Introduction
The tongue squamous cell carcinoma (SCC) is the most
common oral cancer, and even in the early stage, some cases may be
aggressive with poor prognosis. Therefore, the National
Comprehensive Center Network guideline (1) recommends elective neck lymph node
dissection even for clinical N0 cases when the depth of invasion
(DOI) of the tumor is greater than 4 mm. However, more than half of
the patients undergo inappropriate neck dissections (2), and therefore, establishment of a proper
neck lymph node metastasis (NLM) predictor-that can be determined
from the primary lesion-has long been sought. Clinical N status
determination includes various bias related to the influence of the
modality and evaluator. In addition, cervical lymph node metastasis
cannot be assessed at the cellular level with images. In contrast,
pathological results provide a more precise assessment of lymph
node metastasis with little bias, regardless of clinical N
status.
Studies have shown that tumor thickness (3,4), DOI
(5,6), lymphatic vessel invasion (ly) or
vascular vessel invasion (v) (7),
perineural invasion (8), worst
pattern of invasion (9), and YK
classification (10) are
pathological factors that correlate with NLM. Among these, DOI has
been adopted in the recent UICC TNM classification (11), but there is no clear threshold for
predicting NLM, which definitely distinguishes NLM presence from
absence for reliable practical use.
Recently, tumor budding has been drawing
considerable attention. A tumor budding nest, which consists of a
single or less than five cancer cells present on the invasive front
of cancer, closely correlates with NLM and prognosis of colon and
other cancers, and tumor budding grade (TBG) has been incorporated
into the treatment algorithm of colon cancer (12). Some previous studies of SCC in head
and neck have shown the correlation of NLM with TBG, but they were
limited to high TBG cases (13,14). In
our previous study (15), we found
that TBG is the most important prognostic factor for the early
T-stage tongue SCC. High TBG indicates a high risk for NLM, and is
superior to DOI at predicting NLM. The majority of patients with
high TBG showed NLM, and minority of those with low TBG had NLM.
However, in terms of the total number of patients with NLM, they
were almost evenly divided into low and high TBG groups, indicating
that the number of NLM-positive patients in the low TBG group could
not be ignored. Hence, there is a demand for an NLM predictor that
can be combined with TBG for predicting tongue SCC with low
TBG.
Podoplanin has long been known to be a lymphatic
endothelial marker, but has recently been reported to be expressed
in a variety of tumors including SCC (16). In addition, it is a marker of stem
cell, and is associated with tumor lymphangiogenesis and promotes
metastasis by aggregating platelets, consequently preventing immune
attack. It is noteworthy that podoplanin expressed cells have been
reported to be located at the invasive front of tumors, to have a
high metastatic potential (17,18), and
podoplanin expression in dysplastic epithelium could be a
predictive marker for tumorigenesis in precancerous lesions
(19). The expression of podoplanin
at the front of a tumor invasion is observed in routine diagnosis,
but there is no report examining the significance of podoplanin
expression in tumor budding.
The purpose of this study was to investigate whether
podoplanin expression in tumor budding in tongue SCC with low TBG
might be an efficient NLM predictor. This is the routine-based
method of extracting cases with low TGB, but which may potentially
have a high NLM risk. This may contribute to prognosis prediction
and treatment planning for tongue SCC patients.
Materials and methods
Patients and samples
In this retrospective study, 99 patients with tongue
SCC at clinically early T-stages of any clinical N status according
to the UICC TNM seventh edition (20), who underwent surgical resection in
the Saitama Medical University International Medical Center between
2007 and 2016, were enrolled. These patients had to meet the
following criteria: no previous history of neoadjuvant therapy, a
minimum follow-up period of 1 year for surviving cases, adequate
specimens for histological observation and immunohistochemical
analysis, and histologically confirmed invasion. The pathological
stage was determined according to the UICC TNM eighth revised
edition (11), and the cut off
values for DOI and thickness were also based on the
classification.
