Introduction
In recent decades, non-coding RNAs (ncRNAs) were
assumed to be transcripts of junk DNA that had no involvement in
biological processes. However, in emerging studies focusing on the
functions of ncRNAs, they were revealed to be important regulators
of multiple pathways. ncRNAs were also revealed to be involved in
the regular development of organisms, in addition to the
progression of various diseases (1,2).
Furthermore, the development and progression of high-throughput
genomic technologies have allowed scientists to analyze complex
cellular transcriptomes, leading to the discovery of a myriad of
novel ncRNAs (3,4). A significant class of ncRNAs is the
long ncRNAs (lncRNAs), which are defined as transcripts of >200
nucleotides with no protein-coding potential (5). The regulatory roles of lncRNAs have
been demonstrated in a large number of distinct biological
processes (6–8), and multiple lncRNAs have proven their
potential as therapeutic candidates in cancer (9–11). Given
that cancer is largely caused by genetic alterations distributed in
non-coding regions of the genome, the roles of lncRNAs in the
progression of cancer have been increasingly emphasized in the last
few decades (12–14).
lncRNA prostate cancer (PCa)-associated transcript 1
(PCAT-1) has been reported as an oncogenic factor in PCa (15,16).
Upregulation of lncRNA PCAT-1 promoted the proliferation of PCa
cells and was associated with poor prognosis in patients with PCa
(17). Regarding its role in other
cancer types, the upregulation of lncRNA PCAT-1 was also indicated
in esophageal squamous carcinoma (18). Additionally, in a study by Qiao et
al (19), the inhibition of
lncRNA PCAT-1 suppressed the multidrug resistance and
aggressiveness of colorectal cancer cells.
In spite of the widely accepted oncogenic role of
lncRNA PCAT-1, the underlying mechanisms of this role remain to be
fully elucidated. A comprehensive investigation of the downstream
signaling of lncRNA PCAT-1 in different cancer types and stages may
lead to the development of lncRNA PCAT-1-based anti-tumor
therapies. Huang et al (20)
reported that lncRNA PCAT-1 acted as an oncogene in osteosarcoma by
reducing p21 levels; given that p21 is involved in the anti-tumor
effects of multiple agents (20–22),
lncRNA PCAT-1 inhibition may represent a potential treatment
strategy by restoring the levels of p21 in numerous cancer
types.
Tongue squamous cell carcinoma (TSCC) is the most
prevalent malignancy of the oral cavity, accounting for ~30% of all
oral cancer cases worldwide (23).
TSCC is a rapid-growth tumor type with a high risk of regional and
distant metastasis (24), thus,
early prediction and diagnosis are key to the successful management
of TSCC (24). According to a study
by Gao et al (25), multiple
lncRNAs, including lnc-PPP2R4-5, SPRR2D-1, MAN1A2-1 and FAM46A-1
are dysregulated in TSCC, indicating a close interaction between
lncRNAs and the oncogenesis of tongue cells. Regarding the role of
lncRNA PCAT-1 in the onset and progression of TSCC, initial
clinical investigations in the present study identified the
upregulation of lncRNA PCAT-1 in TSCC tissues. It was therefore
hypothesized that the inhibition of lncRNA PCAT-1 may impair the
proliferation and metastasis of TSCC associated with 21
upregulation.
To verify this hypothesis, lncRNA PCAT-1 was knocked
down in TSCC cell lines and the subsequent effects on the
proliferation, apoptosis, motility and p12 were assessed. The
results indicated that depletion of lncRNA PCAT-1 impaired the
growth, increased the apoptotic rate and reduced the metastatic and
invasive potential of TSCC cells, accompanied by an increase in the
expression levels of p21.
