Introduction
Nitric oxide (NO) is one of the most important
messengers for a number of physiological processes, including
vasodilation, smooth muscle relaxation, inhibition of platelet
aggregation and regulation of neurotransmission (1). Endogenous NO, synthesized from
L-arginine via a family of nitric oxide synthases (NOSs), which
comprises neuronal NOS (nNOS; NOS1), inducible NOS (iNOS; NOS2) and
endothelial NOS (eNOS; NOS3). NOS1 and NOS3 are constitutive and
produce short bursts of NO for signaling or messenger functions in
a calcium-dependent manner, whereas NOS2 is expressed in response
to immunological stimuli and is capable of sustained release of NO
in a calcium-independent manner (2).
The production of NO and expression of NOS are
ubiquitous in malignant tumors and possess both pro-tumor and
antitumor effects (3). One of the
primary determinants that account for the paradoxical behavior of
NO in tumor biology are its concentration in tumor milieu, exposure
time and cellular adaptation to NO. Generally, NO favors cell
survival and proliferation at lower NO concentrations (<100 nM).
By contrast at a higher level of NO (>300 nM), NO promotes cell
cycle arrest, apoptosis and senescence (4,5).
Therefore, higher concentrations produced by NOS2, a potential
cytostatic/cytotoxic factor in immune functions, mediate antitumor
activity, whereas chronic induction of NOS2 may contribute to the
initiation of cancer (6).
Furthermore, low concentrations of NO produced by NOS1 and NOS3
facilitated tumor progression by modulating cancer-associated
events, including angiogenesis, apoptosis, cell cycle, invasion and
metastasis (7). Among the three
NOSs, NOS1 was also observed to be aberrantly expressed in human
tumors, including the brain (8),
lung (9) and glioma (10). Despite the discrepancies regarding
the concentration of NO and its cellular effects, a study by Kotake
et al (11) has reported that
low levels of NO formed by NOS1, triggers cell proliferation
primarily via the soluble guanylate cyclase-cyclic guanosine
monophosphate (sGC-cGMP) dependent mechanism. Furthermore, NOS1
expression in melanoma mediated the dysfunction of response to
adoptive T cell therapy (12).
Previous studies suggested that NOS isoforms were
highly expressed in ovarian cancer (13,14). The
function of NOS isoforms on ovarian tumor development is highly
complex, with both tumor-promoting and inhibiting actions having
been described (15). It has also
been demonstrated that the level of NOS2 expression was associated
with differential status, whereas NOS1 and NOS3 were mainly
expressed in poorly differential samples (14). Previously, it was reported that NOS
expression was associated with responsiveness of DDP treatment. The
level of NOS1 expression was associated with DDP-resistance,
whereas NOS2 was highly expressed in sensitive ovarian cancer cell
lines (16). These results indicated
that the expression of NOS isoforms serve a critical role in the
progression of ovarian cancer and have an effect on the sensitivity
of chemotherapy. However, the functional role of individual NOSs,
particularly NOS1, on the biological behaviors of ovarian cancer
remains unclear.
The present study analyzed the gene expression
profiles of ovarian cancer downloaded from the Gene Expression
Omnibus (GEO) database and revealed that there was a higher
expression of NOS1 in ovarian cancer tissues compared with normal
ovarian tissues. Using the NOS inhibitor NG-nitro-L-arginine methyl
ester (L-NAME) or NOS1 knockdown by short hairpin (sh)RNA, the
present study verified that NOS1 serves multiple functions in the
promotion of tumor development, including proliferation, migration
and invasion, as well as drug resistance in OVCAR3 cells. The
results of the present study provide a suggestion for the
improvement of ovarian cancer therapy.
Materials and methods
Chemicals and reagents
Unless otherwise stated, all chemicals were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
GEO database
Analysis of gene expression profiles of NOS isoforms
in ovarian cancer tissues. Expression data was downloaded from GEO
(accession no. GSE14407).