The Institutional Review Board at the Saitama
Medical University International Medical Center approved this study
(approval no. 17-201). All methods were performed in accordance
with the 1975 Declaration of Helsinki.
Histopathological evaluation of
TBG
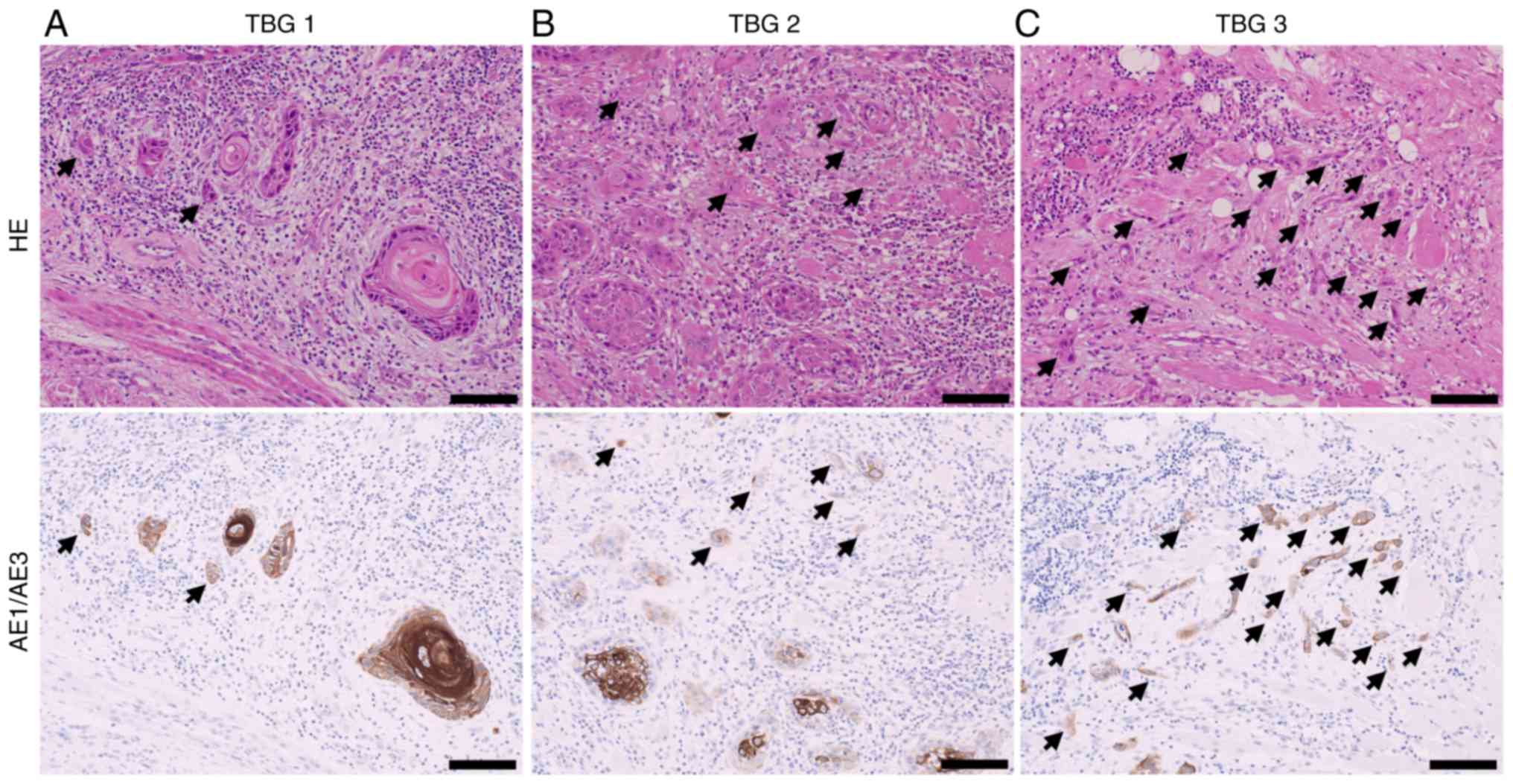
According to the previous study (21,22),
specimens were scanned to determine the area with the highest
density of budding and observed under a ×20 objective lens and a
×10 ocular lens. TBG was classified as 1–3 according to the number
of tumor buds: 1, 0 to 4 buds; 2, 5 to 9 buds; and 3, ≥10 buds
(Fig. 1). Two authors (HM and NK)
independently evaluated each case according to the criteria
mentioned above using hematoxylin-eosin-stained and cytokeratin
AE1/3-stained slides. Disagreements between the two assessors were
resolved by re-reviewing them or having them reviewed by another
assessor (YM). Cytokeratin AE1/3-staining was useful for a less
experienced pathologist (HM), as previously reported (23). The cases were divided into two
groups: high TBG (TBG3) and low TBG (TBG1/2) based on previous
studies (14,15).
