% CI) | P-value |
|---|
| 0 | No | 285 | 67,262 | Reference |
|
| 1 | No | 189 | 12,406 | 5.047
(4.062–6.270) | <0.001 |
| 0 | Yes | 191 | 7,000 | 4.902
(3.967–6.059) | <0.001 |
| 1 | Yes | 509 | 8,151 | 16.097
(13.415–19.317) | <0.001 |
| RERI |
|
| 6.425
(4.543–8.307) |
|
| AP |
|
| 0.410
(0.323–0.498) |
|
| SI |
|
| 1.781
(1.511–2.098) |
|
The synergistic effect between ETE and tumor size on
the incidence of DM was also evaluated (Table VI). The probability of DM was the
highest in patients with large tumors (>10 mm) and ETE
(OR=9.727; 95% CI, 7.295–12.969; P<0.001) compared with those
with other combinations of tumor size and ETE. In addition, the
probability of DM was significantly higher in patients with larger
tumors (>10 mm) without ETE (OR=2.537; 95% CI, 1.903–3.383;
P<0.001) and in patients with small tumors (≤10 mm) with ETE
(OR=4.430; 95% CI, 2.678–7.328; P<0.001) compared with that in
patients with small tumors without ETE.
 | Table VI.Measures for estimation of
synergistic effect between tumor size and ETE for the risk of
distant metastasis in differentiated thyroid cancer. |
Table VI.
Measures for estimation of
synergistic effect between tumor size and ETE for the risk of
distant metastasis in differentiated thyroid cancer.
| Tumor size | ETE | M1 cases | Total cases | OR (95% CI) | P-value |
|---|
| ≤10 mm | No | 63 | 29,774 | Reference |
|
| >10 mm | No | 388 | 47,029 | 2.537
(1.903–3.383) | <0.001 |
| ≤10 mm | Yes | 27 | 1,518 | 4.430
(2.678–7.328) | <0.001 |
| >10 mm | Yes | 613 | 13,204 | 9.727
(7.295–12.969) | <0.001 |
| RERI |
|
| 76.973
(50.238–103.708) |
|
| AP |
|
| 0.864
(0.823–0.905) |
|
| SI |
|
| 7.930
(5.833–10.780) |
|
Further analysis indicated a 76.973 (95% CI,
50.238–103.708) relative excess risk contributed by the additive
synergistic effect of tumor size and ETE on the incidence of DM.
The AP was 0.864 (95% CI, 0.823–0.905), which suggested that 86.4%
of DM cases exhibiting the two risk factors may have been caused by
the synergistic effect. In addition, the SI was 7.930 (95% CI,
5.833–10.780), indicating a synergistic effect between tumor size
and ETE on the incidence of DM.
Synergistic effects of ETE and
histologic subtype, tumor size or LNM status on patient
survival
The synergistic effects of ETE and tumor size,
histologic subtype or LNM status on survival of patients with DTC
were evaluated using Kaplan-Meier analysis. Among the four groups
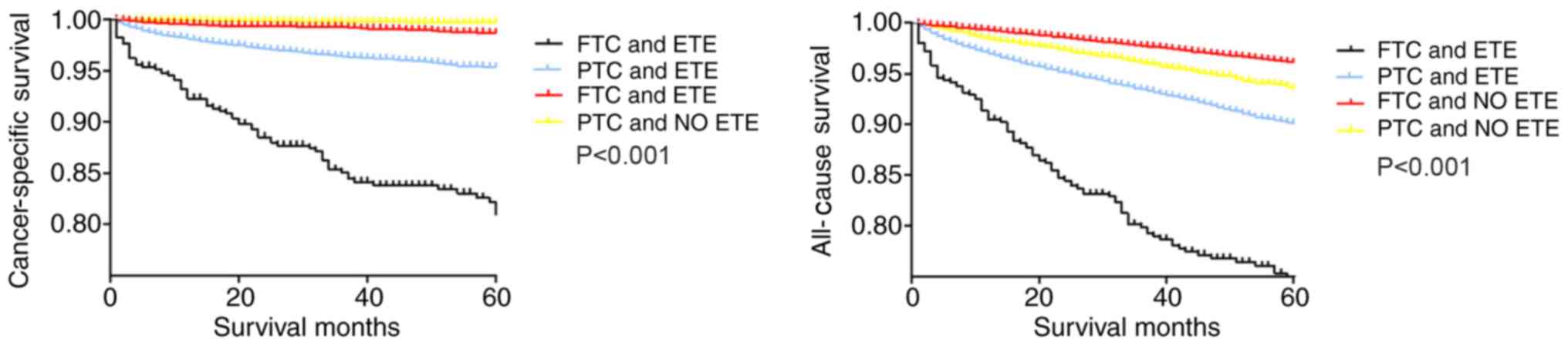
categorized according to ETE and histologic subtype (Fig. 1), the cancer-specific survival curves
were relatively flat for patients without ETE, whereas patients
with PTC and ETE exhibited a modest decline in the survival curve.