Cell culture and transfection
Ovarian cancer cells lines of OVCAR3, SKOV3 and ES-2
were obtained from Southern Medical University Cancer Institute
(Guangzhou, China). Cells were grown in Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The cells were incubated at 37°C in a 95%
air-5% CO2 gas mixture. The medium was replaced every 2
days. OVCAR3 Sh-NOS1 cells were transfected with NOS1 shRNA
(GeneCopoeia, Guangzhou, China). A nonspecific control was used as
non-targeting shRNAs. Transfections were performed using
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) using 1–2 mg of expression vector/ml serum-free medium as
described by the manufacturer. The transfected cells were incubated
at 37°C for 24 h and harvested for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
RT-qPCR
Total cellular RNA from cells was extracted using
TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific, Inc.), according to the manufacturers' protocol. cDNA
was synthesized from 1 µg total RNA using the RNeasy mini kit
(Qiagen GmbH, Hilden, Germany). cDNA was amplified using the KAPA
SYBR Fast universal qPCR kit (Tiangen Biotec Co., Ltd., Beijing,
China) using the following primers: Human NOS1 forward,
5′-CAGAGGATGGCAGTCTGTTTC-3′ and reverse,
5′-CTCAAGAGCACTGGATCTCAG-3′; human GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCTTA-3′. The reaction time was as follows: 2
min at 95°C for initial denaturation, 30 sec at 95°C and 32 sec at
60°C for 40 cycles. Gene expression levels were normalized to those
of GAPDH. RT-qPCR was performed using the Mx3005p system
(Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA).
Relative quantification of mRNA was determined by the
2−∆∆Cq method (17). The
experiments were done at least thrice independently and all samples
were in triplicate.
Western blot analysis
The harvested cells were lysed with lysis buffer
containing 50 mM Tris/HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate, 0.1% SDS, 50 mM NaF, 1 mM
Na3VO4 and protease inhibitor (Hangzhou Fude
Biological Technology Co., Ltd., Hangzhou, China). Protein
concentrations in the cell lysates were quantified using the BCA
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China). Proteins (50 µg) were separated on 10% SDS-PAGE gels and
transferred to a nitrocellulose membrane (EMD Millipore, Billerica,
CA, USA). Following blocking with 5% bovine serum albumin in TBS
supplemented with 1% Tween-20 at 28°C for 1 h, the membranes were
incubated with the appropriate primary antibodies: NOS1 (cat. no.
ab76067; 1:1,000; Abcam, Cambridge, UK), NOS2 (cat. no. ab15323;
1:1,000; Abcam), NOS3 (cat. no. ab76198; 1:1,000; Abcam) and GAPDH
(cat. no. G9545; 1:5,000; Sigma Aldrich; Merck KGaA), at 4 °C
overnight, followed by 2 h incubations at room temperature with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (cat. no. FD0128; 1:5,000; Hangzhou Fude Biological
Technology Co., Ltd.) and goat anti-mouse IgG secondary antibody
(cat. no. FDM007; 1:5,000; Hangzhou Fude Biological Technology Co.,
Ltd.). The protein bands were visualized using an Enhanced
Chemiluminescence Western Blot Detection system.
Griess assay
OVCAR3 cells were treated with NOS extensive
inhibitor [NG-nitro-L-arginine methyl ester (L-NAME)] (1 mM) and NO
donor [DETA-NONOate, (DETA-NO)] (50 µM) for 24 h. Accumulation of
NO in cultured media samples was analyzed by Griess assay-through
measuring levels of nitrite expression using a nitrate/nitrite
colorimetric assay kit. Briefly, OVCA3 cells were seeded in 96-well
plates at a density of 5,000 cells/well, and then supernatant of
the cultured cells was collected following the indicated time. The
culture supernatant (20 µl) was mixed with 60 µl assay buffer, 10
µl enzyme cofactor and 10 µl nitrate reductase. Following
incubation for 10 min at room temperature, 10 µl DAN and 20 µl NaOH
was added to each well and the fluorescence was determined at an
excitation wavelength of 365 nm and an emission wavelength of 430
nm. The amount of nitrite was evaluated using a NaNO2
standard curve.