Immunohistochemistry and
evaluation
Paraffin sections of 4-µm thickness were
immunostained with the AE1/3 antibody (PCK26, cocktail antibody,
Ventana; Roche Tissue Diagnostics Japan; cat. no. 760-2595) and the
podoplanin antibody (clone D2-40, Dako Agilent Technologies, Inc.;
cat. no. M3619), respectively, using an automated immunostainer
(VENTANA BenchMark ULTRA system; Ventana Medical Systems, Inc.)
according to the manufacturers' protocol. The incubation with
secondary antibodies and detection were carried out using the
I–VIEW DAB Universal kit (Ventana Medical Systems, Inc.; cat. no.
760-041) and Endogenous Biotin Blocking kit (Ventana Medical
Systems, Inc.; cat. no. 760-050).
Podoplanin expression in budding cells, which was
confirmed by AE1/3 expression, was evaluated independently by the
same authors mentioned above (HM and NK). The results were scored
from 0 to 3 based on the intensity of the staining at the membrane
or in the cytoplasm: 0, no reactivity; +1, weak; +2, moderate; and
3, marked, that is, overexpression (Fig.
2). For analysis, the results were divided into 2 groups:
negative (score 0) and positive (from +1 to +3). The ly of the
tumor tissue was evaluated using hematoxylin and eosin staining or
podoplanin staining for detecting lymphatic vessels (negative;
positive).
 | Figure 2.Scoring of PDPN in tumor budding cells
by immunohistochemical stain analysis with D2-40. After confirming
budding cells by Pan-Cytokeratin AE1/3 staining (upper figures),
the PDPN score was judged in the same budding cells (lower
figures). (A) was judged to be score 0 and (B), (C), and (D) were
judged to be scores 1, 2, and 3, respectively. For statistical
analysis (A) is determined to be negative. (B), (C), and (D) are
determined to be positive. The arrows indicate each bud and each
field at magnification, 60×10. Scale bar, 40 µm. PDPN,
podoplanin. |
Statistical analysis
The clinicopathological characteristics of the
patients were compared using the Chi-square test or Fisher's exact
test. Disease-specific survival (DSS) rate was compared using
Kaplan-Meier method with log-rank test and Cox proportional hazards
model for a multivariate analysis. The independent prognostic
strength of NLM was determined using a logistic regression model.
All statistical analyses were performed using IBM SPSS software
(version 24.0; IBM Corp.), and P<0.05 was considered to indicate
a statistically significant difference.