By contrast, patients with FTC and ETE exhibited a sharp decline in
the survival curve. The all-cause survival curve of patients with
FTC without ETE was relatively flat, and the survival curve of
patients with FTC and ETE exhibited a sharp decline. Patients with
PTC with and without ETE exhibited a modest decline in the survival
curve (Fig. 1).
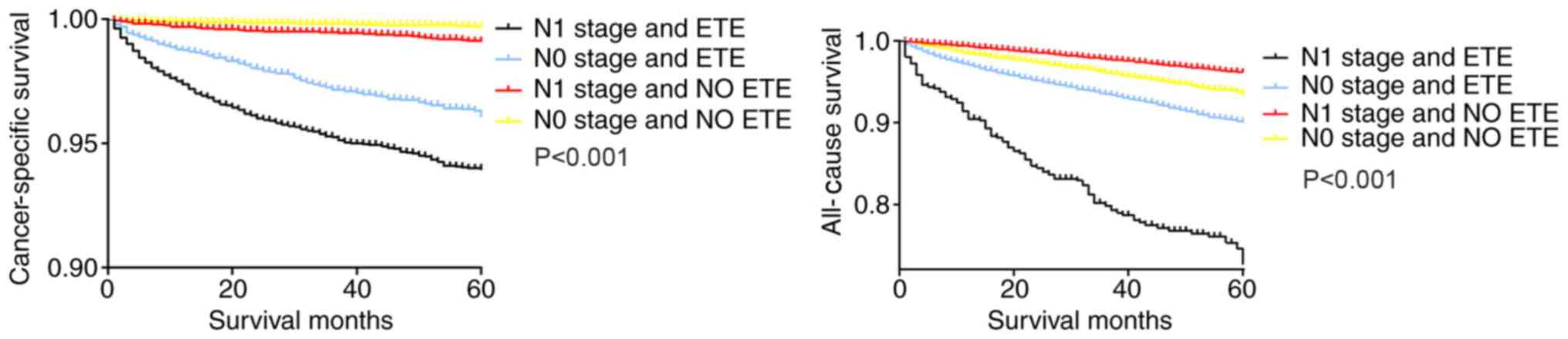
Similarly, in the Kaplan-Meier analysis of the four
groups categorized according to ETE and LNM status, patients with
N1 stage tumors and ETE exhibited a sharp decline in the
cancer-specific survival curve and all-cause survival curve,
compared with patients with only ETE or N1 stage tumors and
patients with N0 stage tumors without ETE (Fig. 2).
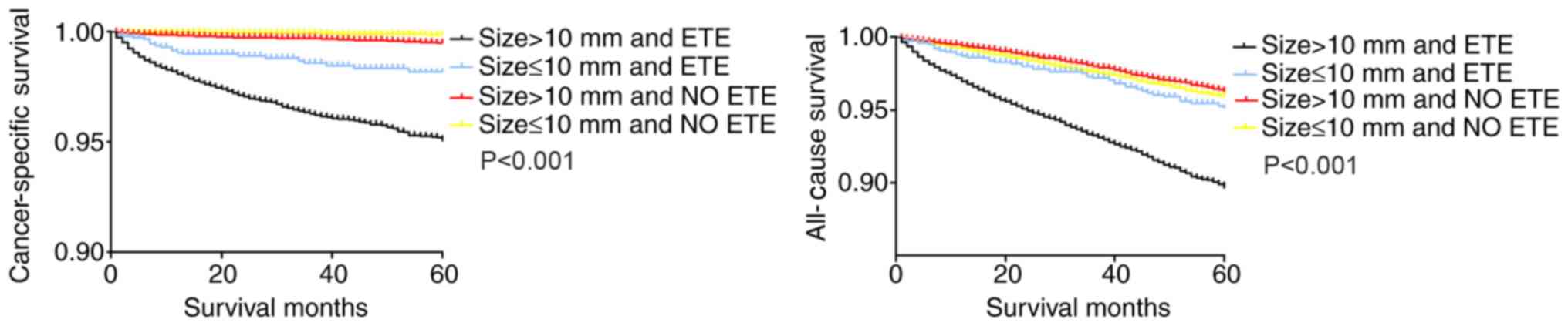
In the Kaplan-Meier analysis of the four groups
categorized according to ETE and tumor size, the cancer-specific
and all-cause survival curves of patients with larger tumors
(>10 mm) and ETE exhibited a decline in survival compared with
those of patients with either factor alone, as well as patients
with smaller tumors (≤10 mm) without ETE (Fig. 3).