Cell proliferation assay
The cells were seeded into 96-well plates at a
density of 5,000 cells/well and then incubated at 37°C with 5%
CO2. Following 24 h, 10 µl MTT (5 mg/ml) was added to
each well and the cells were further incubated at 37°C for 4 h.
Subsequently, all supernatant was discarded, 100 µl DMSO was added
to each well and 96-well plates were agitated for 15 min at room
temperature. The absorbance of cells was analyzed at 490 nm using a
microplate reader.
Colony formation assay
The cells were trypsinized, counted and plated in
6-well plates at a density of 500 cells per well. Following two
weeks, the cells were washed with PBS, fixed in 10% methanol for 15
min and stained with Giemsa for 10 min at room temperature. The
colonies were then imaged and counted with a light microscope. All
experiments were performed in triplicate.
Scratch-wound assay
The cells at a density of 5×105
cells/well were seeded into a six-well plate and cultured to 80%
confluence in medium supplemented with 10% FBS at 37°C. Cell
monolayers were scratched using a plastic tip (1 mm) and incubated
in serum-containing medium (2% serum) for 24 h at 37°C. The
migration distance of the cells was determined at three sites using
Photoshop (Adobe Systems, Inc., San Hose, CA, USA).
Invasion assay
The invasive potential of the cancer cells was
assessed in vitro using Transwell chambers (Corning
Incorporated, Corning, NY, USA). The upper chambers were coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA).
Subsequently 1×105 cells in serum-free medium were added
to the upper chambers and 10% FBS was added to the bottom chambers.
Cells were incubated for 24 h at 37°C. The cells on the lower side
of the filter were fixed with 75% methyl alcohol for 15 min at room
temperature and where then stained with hematoxylin for 10 min at
room temperature, and counted under a fluorescence microscope in 5
different fields (×400 magnification). Equal amounts of PBS were
used as the control.
Statistical analysis
All experiments were repeated at least three times.
The results are presented as the mean ± standard deviation. The
statistical significance of differences was determined by Student's
t-test when comparing two groups, and one-way analysis of variance
was used to compare multiple groups. P<0.05 was considered to
indicate a statistically significant difference. All data were
analyzed using SPSS version 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Expression of NOS1 in ovarian
cancer
In order to evaluate the effect of NOS1 on ovarian
cancer progression, the present study first analyzed the expression
level of NOS isoforms, NOS1, NOS2 and NOS3, in gene expression
profiles including 12 pairs of ovarian cancer tissues and normal
ovarian tissues downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/, accession no.
GSE14407). Expression levels of NOS isoforms mRNA were revealed to
be increased in ovarian cancer tissues compared with normal ovarian
tissues. However, only the alteration of NOS1 expression level was
statistically significant (Fig. 1).
The level of NOS1 expression was also increased in the
DDP-resistant group compared with the DDP-sensitive group (data not
shown). These results indicated that NOS1 may serve an important
role in ovarian cancer progression.
Production of NO by NOS1 in ovarian
cancer cells
To verify the role of NOS1 in ovarian cancer, the
present study evaluated the expression levels of three NOS enzymes
in a human ovarian cancer line by RT-qPCR and western blot
analysis. The mRNA expression levels of the three NOSs were
detectable in all analyzed ovarian cancer cell lines: SKOV3, OVCAR3
and ES-2 (Fig. 2A). Protein
expression of three NOSs was also detected in SKOV3 and OVCAR3
(Fig. 2B). Subsequently, the present
study treated OVCAR3 cells with NOS extensive inhibitor
[NG-nitro-L-arginine methyl ester (L-NAME)] and NO donor
[DETA-NONOate, (DETA-NO)] for 24 h, and examined nitrates (NOx) in
the culture medium by Griess assay. The concentration of NO
released by OVCAR3 was relatively low and the value of was ~24 nM
compared with the concentration released by NO donor DETA-NO 50 µM.