Materials and methods
TSCC specimen collection
A total of 23 pairs of TSCC and corresponding
peri-tumor samples were obtained from volunteers at the People's
Hospital of Tongliang District Chongqing City (Chongqing, China)
between January and December 2015. The cohort included 14 males
(60.9%) and 9 females (39.1%), with an average patient age of 51.5
years (range, 23–75 years). The patients were included based on the
following criteria: i) Patients were diagnosed with primary tongue
squamous cell carcinoma according to American Joint Committee on
Cancer; ii) no prior history of chemotherapy or radiotherapy; and
iii) patients underwent radical tumor resection. The peri-tumor
tissues were collected from regions 1.5 cm from the tumor.
Following dissection, the samples were stored at −80°C prior to
analysis using reverse transcription-quantitative PCR (RT-qPCR).
All patients provided written informed consent for the use of their
tissues and the study was approved by the ethics committee of the
People's Hospital of Tongliang District Chongqing City (Chongqing,
China); all procedures were performed in accordance with the
Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/).
Cell culture
The TSCC cell line CAL27 (cat. no. ZQ0606) was
obtained from OriGene Technologies, Inc. and the human TSCC cell
line Tca-8113 (cat. no. TCHu 77) was purchased from the cell bank
of the Type Culture Collection of the Chinese Academy of Sciences.
The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM) with 10% fetal bovine serum (both from Thermo Fisher
Scientific, Inc.) at 37°C and 5% CO2.
Construction of lncRNA PCAT-1 small
hairpin (sh)RNA vectors and transfection
shRNAs targeting PCAT-1 (shRNA-1,
5′-GCTCACGCCTGTAATCTCA-3′; and shRNA-2, 5′-GAACCTAACTGGACTTTAA-3′)
were synthesized by Sangon Biotech Co., Ltd. and inserted into the
pRNA-H1.1 plasmid (between BamIII and HindIII) to
construct 2 PCAT-1 suppression vectors (shRNA-1 and shRNA-2,
respectively). A non-targeting shRNA was employed as a negative
control (NC;
5′-GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT-3′).
Transfections were performed using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol, using 2 µg plasmid. Cells with stable lncRNA PCAT-1
knockdown were selected using 500 µg/ml G418 (Invitrogen; Thermo
Fisher Scientific Inc.). Subsequent experiments were conducted 48 h
following transfection.
MTT assay
The viability of PCAT-1-knockdown TSCC cells was
determined using an MTT assay. Briefly, cells were seeded into a
96-well plate and cultured for 96 h at 37°C; at 24-h intervals, 5
mg/ml MTT was added to 3 wells from each group, and the cells were
incubated for an additional 4 h at room temperature, leading to the
formation of a colored precipitate. DMSO was added to the wells to
dissolve the purple formazan crystals. The optical density at 490
nm (OD490) was detected using a microplate reader
(ELX-800; Biotek Instruments, Inc.).
Flow cytometry detection of
apoptosis
The effects of PCAT-1 knockdown on TSCC cell
apoptosis were detected using an Apoptosis Detection kit (cat. no.
KGA106; Nanjing KeyGen Biotech Co., Ltd.), using 1×105
cells/well, according to the manufacturer's protocol. The apoptotic
cells were detected using a FACScan flow cytometer (BD Biosciences)
and analyzed using the FlowJo 7.6.1 software (Tree Star, Inc.) The
total apoptotic rate was determined as the sum of the late and the
early apoptotic rates.
Wound healing assay
The effects of lncRNA PCAT-1 inhibition on cell
motility was determined using a wound-healing assay. TSCC cells
(2×104 cells/well) were seeded in a 24-well plate and
reference points were recorded to ensure the acquisition of the
identical area for imaging. After culturing for 2 days at 37°C (5%
CO2), the cell monolayers were scratched to generate a
cell-free wound and rinsed with PBS to remove cell debris from the
wound edges. The cells were incubated in DMEM once more, and the
migration distances (as the percentage of gap closure) were
measured at 0-, 12- and 24-h time points.