Discussion
DTC exhibits a lower mortality rate compared with
other carcinomas, but certain types of DTC, such as radioiodine
refractory differentiated thyroid carcinoma, are difficult to
diagnose at an early stage (23,24).
Patients with DTC have poor prognosis due to DM (25–27).
Previous studies have indicated that LNM, tumor size, histologic
subtype and ETE are risk factors that influence the prognosis of
patients with DTC (20,28,29).
However, the majority of these studies were single-center,
small-sample studies or meta-analyses, and the synergistic effects
of these risk factors have not been sufficiently elaborated.
The SEER database contains data encompassing
approximately 28% of the US population (30). SEER registries hold the highest level
of certification of data quality provided by the North American
Association of Central Cancer Registries (31). In addition, the index of completeness
of case ascertainment of the program is 98%, making it the gold
standard database for cancer analysis in the US (32–34).
Therefore, the SEER database allows in-depth analysis of certain
clinical issues.
The presence of DM is considered to be a significant
factor at diagnosis and during staging according to the
Tumor-Node-Metastasis (TNM) classification (35,36), and
a confirmed diagnosis of DM is associated with mortality or poor
prognosis in patients with FTC and PTC (37). Predicting the incidence of DM in
patients with DTC may help develop an appropriate treatment
strategy. The treatment for patients with DTC without DM includes
local therapeutic measures, such as surgery and radiofrequency;
however, in patients with DM, radioiodine therapy is considered the
most effective treatment (38).
Therefore, predicting the most unfavorable prognostic factors for
DM is crucial for determining treatment strategies for patients
with DTC.
The present study used data from patients with DTC
and DM from the SEER database to evaluate the risk factors and
analyze the synergistic effects of ETE and the other risk factors.
The results of the univariate and multivariate analyses determined
that ETE, histologic subtype, tumor size and LNM status were
independent factors that influenced the incidence of DM. Among
these independent factors, ETE has been emphasized in previous
studies. The American Joint Committee on Cancer (AJCC) Cancer
Staging Manual (8th edition) (35)
has reported that gross ETE may be identified clearly as T3b, T4a,
or T4b based on imaging or intraoperative findings. In addition,
due to the poor survival outcomes associated with gross ETE, its
presence is considered to be a significant reference index for the
staging category (35,39). Thus, ETE was selected as the key
factor in the present study, and its interaction with the three
other factors was investigated.
The preliminary analyses of the present study
demonstrated that patients with FTC and ETE obtained the highest
incidence of DM compared with the other subgroups (patients with
FTC and ETE, patients with FTC without ETE, and patients with PTC
and ETE). Patients with ETE and large tumor size exhibited the
highest incidence of DM. Patients with ETE and LNM also exhibited a
higher incidence of DM compared with the other subgroups.
Therefore, the results of the present study suggest synergistic
effects of ETE and histologic subtype, LNM status or tumor size on
the incidence of DM in patients with DTC. Furthermore, the analyses
of survival curves using the Kaplan-Meier method also demonstrated
synergistic effects of ETE and the other risk factors on survival;
a similar tendency was observed in both cancer-specific and
all-cause survival
More specifically, the evaluation of the synergistic
effects based on RERI, AP and SI indicated that the FTC histologic
subtype, large tumor size or presence of LNM may contribute an
added risk of DM to patients with DTC who also exhibit ETE.