The cells treated with 50 µM DETA-NO released 50 nM NO (Fig. 2C).
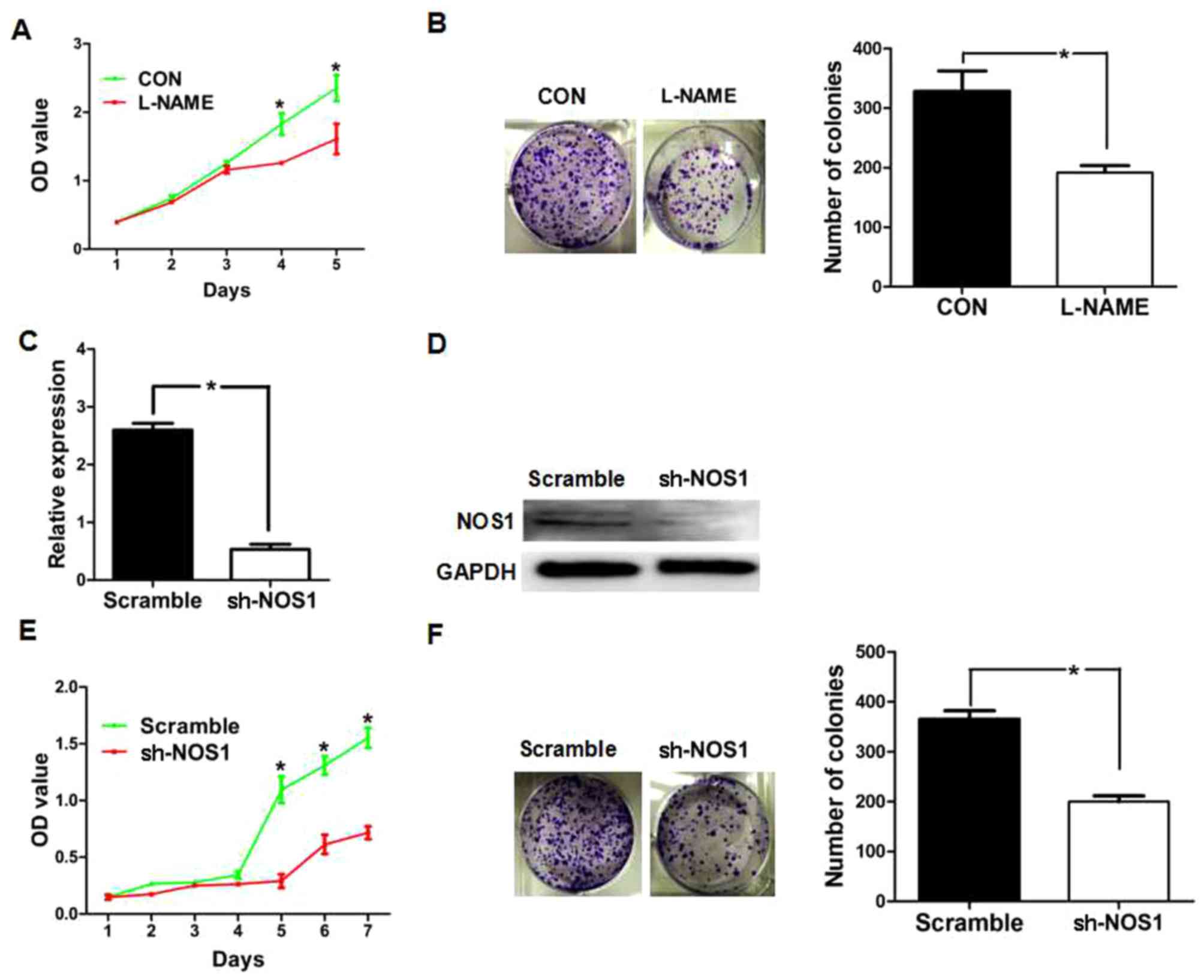
NOS1 promotes proliferation of OVCAR3
cells
In order to investigate the role of NOS1 in cell
proliferation, the present study first investigated the effect of
NOSs inhibitor L-NAME on cell proliferation using MTT and colony
formation assays. The results revealed that cells treated with
L-NAME demonstrated a significantly lower proliferation rate
compared with the control cells (Fig. 3A
and B). Subsequently, the present study examined the effect of
shRNA-mediated NOS1 knockdown on cell proliferation. OVCAR3 cells
were either transfected with nonspecific shRNA (scramble) or NOS1
shRNA (Sh-NOS1). Following transfection for 24 h, RT-qPCR and
western blotting were performed to analyze the levels of NOS1 mRNA
and protein expression. As presented in Fig. 3C and D, the expression of NOS1 mRNA
and protein was markedly decreased in NOS1 shRNA-transfected cells
compared with the control shRNA-transfected cells (P<0.01). The
results of the MTT and colony formation assays demonstrated that
NOS1 shRNA-transfected cells revealed a significantly lower
proliferation rate compared with the control shRNA-transfected
cells (Fig. 3E and F).