Results
The 99 patients comprised 72 males and 27 females
with a median age of 63 years old (range: 20–89 years) and median
follow-up of 39 months (range: 6–121 months) (Table I). Forty-three patients were
categorized as wait and watch and neck dissection was performed for
56 patients. After primary resection, 14 patients without neck
dissection were classified as doubtful NLM and subsequently
subjected to therapeutic neck dissection. Pathological lymph node
metastasis was present in 39 cases consisting of 27 cases with
initial neck dissection and 12 cases with therapeutic neck
dissection. In clinical N0 patients, there was no difference in the
disease specific survival rate between patients in the wait and
watch group and the elective neck dissection group (P=0.883). At
that time, elective neck dissection was recommended for patients
with late T2 (diameter of ≥30 mm) tumors. In regard to intermediate
tumors that were between superficial tumors and late T2 tumors,
physicians individually considered tumor thickness, localization,
and the patient's health condition.
 | Table I.Patient characteristics and NLM
analysis. |
Table I.
Patient characteristics and NLM
analysis.
|
| All cases (n=99) | Low TBG cases
(n=77) |
|---|
|
|
|
|
|---|
|
|
| NLM |
|
| NLM |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Characteristics | No. | - (%) 60 (61) | + (%) 39 (39) | P-valuea | No. | - (%) 54 (70) | + (%) 23 (30) | P-valuea |
|---|
| Age
(20–89)b |
|
|
| 0.077 |
|
|
| 0.332 |
|
<63 | 50 | 26 (52) | 24 (48) |
| 37 | 24 (65) | 13 (35) |
|
| ≥63 | 49 | 34 (69) | 15 (31) |
| 40 | 30 (75) | 10 (25) |
|
| Sex |
|
|
| 0.769 |
|
|
| 0.813 |
| Male | 72 | 43 (60) | 29 (40) |
| 55 | 39 (71) | 16 (29) |
|
|
Female | 27 | 17 (63) | 10 (37) |
| 22 | 15 (68) | 7
(32) |
|
| pT |
|
|
|
0.004a |
|
|
| 0.109 |
| pT1 | 29 | 24 (83) | 5
(17) |
| 25 | 21 (84) | 4
(16) |
|
|
≥pT2 | 70 | 36 (51) | 34 (49) |
| 52 | 33 (63) | 19 (37) |
|
| pDiameter (7–53
mm)b |
|
|
|
0.012a |
|
|
| 0.076 |
| ≤20
mmc | 51 | 37 (73) | 14 (27) |
| 42 | 33 (79) | 9
(21) |
|
| >20
mm | 48 | 23 (48) | 25 (52) |
| 35 | 21 (60) | 14 (40) |
|
| DOI (0–25
mm)b |
|
|
|
0.024a |
|
|
| 0.467 |
| ≤5
mmc | 39 | 29 (74) | 10 (26) |
| 35 | 26 (74) | 9
(26) |
|
| >5
mm | 60 | 31 (52) | 29 (48) |
| 42 | 28 (67) | 14 (33) |
|
|
Differentiation |
|
|
| 0.170 |
|
|
| 0.113 |
|
Well | 78 | 50 (64) | 28 (36) |
| 62 | 46 (74) | 16 (26) |
|
| Mod and
Por | 21 | 10 (48) | 11 (52) |
| 15 | 8
(53) | 7
(47) |
|
| ly |
|
|
| 0.001a |
|
|
| 0.008a |
|
(−) | 87 | 58 (67) | 29 (33) |
| 69 | 52 (75) | 17 (25) |
|
|
(+) | 12 | 2
(17) | 10 (83) |
| 8 | 2
(25) | 6
(75) |
|
| v |
|
|
| 0.001a |
|
|
| 0.055 |
|
(−) | 67 | 48 (72) | 19 (28) |
| 58 | 44 (76) | 14 (24) |
|
|
(+) | 32 | 12 (38) | 20 (63) |
| 19 | 10 (53) | 9
(47) |
|
| neu |
|
|
| 0.072 |
|
|
| 0.117 |
|
(−) | 82 | 53 (65) | 29 (35) |
| 68 | 50 (74) | 18 (26) |
|
|
(+) | 17 | 7
(41) | 10 (59) |
| 9 | 4
(44) | 5
(56) |
|
| TBG |
|
|
|
<0.001a |
|
|
|
|
|
1+2 | 77 | 54 (70) | 23 (30) |