However, there were several limitations to this
study that need to be addressed. First, the study was
retrospective, and thus, selection bias was inevitable. Second, in
the 8th edition of the TNM/AJCC classification system, ETE was
classified into minor ETE and gross ETE, and only gross ETE was
considered as a risk factor for staging since gross extrathyroidal
extension can be identified clearly by imaging or intraoperative
findings, whereas minor ETE is difficult to identify (13,35).
Thus, more data containing gross ETE, and not minor ETE, should be
included in future research. Third, all data on ETE from the SEER
database were based on pathological diagnosis. However, the
majority of the data on ETE in clinic are provided by
ultrasonologists during diagnosis. Therefore, in clinical
application, the conclusion of this study may be subjected to the
subjective influence of ultrasonologists and may vary by
institution or provider (40). In
addition, the SEER database may contain certain bias resulting from
the sole inclusion of patients registered with Medicare, with data
based only on diagnoses or procedures covered by the insurance.
The synergistic effects of histologic subtype, tumor
size, ETE and LNM status on DM might benefit cancer management; if
two of the four variables are present, this may suggest that the
patients with DTC may be more likely to exhibit DM, which is
difficult to diagnose at an early stage. Thus, aggressive treatment
may be recommended, such as surgical treatment, thyroid-stimulating
hormone suppression, radioiodine, molecular targeted or biological
therapy (4,5).
In summary, histologic subtype, tumor size, ETE and
LNM status were identified in the present study as independent risk
factors of DM in patients with DTC. The presence of ETE and FTC,
ETE and large tumor size or ETE and LNM may have a synergistic
effect of increasing the risk on DM in patients with DTC. Study of
the associations between these parameters and DM may be helpful for
clinicians for predicting prognosis and developing appropriate
treatment strategies. To improve data accuracy, more precise
evaluations can be performed in patients with DTC by including more
risk factors in the analysis. DM, as a significant independent
factor for survival, may also have synergistic effects with other
associated factors. This may provide guidance for further research
on the associations among demographic factors in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Authors' contributions
ZML, LG conceived the idea and designed the study.
DYC, LH, YHH, DH, WZe, MW, WZh and HFF undertook the data
collection and prepared the tables. DYC, LH, WW and CZ performed
the data analysis. DYC, LH, SCC contributed to the interpretation
of data and prepared the figures. LH, DYC, LG drafted the
manuscript. All authors revised the manuscript critically and
approved the final version.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
This study's retrospective protocol was approved by
the Ethics Committee of Zhongnan Hospital of Wuhan University.
Informed consent has been obtained from each patient after full
explanation of the purpose and nature of all procedures used, and
patient records were anonymized and de-identified prior to the
analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, et al:
SEER Cancer Statistics Review, 1975–2014. National Cancer
Institute; Bethesda, MD: April. 2017
|
|
2
|
Fardella C, Jimenez M, Gonzalez H, León A,
Goñi I, Cruz F, Solar A, Torres J, Mosso L, González G, et al:
Pathological characteristics of thyroid microcarcinoma. A review of
402 biopsies. Rev Med Chil. 133:1305–1310. 2005.(In Spanish).
PubMed/NCBI
|
|
3
|
Galuppini F, Vianello F, Censi S, Barollo
S4, Bertazza L, Carducci S, Colato C, Manso J, Rugge M, Iacobone M,
et al: Differentiated thyroid carcinoma in pediatric age: Genetic
and clinical scenario. Front Endocrinol (Lausanne). 10:5522019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zanotti-Fregonara P, Hindie E, Faugeron I,
Moretti JL, Ravasi L, Rubello D and Toubert ME: Update on the
diagnosis and therapy of distant metastases of differentiated
thyroid carcinoma. Minerva Endocrinol. 33:313–327. 2008.PubMed/NCBI
|
|
5
|
Tuttle RM, Ball DW, Byrd D, Dilawari RA,
Doherty GM, Duh QY, Ehya H, Farrar WB, Haddad RI, andeel F, et al:
Thyroid carcinoma. J Natl Compr Canc Netw. 8:1228–1274. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin JJ and Milas M: Detection of disease
recurrence in differentiated thyroid cancer. Minerva Chir.