Transwell assay
The effect of lncRNA PCAT-1 inhibition on cell
invasion potential was detected using a Transwell assay. TSCC cells
(1×105 cells/well) suspended in serum-free DMEM were
added to the upper chamber of the Transwell inserts (membranes were
pre-coated with 40 µl Matrigel for 2 h at 37°C) and incubated for 2
h at 37°C; the cells were allowed to penetrate through the porous
membrane to the lower chamber [supplemented with 30% FBS (HyClone;
Thermo Fisher Scientific Inc.)] for 4 h. After removal of the cells
on the upper surface, the cells in the lower chamber were stained
with 0.5% (w/v) crystal violet for 5 min at room temperature.
Images were captured (magnification, ×200) under an inverted light
microscope (AE31; Motic) and the number of invaded cells was
calculated using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc.).
RT-qPCR
The total RNA of TSCC cells was extracted using the
RNApure High-purity Total RNA Rapid Extraction kit (cat. no.
RP1201; BioTeke Corporation) according to the manufacturer's
instructions. The cDNA templates were synthesized using Super M-MLV
(cat. no. PR6502, BioTeke Corporation) according to the
manufacturer's instruction; cDNA synthesis was conducted at 70°C
for 5 min with 1 µl oligo(dT)15, 1 µl random primers and
2 µl dNTPs (2.5 mM). The PCR system contained 10 µl
SYBR® Green master mix (Beijing Solarbio Science &
Technology Co., Ltd.), 0.5 µl of each primer (PCAT-1 forward,
5′-ACAGGCTGAGGCAGGAGAAT-3′; PCAT-1 reverse;
5′-CTTTGGGAAGTGCTTTGGAG-3′; β-actin forward,
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′; and β-actin reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′), 1 µl cDNA template and 8 µl
double-distilled H2O. The amplification conditions were
as follows: Denaturation at 95°C for 10 min, followed by 40 cycles
of amplification at 95°C for 10 sec, 60°C for 20 sec and 72°C for
30 sec. The reaction was terminated at 25°C for 5 min. The relative
expression levels of lncRNA PCAT-1 were determined using a
Real-time PCR system (Exicycler 96; Bioneer Corporation) according
to the 2−∆∆Cq method (26), and β-actin was used as the internal
reference.
Western blot analysis
Total protein was extracted from TSCC cells using
RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) and collected by centrifugation at 10,000 × g for 10
min at 4°C. SDS-PAGE (using a 12% gel) was performed with 40 µg
protein/sample. The proteins were transferred to PVDF membranes
that were subsequently blocked for 1 h at room temperature using 5%
skimmed milk powder. The membranes were then incubated with primary
antibodies against p21 (1:500 dilution; cat. no. D153319; Sangon
Biotech Co., Ltd.) and β-actin (1:1,000 dilution; cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.) at 4°C overnight,
followed by incubation with secondary horseradish
peroxidase-conjugated IgG antibodies (1:5,000 dilution; cat. no.
A0216 and A0208; Beyotime Institute of Biotechnology) at 37°C for
45 min. The membranes were developed using ECL Plus reagent (cat.
no. P0018; Beyotime Institute of Biotechnology) and the relative
expression levels were quantified using the Gel-Pro-Analyzer
software version 4.0 (Media Cybernetics, Inc.) with β-actin as the
internal reference.