|
|
|
|
|
| 3 | 22 | 6
(27) | 16 (73) |
|
|
|
|
|
| PDPN |
|
|
|
0.007a |
|
|
| 0.002a |
|
(−) | 39 | 30 (77) | 9
(23) |
| 34 | 30 (88) | 4
(12) |
|
|
(+) | 60 | 30 (50) | 30 (50) |
| 43 | 24 (56) | 19 (44) |
|
| Status |
|
|
|
|
|
|
|
|
|
NED | 82 | 56 (68) | 26 (32) |
| 68 | 52 (76) | 16 (24) |
|
|
DOD | 17 | 4
(24) | 13 (76) |
| 9 | 2
(22) | 7
(78) |
|
TBG evaluation resulted in 41 patients (41%) for
TBG1, 36 (36%) for TBG2, and 22 (22%) for TBG3. Based on podoplanin
expression, 39 patients (39%) were negative and 60 patients (61%)
were positive: weak (+1), 35 patients; moderate (+2), 14 patients;
marked (+3), 11 patients. The final outcome was survival in 76
patients and death in 23 patients: disease-specific death, 17
patients; other causes of death, 6 patients (1 heart
disease-related death, 2 had carcinomas apart from the head and
neck, and 3 had pneumonia). DSS rate was 77% at 5 years (Fig. 3A).
In the low TBG group, there were significant
differences in the DSS between the NLM-positive and NLM-negative
groups (P=0.010; Fig. 3B) and
between the podoplanin-positive and podoplanin-negative groups
(P=0.006). In the low TBG with clinical N0 group (n=53), there was
no difference of disease specific survival rate between patient
with wait and watch group (n=36) and with elective neck dissection
group (n=17) (P=0.803; Fig. 3C).
Correlations between NLM and variable
clinicopathological parameters such as pT (P=0.004), pathological
diameter (pDiameter) (P=0.012), DOI (P=0.024), ly (P=0.001), v
(P=0.001), TBG (P<0.001), and podoplanin (P=0.007) were found to
be significant in all the patients (Table I). However, in the low TBG group, ly
(P=0.008) and podoplanin (P=0.002) were significant for NLM.
The multivariate analysis of NLM was performed by
using a logistic regression model with pDiameter, DOI and ly, TBG,
and podoplanin (T factor itself was not included since pDiameter
and DOI were components of T factor). The results showed that ly
[odds ratio (OR)=11.5, 95% confidence interval (CI): 1.50–87.6;
P=0.02] and podoplanin (OR=7.07, 95% CI: 1.80–27.7; P=0.005) were
independent predictors of NLM (Table
II). In the low TBG group, ly (P=0.019) and podoplanin
(P=0.005) presented a significant difference.
 | Table II.Multivariate analysis of NLM
risk. |
Table II.
Multivariate analysis of NLM
risk.
|
|
| All cases
(n=99) | Low TBG cases
(n=77) |
|---|
|
|
|
|
|
|---|
|
Characteristics | Value | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| DOI | ≤5 vs. >5
mma | 1.27 | 0.41–3.89 | 0.678 |
0.88 | 0.26–3.01 | 0.834 |
| pDiameter | ≤20 vs. >20
mma | 2.63 | 0.94–7.40 | 0.067 |
1.97 | 0.61–6.37 | 0.257 |
| ly | (−) vs. (+) | 9.78 | 1.68–56.8 |
0.011b | 11.5 | 1.50–87.6 |
0.019b |
| TBG | 1+2 vs. 3 | 4.71 | 1.47–15.1 |
0.009b |
|
|
|
| PDPN | (−) vs. (+) | 3.47 | 1.18–10.2 |
0.023b |
7.07 | 1.80–27.7 |
0.005b |
We analyzed the predictive value of NLM. The ratio
of NLM was 30% in the low TBG group (Fig. 4A). DOI was not significant for
stratification of NLM (Fig. 4B).