65:101–116. 2010.PubMed/NCBI
|
|
7
|
Goffredo P, Sosa JA and Roman SA:
Differentiated thyroid cancer presenting with distant metastases: A
population analysis over two decades. World J Surg. 37:1599–1605.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mazzaferri EL and Kloos RT: Clinical
review 128: Current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hay ID: Papillary thyroid carcinoma.
Endocrinol Metab Clin North Am. 19:545–576. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schlumberger M, Challeton C, De Vathaire
F, Travagli JP, Gardet P, Lumbroso JD, Francese C, Fontaine F,
Ricard M and Parmentier C: Radioactive iodine treatment and
external radiotherapy for lung and bone metastases from thyroid
carcinoma. J Nucl Med. 37:598–605. 1996.PubMed/NCBI
|
|
11
|
Toubert ME, Hindie E, Rampin L, Al-Nahhas
A and Rubello D: Distant metastases of differentiated thyroid
cancer: Diagnosis, treatment and outcome. Nucl Med Rev Cent East
Eur. 10:106–109. 2007.PubMed/NCBI
|
|
12
|
Andersen PE, Kinsella J, Loree TR, Shaha
AR and Shah JP: Differentiated carcinoma of the thyroid with
extrathyroidal extension. Am J Surg. 170:467–470. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SY, Kim HI, Kim JH, Kim JS, Oh YL,
Kim SW, Chung JH, Jang HW and Kim TH: Prognostic significance of
gross extrathyroidal extension invading only strap muscles in
differentiated thyroid carcinoma. Br J Surg. 105:1155–1162. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim H, Shin JH, Hahn SY, Oh YL, Kim SW,
Park KW and Lim Y: Prediction of follicular thyroid carcinoma
associated with distant metastasis in the preoperative and
postoperative model. Head Neck. 41:2507–2513. 2019.PubMed/NCBI
|
|
15
|
Aboelnaga EM and Ahmed RA: Difference
between papillary and follicular thyroid carcinoma outcomes: An
experience from Egyptian institution. Cancer Biol Med. 12:53–59.
2015.PubMed/NCBI
|
|
16
|
Mizukami Y, Michigishi T, Nonomura A,
Hashimoto T, Terahata S, Noguchi M, Hisada K and Matsubara F:
Distant metastases in differentiated thyroid carcinomas: A clinical
and pathologic study. Hum Pathol. 21:283–290. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barbosa MP, Momesso D, Bulzico DA, Farias
T, Dias F, Lima RA, Corbo R, Vaisman M and Vaisman F: Metastatic
lymph node characteristics as predictors of recurrence/persistence
in the neck and distant metastases in differentiated thyroid
cancer. Arch Endocrinol Metab. 61:584–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mercante G, Frasoldati A, Pedroni C,
Formisano D, Renna L, Piana S, Gardini G, Valcavi R and Barbieri V:
Prognostic factors affecting neck lymph node recurrence and distant
metastasis in papillary microcarcinoma of the thyroid: Results of a
study in 445 patients. Thyroid. 19:707–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeon MJ, Kim WG, Kim TH, Kim HK, Kim BH,
Yi HS, Kim ES, Kim H, Kim YN, Kim EH, et al: Disease-Specific
mortality of differentiated thyroid cancer patients in Korea: A
Multicenter Cohort Study. Endocrinol Metab (Seoul). 32:434–441.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vuong HG, Duong UNP, Pham TQ, Tran HM,
Oishi N, Mochizuki K, Nakazawa T, Hassell L, Katoh R and Kondo T:
Clinicopathological risk factors for distant metastasis in
differentiated thyroid carcinoma: A meta-analysis. World J Surg.
42:1005–1017. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fritz A, Percy C, Jack A, Shanmugaratnam
K, Sobin L, Parkin DM and Whelan S: Third edition, World Health
Organization. International Classification of Diseases for
Oncology. 2001.