NOS1 promotes invasion and migration
of OVCAR3 cells
The present study assessed the effect of NOS1 on
tumor cell metastasis. Cellular migration was analyzed by
scratch-wound assay and invasion was analyzed by Transwell assay.
The results of scratch-wound assay demonstrated that NOSs inhibitor
exhibited a significant decrease in cellular migration compared
with the control (P<0.05; Fig.
4A). In the in vitro invasion assays, the number of
cells invaded through the Transwell membrane in the L-NAME-treated
group was significantly lower compared with the control (P<0.05;
Fig. 4B). Subsequently, the present
study determined the effect of shRNA-mediated NOS1 knockdown on
cell invasion ability in OVCAR3, respectively. The results of the
scratch-wound assay demonstrated a significant reduction in
motility of NOS1 shRNA-transfected cells compared with the control
(P<0.01; Fig. 4C). In the in
vitro invasion assays, the number of cells invaded through the
Transwell membrane in NOS1 shRNA-transfected group was
significantly lower compared with the control (P<0.01; Fig. 4D).
NOS1 inhibition increases sensitivity
of OVCAR3 to DDP-induced cell death
The present study investigated the role of NOS1 in
chemoresistance in ovarian cancer. Primarily, the cytotoxic effect
of DDP on ovarian cancer cells (OVCAR3) analyzed by MTT assay
demonstrated cytotoxic activity with an IC50 value of 5
µmol/l (Fig. 5A). Next, the effect
of NOSs inhibitor combined with DDP (2 µmol/l) on cell
proliferation was evaluated by analyzing cell viability. The
results revealed that the combination of L-NAME treatment and
DDP-mediated knockdown effectively promoted cell death of OVCAR3
cells compared with treatment with DDP alone (Fig. 5B). Similarly, NOS1 knockdown
significantly reduced cell viability compared with treatment with
DDP alone (Fig. 5C).
Discussion
Epithelial ovarian cancer has the highest mortality
rate of all gynecological malignancies and is the fifth leading
cause of cancer mortality in females (18,19).
Current therapeutic approaches for ovarian cancer are relatively
effective for early-stage disease with 5-year survival rates of 84%
for stage I and 66% for stage II disease, whereas the 5-year
survival rates for stage III or IV disease are <30% (20,21).
More than two-thirds of females with advanced-stage epithelial
ovarian cancer experience recurrence despite achieving clinical
remission following completion of initial treatment (22). Chemoresistance to standard treatment
is crucial for recurrence of ovarian cancer. Therefore,
understanding the molecular mechanism underlying ovarian cancer
development and providing effective predictive markers for
recurrence or chemoresistance are urgently required for more
effective management and for developing ovarian cancer therapies.