Lymphatic vessel invasion had a negative predictive value (NPV) of
75% and positive predictive value (PPV) of 75% (Fig. 4C). Podoplanin had NPV of 88% and PPV
of 44% (Fig. 4D).
Discussion
In this study, podoplanin expression was found to
serve as a malignant index in the low TBG tongue SCC patients. In
these patients, DOI was considered less useful, whereas ly and
podoplanin were expected to become independent predictors of
NLM.
For patients with the low TBG, because of the high
PPV, neck lymph node dissection is recommended if they are positive
for ly. Lymphatic angiogenesis and expansion within a tumor is
considered to promote tumor invasion and metastasis, based on the
presumed passive metastasis mechanism (24). However, positivity for ly was only
found in eight cases (10% in low TBG cases), and 74% of
NLM-positive cases in the low TBG cases were negative for ly.
Hence, in treating ly-negative patients, it may not be practical to
adopt a wait and watch policy without neck lymph node
dissection.
Moreover, the NPV of podoplanin (88%) was superior
to its PPV (56%). Only four (12%) podoplanin-negative cases were
found to be NLM-positive, implying that podoplanin, compared to ly,
has the advantage of fractionation in number. For
podoplanin-negative in the low TBG patients, the wait and watch
policy should be considered. This policy could reduce unnecessary
neck dissections. Where high TBG can be distinguished by
morphological appearance, malignant risks of low TBG cases can be
distinguished by the combined use of immunohistochemical podoplanin
expression. Neck dissection poses risks of complications and
sequelae. It also causes prolonging of operation time or
hospitalization period, in addition to cosmetic or functional
problems, thus affecting patients' quality of life. Hence,
selecting patients not requiring neck dissection is beneficial.
In our pilot study, we evaluated the degree of
inflammation surrounding the buddings and analyzed its correlation
with podoplanin expression using the Chi-squared test, resulting in
a significant difference (P<0.05, data not shown). This result
is consistent with previous reports that inflammation is associated
with podoplanin expression (16). In
addition, podoplanin expression is thought to involve not only
inflammation but also multiple factors like EMT. It is known that
downregulation of E-cadherin and upregulation of vimentin occur
when EMT is developed (25).
Immunohistochemical staining of E-cadherin and vimentin was also
conducted in our pilot study (data not shown). However,
quantitative immunohistochemical evaluation of staining intensity,
which means “increase and decrease” in these markers, is easily
affected by various conditions, such as fixation and thickness of
the section, which make reproducible and objective staining
evaluation difficult. Moreover, vimentin is expressed on various
non-epithelial cells, especially mesenchymal cells, including
fibroblasts, endothelial cells, lymphocytes, and macrophages. This
raises the difficulty in evaluating vimentin expression in buddings
because of the positive reactions for vimentin in various cells.
However, podoplanin showed the following advantages: it was
expressed in fewer cells, and it was already widely established as
a marker for detecting lymphatic invasion of tumor cells. Moreover,
lymphovascular invasion was evaluated simultaneously when
podoplanin expression in buddings was examined.
The limitations of this study were the small sample
size and the lack of understanding of the mechanism underlying
podoplanin expression in tumor buddings. Future research should
expand the sample size to strengthen the relevance of the data
presented in this study. The detailed mechanism of podoplanin
expression in tumor budding cells has also yet to be elucidated.
However, it is reported that podoplanin is affected by various
phenomena, such as inflammation and wound healing, and is known to
be associated with immune cells, cytokines, and EMT or non-EMT
pathways (16,19). Future comprehensive analyses,
including investigation of variable podoplanin-related factors and
standardization of the evaluation procedure, are expected to make
podoplanin more useful.