|
|
22
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American Thyroid Association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim M, Kim TH, Shin DY, Lim DJ, Kim EY,
Kim WB, Chung JH, Shong YK, Kim BH, Kim WG, et al: Tertiary care
experience of sorafenib in the treatment of progressive
radioiodine-refractory differentiated thyroid carcinoma: A Korean
multicenter study. Thyroid. 28:340–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de la Fouchardiere C, Alghuzlan A, Bardet
S, Borget I, Borson Chazot F, Do Cao C, Godbert Y, Leenhardt L,
Zerdoud S and Leboulleux S: The medical treatment of
radioiodine-refractory differentiated thyroid cancers in 2019. A
TUTHYREF® network review. Bull Cancer. 106:812–819.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao Y and Xing M: Recent incidences and
differential trends of thyroid cancer in the USA. Endocr Relat
Cancer. 23:313–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM,
Sung TY, Yoon JH, Chung KW, Hong SJ, Kim TY, et al: Features
predictive of distant metastasis in papillary thyroid
microcarcinomas. Thyroid. 26:161–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nixon IJ, Whitcher MM, Palmer FL, Tuttle
RM, Shaha AR, Shah JP, Patel SG and Ganly I: The impact of distant
metastases at presentation on prognosis in patients with
differentiated carcinoma of the thyroid gland. Thyroid. 22:884–889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ito Y, Tomoda C, Uruno T, Takamura Y, Miya
A, Kobayashi K, Matsuzuka F, Kuma K and Miyauchi A: Prognostic
significance of extrathyroid extension of papillary thyroid
carcinoma: Massive but not minimal extension affects the
relapse-free survival. World J Surg. 30:780–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bellantone R, Lombardi CP, Boscherini M,
Ferrante A, Raffaelli M, Rubino F, Bossola M and Crucitti F:
Prognostic factors in differentiated thyroid carcinoma: A
multivariate analysis of 234 consecutive patients. J Surg Oncol.
68:237–241. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacobs AJ, Morris CD and Levin AS:
Synovial Sarcoma is not Associated with a higher risk of lymph node
metastasis compared with other soft tissue sarcomas. Clin Orthop
Relat Res. 476:589–598. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warren JL, Klabunde CN, Schrag D, Bach PB
and Riley GF: Overview of the SEER-Medicare data: Content, research
applications, and generalizability to the United States elderly
population. Med Care. 40 (Suppl 8):IV-3:–IV18. 2002. View Article : Google Scholar
|
|
32
|
Liu Y, Liu Z, Zhao Q, Hua T, Chi S, Huang
T and Wang H: Propensity score matching analysis of the prognosis
for the rare insular subtype of thyroid cancer based on SEER
database. Oncotarget. 8:101623–101633. 2017.PubMed/NCBI
|
|
33
|
Liu C, Chen T, Zeng W, Wang S, Xiong Y,
Liu Z and Huang T: Reevaluating the prognostic significance of male
gender for papillary thyroid carcinoma and microcarcinoma: A SEER
database analysis. Sci Rep. 7:114122017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiong Y, Zhao Q, Liu C, Wang S, Liu Z and
Huang T: Prognosis of patients with TX stage differentiated thyroid
cancer: Propensity scored matching analysis of the SEER database
2004–2013. Am J Transl Res. 10:2004–2014. 2018.PubMed/NCBI
|
|
35
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. 8th edition. Vol.
Springer International Publishing; Chicago: 2017
|
|
36
|
Tam S, Boonsripitayanon M, Amit M, Fellman
BM, Li Y, Busaidy NL, Cabanillas ME, Dadu R, Sherman S, Waguespack
SG, et al: Survival in differentiated thyroid cancer: Comparing the
AJCC cancer staging seventh and eighth editions. Thyroid.
28:1301–1310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Melo TG, Zantut-Wittmann DE, Ficher E
and da Assumpcao LV: Factors related to mortality in patients with
papillary and follicular thyroid cancer in long-term follow-up. J
Endocrinol Invest. 37:1195–1200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kreissl MC, Janssen MJR and Nagarajah J:
Current treatment strategies in metastasized differentiated thyroid
cancer. J Nucl Med. 60:9–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shteinshnaider M, Muallem Kalmovich L,
Koren S, Or K, Cantrell D and Benbassat C: Reassessment of
differentiated thyroid cancer patients using the eighth TNM/AJCC
classification system: A Comparative Study. Thyroid. 28:201–209.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuo EJ, Thi WJ, Zheng F, Zanocco KA,
Livhits MJ and Yeh MW: Individualizing surgery in papillary thyroid
carcinoma based on a detailed sonographic assessment of
extrathyroidal extension. Thyroid. 27:1544–1549. 2017. View Article : Google Scholar : PubMed/NCBI
|