The present study analyzed the gene expression profiles of ovarian
cancer downloaded from the GEO database and revealed that NOS1
expression level was higher in ovarian cancer compared with paired
normal ovarian tissues. The in vitro experiment verified
that NOS1 promoted proliferation, invasion and migration, as well
as chemoresistance in ovarian cancer cells. The present study
provides a candidate marker for prognosis of ovarian cancer and an
implication for improvements in ovarian cancer therapy.
Endogenous NO is produced in mammalian cells by the
three NOSs (NOS1, NOS2 and NOS3). Typically, NOS1 and NOS3 produce
NO at low or physiological levels. By contrast, NOS2 produces high
levels of NO with a toxicity effect. Three isoforms of NOS were
expressed increasingly in tumors and serve a dual role in tumor
development as the effects of NO are strictly
concentration-dependent (4). A study
has demonstrated that low/intermediate concentrations of NO that
slight higher than physiological dose was able to promote primary
tumor growth and stimulate metastasis, whereas other studies
revealed that metastasis was suppressed by higher levels of NO
(4,23). The present study demonstrated that
NOS1 protein and mRNA were highly expressed in OVCAR3 cells. Griess
assay revealed that the NO formed by NOSs was relatively low when
compared with NOS1 with a concentration of ~24 nM in OVCAR3 cell
(5), therefore NOSs might serve a
promoting role on a number of cellular functions, including cell
proliferation, migration and invasion. However, the underlying
molecular mechanism by which NOS1 promotes cell functions remains
unclear and requires further study.
Chemotherapies are the most common treatments in
advanced and recurrent ovarian cancer (24). The clinical response to cisplatin
(DDP), one of first-line chemotherapeutic agents, is ~80% in
patients with ovarian cancer, but most patients with advanced
disease will eventually relapse and succumb to the disease due to
acquired drug resistance (25). A
previous study demonstrated that combination therapies of DDP with
other drugs were benefit to overcome drug-resistance and reduce
toxicity (26). The present study
identified that NOS1 expression contributed to DDP resistance in
OVCAR3 cells, and inhibition of NOS1 by chemical inhibitor or NOS1
shRNA increased the sensitivity of cells to DDP-induced cell death,
suggesting that therapeutic targeting of NOS1 in combination with
conventional chemo-therapeutic agents may increase the efficacy of
ovarian cancer therapy. In summary, the results of the present
study suggested that NOS1 expression may be an indicator of
response to chemotherapy. NOS1 may be an appropriate target for
reducing chemoresistance to ovarian cancer therapy.
Acknowledgements
The authors would like to thank Dr Yaru Wang (Cancer
Research Institute, Southern Medical University, Guangzhou,
Guangdong, China) for the critical discussion during the
experiments.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472834), Guangzhou
Science and Technology Research Project (grant no. 201400000001-1)
and the Ministry of Education Key Laboratory Open Project (grant
no. 2013jsz105).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZWZ and XXL participated in the design of the study,
performed all experiments and ZWZ wrote the manuscript. YLS, LQZ
and ZZ assisted performing cells cultures, western blotting and
invasion and migration assays. LLL, QBZ, MW and QLW helped with the
statistical analysis. ZJL, BTH, YFW, YFP and KTY participcated in
the design of the study. QZL conceived the study and assisted
editing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rapozzi V, Della Pietra E and Bonavida B:
Dual roles of nitric oxide in the regulation of tumor cell response
and resistance to photodynamic therapy. Redox Biol. 6:311–317.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukumura D, Kashiwagi S and Jain RK: The
role of nitric oxide in tumour progression. Nat Rev Cancer.
6:521–534. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choudhari SK, Chaudhary M, Bagde S,
Gadbail AR and Joshi V: Nitric oxide and cancer: A review. World J
Surg Oncol. 11:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambs S and Glynn SA: Candidate pathways
linking inducible nitric oxide synthase to a basal-like
transcription pattern and tumor progression in human breast cancer.