In conclusion, the results of the present study
emphasized that risk stratification by podoplanin expression
associated with low TBG among patients with tongue SCC could
provide a beneficial outcome. It could be expected to find the low
TBG cases having a high NLM risk, based on the podoplanin-positive
reaction. In general, these cases are difficult to predict the risk
only morphologically. When the patients have not had a neck
dissection, the podoplanin expression would be helpful for
consideration of an additional treatment plan. This approach may be
proposed as an accurate treatment algorithm for tongue SCC
patients.
Acknowledgements
The authors would like to thank Mr. Kouichi Kamada
and Ms. Akemi Miyata (Department of Pathology, Saitama Medical
University International Medical Center) for their technical
support.
Funding
The current study was supported by the Hidaka
Research Projects from the Saitama Medical University (grant no.
31-D-1-11) and the Japan Society for the Promotion of Science
KAKENHI Grant-in-Aid for Scientific Research C (grant no.
17K11404).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH took part in conception, design, acquisition,
analysis and interpretation of data, and drafted the manuscript.
MYasuda took part in conception, design, critical revision of the
manuscript for important intellectual content and supervision of
the study. YE took part in conception, design, acquisition and
drafted the manuscript. KN and MYano took part in analysis and
interpretation of data and drafted the manuscript. YK took part in
acquisition of data and important intellectual content. MN, MS and
HN took part in acquisition of data and supervision.
Ethics approval and consent to
participate
The current study research was a retrospective study
with approval of the Institutional Review Board at the Saitama
Medical University International Medical Center (approval no.
17-201) and the contents of this study are open to the public at
our hospital. All methods were performed in accordance with the
1975 Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DOI
|
depth of invasion
|
|
DSS
|
disease-specific survival
|
|
EMT
|
epithelial mesenchymal
transformation
|
|
ly
|
lymphatic vessel invasion
|
|
NLM
|
neck lymph node metastasis
|
|
NPV
|
negative predictive values
|
|
pDiameter
|
pathological diameter of tumor
|
|
TBG
|
tumor budding grade
|
|
v
|
vascular vessel invasion
|
References
|
1
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology: Head and Neck
Cancers. Version 1.2019. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdfMarch
6–2019
|
|
2
|
Monroe MM and Gross ND: Evidence-based
practice: Management of the clinical node-negative neck in
early-stage oral cavity squamous cell carcinoma. Otolaryngol Clin
North Am. 45:1181–1193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang SH, Hwang D, Lockwood G, Goldstein
DP and O'Sullivan B: Predictive value of tumor thickness for
cervical lymph-node involvement in squamous cell carcinoma of the
oral cavity: A meta-analysis of reported studies. Cancer.
115:1489–1497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Massano J, Regateiro FS, Januário G and
Ferreira A: Oral squamous cell carcinoma: Review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitani S, Tomioka T, Hayashi R, Ugumori T,
Hato N and Fujii S: Anatomic Invasive Depth Predicts Delayed
Cervical Lymph Node Metastasis of Tongue Squamous Cell Carcinoma.
Am J Surg Pathol. 40:934–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kane SV, Gupta M, Kakade AC and D'Cruz A:
Depth of invasion is the most significant histological predictor of
subclinical cervical lymph node metastasis in early squamous
carcinomas of the oral cavity. Eur J Surg Oncol. 32:795–803. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adel M, Kao HK, Hsu CL, Huang JJ, Lee LY,
Huang Y, Browne T, Tsang NM, Chang YL and Chang KP: Evaluation of
Lymphatic and Vascular Invasion in Relation to Clinicopathological
Factors and Treatment Outcome in Oral Cavity Squamous Cell
Carcinoma. Medicine (Baltimore). 94:e15102015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Binmadi NO and Basile JR: Perineural
invasion in oral squamous cell carcinoma: A discussion of
significance and review of the literature. Oral Oncol.