Statistical analysis
The data are presented as the mean ± standard
deviation of three replicates. Statistical analyses were performed
using SPSS version 19.0 (IBM Corp.). One-way analysis of variance
and Duncan's multiple range test were conducted for comparisons
between >3 variables. The differences in lncRNA PCAT-1
expression levels between TSCC and peri-tumor tissue groups were
analyzed using paired Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
lncRNA PCAT-1 is upregulated in TSCC
specimens
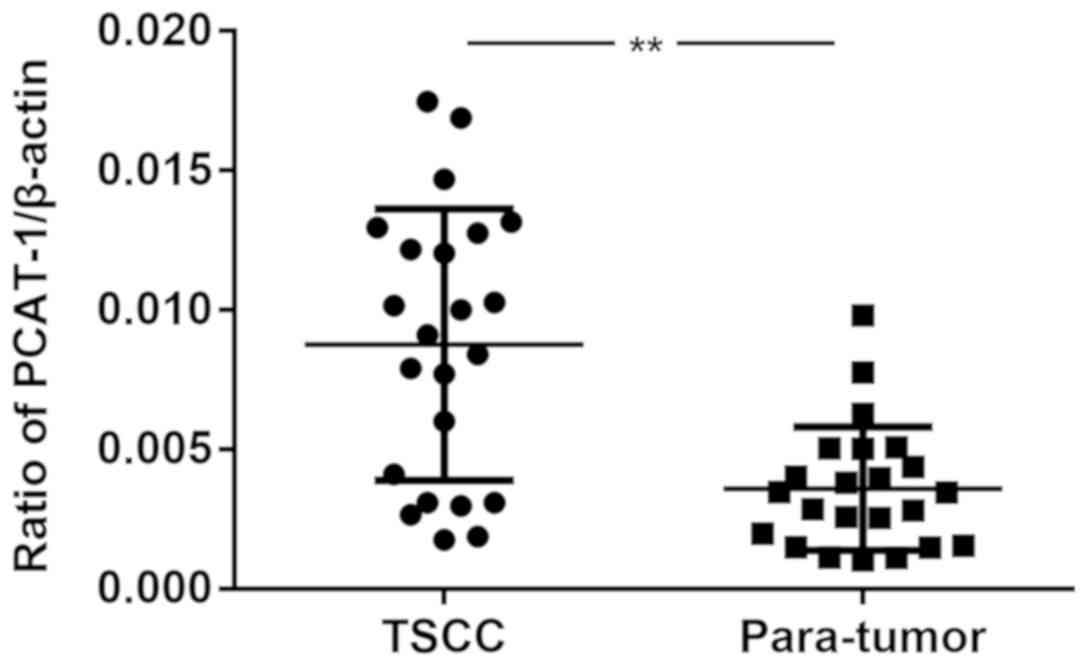
The expression levels of lncRNA PCAT-1 were detected
in 23 pairs of TSCC specimens and corresponding peri-tumor tissues
using RT-qPCR. As presented in Fig.
1, the expression levels of lncRNA PCAT-1 were significantly
upregulated in tumor tissues compared with those in the peri-tumor
tissues. The results demonstrated a possible positive association
between lncRNA PCAT-1 expression levels and TSCC progression.
Inhibition of lncRNA PCAT-1 suppresses
proliferation and induces apoptosis in TSCC cells
The TSCC cell lines CAL27 and Tca-8113 were
transfected with lncRNA PCAT-1-shRNA vectors and the knockdown was
confirmed using RT-qPCR (Fig. 2).
MTT and flow cytometry assays were subsequently performed to
establish the influence of lncRNA PCAT-1 inhibition on cell
proliferation and apoptosis. For each cell line, the
OD490 values of the knockdown groups were significantly
lower than those of the NC-transfected groups (Fig. 3A and B). Furthermore, lncRNA PCAT-1
knockdown resulted in a marked increase in apoptotic rate compared
with the NC group (Fig. 3C and D).
Taken together, these results confirmed that lncRNA PCAT-1
inhibition impaired proliferation and induced apoptosis in TSCC
cells.