Cell Cycle. 10:619–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Switzer CH, Glynn SA, Cheng RY, Ridnour
LA, Green JE, Ambs S and Wink DA: S-nitrosylation of EGFR and Src
activates an oncogenic signaling network in human basal-like breast
cancer. Mol Cancer Res. 10:1203–1215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vannini F, Kashfi K and Nath N: The dual
role of iNOS in cancer. Redox Biol. 6:334–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Wang ZF, Xu Y, Ren R, Heng BL and Su
ZX: Association between three eNOS polymorphisms and cancer risk: A
meta-analysis. Asian Pac J Cancer Prev. 15:5317–5324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Broholm H, Rubin I, Kruse A, Braendstrup
O, Schmidt K, Skriver EB and Lauritzen M: Nitric oxide synthase
expression and enzymatic activity in human brain tumors. Clin
Neuropathol. 22:273–281. 2003.PubMed/NCBI
|
|
9
|
Ambs S, Bennett WP, Merriam WG, Ogunfusika
MO, Oser SM, Khan MA, Jones RT and Harris CC: Vascular endothelial
growth factor and nitric oxide synthase expression in human lung
cancer and the relation to p53. Br J Cancer. 78:233–239. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Zhou R, Sulman EP, Scheurer ME,
Boehling N, Armstrong GN, Tsavachidis S, Liang FW, Etzel CJ, Conrad
CA, et al: Genetic modulation of neurocognitive function in glioma
patients. Clin Cancer Res. 21:3340–3346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kotake M, Sato K, Mogi C, Tobo M, Aoki H,
Ishizuka T, Sunaga N, Imai H, Kaira K, Hisada T, et al: Acidic pH
increases cGMP accumulation through the OGR1/phospholipase
C/Ca(2+)/neuronal NOS pathway in N1E-115 neuronal cells. Cell
Signal. 26:2326–2332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Q, Tomei S, Ascierto ML, De Giorgi V,
Bedognetti D, Dai C, Uccellini L, Spivey T, Pos Z, Thomas J, et al:
Melanoma NOS1 expression promotes dysfunctional IFN signaling. J
Clin Invest. 124:2147–2159. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamaoka R, Yaginuma Y, Takahashi T, Fujii
J, Koizumi M, Seo HG, Hatanaka Y, Hashizume K, Ii K, Miyagawa J, et
al: Different expression patterns of nitric oxide synthase isozymes
in various gynecological cancers. J Cancer Res Clin Oncol.
125:321–326. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomsen LL, Sargent JM, Williamson CJ and
Elgie AW: Nitric oxide synthase activity in fresh cells from
ovarian tumour tissue: Relationship of enzyme activity with
clinical parameters of patients with ovarian cancer. Biochem
Pharmacol. 56:1365–1370. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caneba CA, Yang L, Baddour J, Curtis R,
Win J, Hartig S, Marini J and Nagrath D: Nitric oxide is a positive
regulator of the Warburg effect in ovarian cancer cells. Cell Death
Dis. 5:e13022014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leung EL, Fraser M, Fiscus RR and Tsang
BK: Cisplatin alters nitric oxide synthase levels in human ovarian
cancer cells: Involvement in p53 regulation and cisplatin
resistance. Br J Cancer. 98:1803–1809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lawrie TA, Winter-Roach BA, Heus P and
Kitchener HC: Adjuvant (post-surgery) chemotherapy for early stage
epithelial ovarian cancer. Cochrane Database Syst Rev.
12:CD0047062015.
|
|
19
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park B, Park S, Kim TJ, Ma SH, Kim BG, Kim
YM, Kim JW, Kang S, Kim J, Kim TJ, et al: Epidemiological
characteristics of ovarian cancer in Korea. J Gynecol Oncol.
21:241–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goff BA, Mandel L, Muntz HG and Melancon
CH: Ovarian carcinoma diagnosis. Cancer. 89:2068–2075. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vasudevan D and Thomas DD: Insights into
the diverse effects of nitric oxide on tumor biology. Vitam Horm.
96:265–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Apps MG, Choi EH and Wheate NJ: The
state-of-play and future of platinum drugs. Endocr Relat Cancer.
22:R219–R233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|