47:1005–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brandwein-Gensler M, Teixeira MS, Lewis
CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML and Wang BY:
Oral squamous cell carcinoma: Histologic risk assessment, but not
margin status, is strongly predictive of local disease-free and
overall survival. Am J Surg Pathol. 29:167–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto E, Kohama G, Sunakawa H, Iwai M
and Hiratsuka H: Mode of invasion, bleomycin sensitivity, and
clinical course in squamous cell carcinoma of the oral cavity.
Cancer. 51:2175–2180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brierley JD, Gospodarowicz MK and
Wittekind C: UICC TNM Classification of Malignant Tumours. 8th.
Wiley Blackwell; New York, NY: 2017
|
|
12
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
et al Japanese Society for Cancer of the Colon and Rectum, :
Japanese Society for Cancer of the Colon and Rectum (JSCCR)
guidelines 2016 for the treatment of colorectal cancer. Int J Clin
Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Almangush A, Pirinen M, Heikkinen I,
Mäkitie AA, Salo T and Leivo I: Tumour budding in oral squamous
cell carcinoma: A meta-analysis. Br J Cancer. 118:577–586. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Y, Liu H, Xie N, Liu X, Huang H, Wang
C and Hou J: Impact of tumor budding in head and neck squamous cell
carcinoma: A meta-analysis. Head Neck. 41:542–550. 2019.PubMed/NCBI
|
|
15
|
Ebihara Y, Yoshida S, Nakahira M,
Kogashiwa Y, Enoki Y, Kuba K, Inoue H, Minami K, Yasuda M and
Sugasawa M: Importance of tumor budding grade as independent
prognostic factor for early tongue squamous cell carcinoma. Head
Neck. 41:1809–1815. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quintanilla M, Montero-Montero L, Renart J
and Martín-Villar E: Podoplanin in Inflammation and Cancer. Int J
Mol Sci. 20:E7072019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puram SV, Parikh AS and Tirosh I: Single
cell RNA-seq highlights a role for a partial EMT in head and neck
cancer. Mol Cell Oncol. 5:e14482442018.PubMed/NCBI
|
|
18
|
Puram SV, Tirosh I, Parikh AS, Patel AP,
Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, et
al: Single-Cell Transcriptomic Analysis of Primary and Metastatic
Tumor Ecosystems in Head and Neck Cancer. Cell. 171:1611–1624.e24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swain N, Kumar SV, Routray S, Pathak J and
Patel S: Podoplanin - a novel marker in oral carcinogenesis. Tumour
Biol. 35:8407–8413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sobin LH, Gospodarowicz MK and Wittekind
C: UICC TNM Classification of Malignant Tumours. 7th. Wiley; West
Sussex: 2009
|
|
21
|
Lugli A, Kirsch R, Ajioka Y, Bosman F,
Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann
A, et al: Recommendations for reporting tumor budding in colorectal
cancer based on the International Tumor Budding Consensus
Conference (ITBCC) 2016. Mod Pathol. 30:1299–1311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie N, Yu P, Liu H, Liu X, Hou J, Chen X,
Huang H and Wang C: Validation of the International Tumor Budding
Consensus Conference (2016) recommendations in oral tongue squamous
cell carcinoma. J Oral Pathol Med. 48:451–458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kai K, Aishima S, Aoki S, Takase Y,
Uchihashi K, Masuda M, Nishijima-Matsunobu A, Yamamoto M, Ide K,
Nakayama A, et al: Cytokeratin immunohistochemistry improves
interobserver variability between unskilled pathologists in the
evaluation of tumor budding in T1 colorectal cancer. Pathol Int.
66:75–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stacker SA, Williams SP, Karnezis T,
Shayan R, Fox SB and Achen MG: Lymphangiogenesis and lymphatic
vessel remodelling in cancer. Nat Rev Cancer. 14:159–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aiello NM and Kang Y: Context-dependent
EMT programs in cancer metastasis. J Exp Med. 216:1016–1026. 2019.
View Article : Google Scholar : PubMed/NCBI
|