Inhibition of lncRNA PCAT-1 reduces
the metastatic potential of TSCC cells
As presented in Fig. 4A
and B, lncRNA PCAT-1 inhibition reduced the closure of scratch
wounds compared with that in the Control and NC groups (Fig. 4A and B). The differences in the
wound-healing rate between lncRNA PCAT-1-knockdown and NC cells
were statistically significant (P<0.05; Fig. 4A and B). The results suggested that
lncRNA PCAT-1 inhibition significantly suppressed TSCC cell
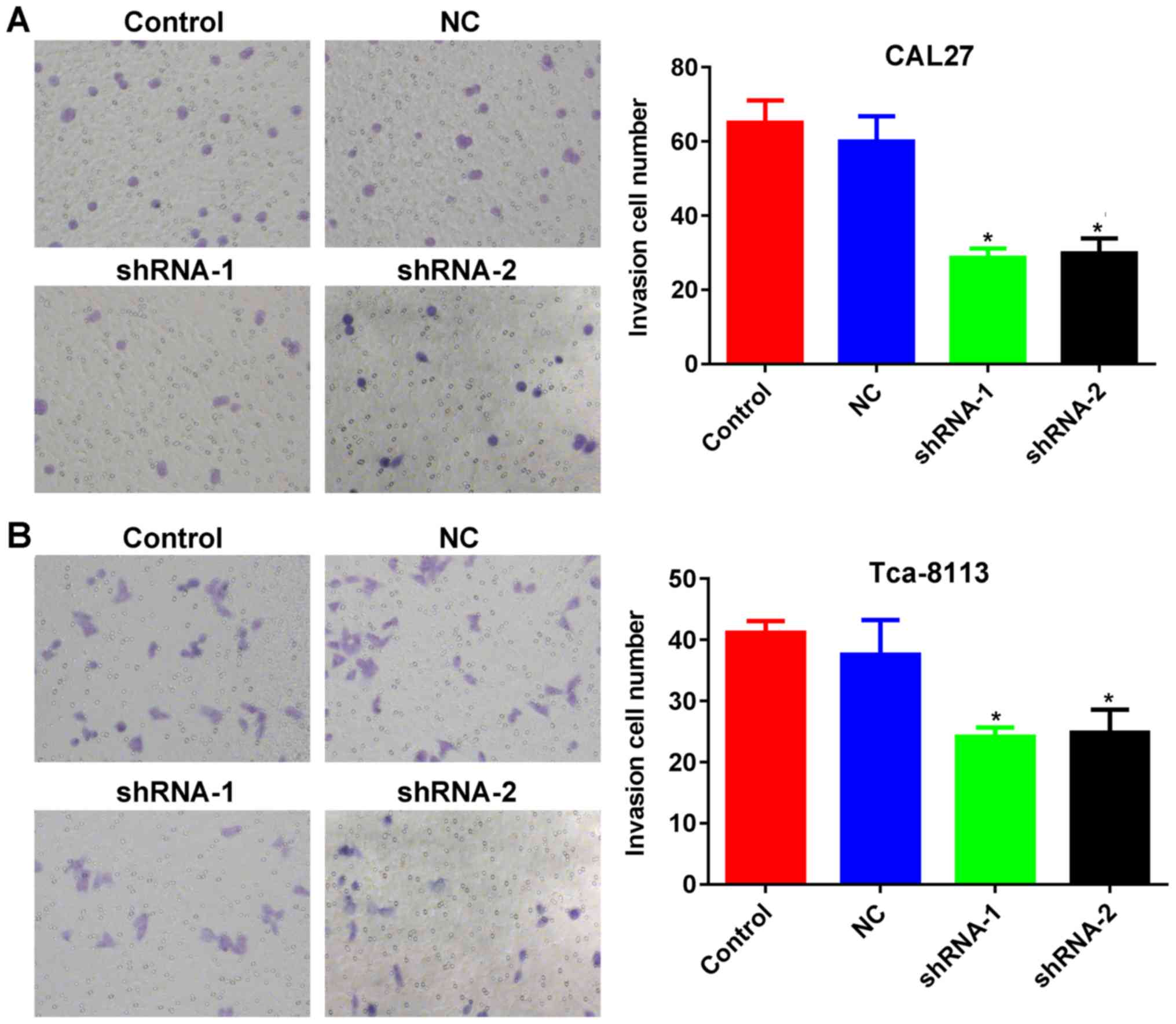
migration. In addition, Transwell assays indicated that the number
of TSCC cells penetrating through the membrane were significantly
lower in the knockdown groups compared with those in the control
groups (P<0.05; Fig. 5A and B),
demonstrating inhibited invasive potential as a result of lncRNA
PCAT-1 inhibition.
Inhibitory effect of lncRNA PCAT-1
knockdown in TSCC cells is associated with the upregulation of
p21
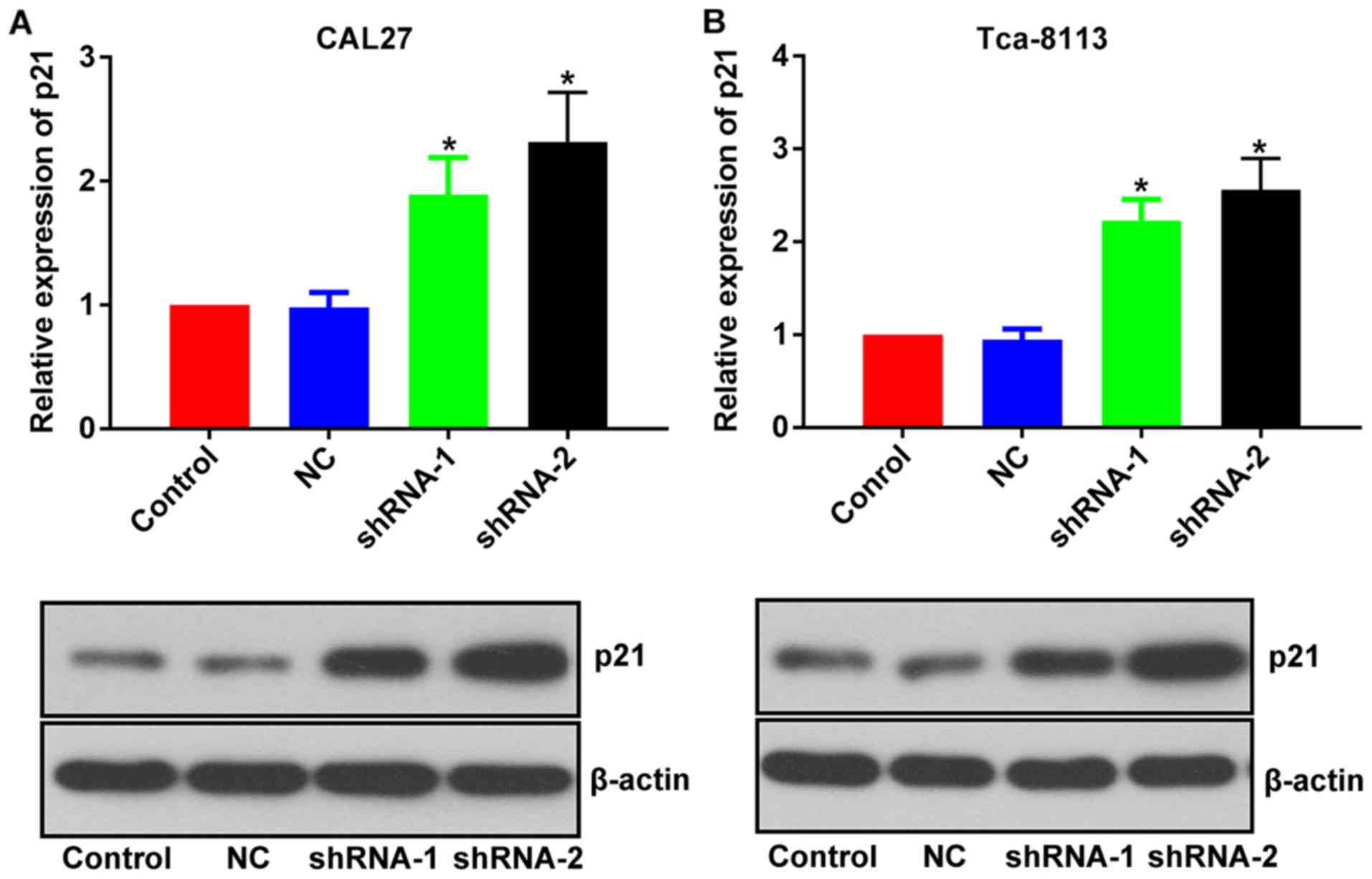
The mechanisms associated with the inhibitory effect
of lncRNA PCAT-1-knockdown were further investigated by focusing on
its influence on p21 expression level. Following the inhibition of
lncRNA PCAT-1, the expression level of p21 was upregulated in both
TSCC cell lines (Fig. 6). Given the
well-documented anti-tumor effect of p21 (20–22), the
anti-TSCC effect of lncRNA PCAT-1-knockdown may be associated with
the activation of p21 signaling.
Discussion
In the past 5 years, TSCC has become the most common
type of oral cancer (23). The
incidence and mortality rates associated with TSCC have been
steadily increasing, and patients with TSCC currently account for
one third of all oral cancer cases worldwide. Furthermore, TSCC is
one of the most aggressive subtypes of oral cancer and in spite of
the rapid progression in diagnostic and therapeutic strategies, the
5-year survival rate has remained unchanged (27). Therefore, it is necessary to explore
novel targets for the development of additional anti-TSCC
therapies.
Advancements in sequencing techniques have resulted
in the identification of multiple cancer-associated lncRNAs in TSCC
(25), and lncRNA-PPP2R4-5,
SPRR2D-1, MAN1A2-1 and FAM46A-1 have been reported to be
dysregulated in TSCC (25). In light
of previous findings, the present study explored the role of lncRNA
PCAT-1 on the growth and metastatic potential of TSCC. Furthermore,
given the fact that lncRNA PCAT-1 is known to inhibit expression of
the anti-tumor protein p21, the present study also explored the
downstream pathway mediating its function, focusing on its
interaction with p21. The results demonstrated that the expression
of lncRNA PCAT-1 was upregulated in clinical TSCC samples, and that
the inhibition of this lncRNA in TSCC cell lines impaired
proliferation, induced apoptosis and suppressed metastatic and
invasive potential. Regarding the downstream mechanisms at the
molecular level, knockdown of PCAT-1 resulted in the upregulation
of p21, indicating that these inhibitory effects on TSCC cells are,
at least in part associated with the activation of p21 and
downstream pathways.
The gene encoding lncRNA PCAT-1 is located on
chromosome 8q24, and it was originally identified as a biomarker
for prostate cancer (15).
Subsequently, altered expression levels of lncRNA PCAT-1 were also
reported in other cancer types; Shi et al (18) reported that the upregulation of
lncRNA PCAT-1 was associated with the development of esophageal
squamous cell carcinoma. A study by Qiao et al (19) reported that inhibition of lncRNA
PCAT-1 impaired the multidrug resistance and aggressiveness of
colorectal cancer cells. Given the involvement of lncRNA PCAT-1 in
the genesis of various different cancer types, and the results of
current clinical investigations with TSCC samples, the present
study hypothesized that lncRNA PCAT-1 may also contribute to the
onset and progression of TSCC. The results of the present study
have supported this hypothesis, where inhibition of lncRNA PCAT-1
not only reduced cell growth and induced apoptosis in TSCC cells,
but also suppressed the metastatic and invasive potential of these
cells. The effects of lncRNA PCAT-1 inhibition demonstrated the
critical function of this lncRNA in maintaining the normal
biological characteristics of TSCC cells. They also inferred that
this inhibition may represent a promising strategy for the
development of anti-TSCC therapies.
Apart from determining the role of lncRNA PCAT-1 in
the progression of TSCC, the present study also attempted to
elucidate its mechanism. Therefore, the expression levels of p21 in
TSCC PCAT-1-knockdown cells were examined. It was revealed that the
expression levels of p21 were significantly upregulated following
lncRNA PCAT-1 inhibition. The results were consistent with the
previously reported effect of lncRNA PCAT-1, acting as an oncogene
by reducing p21 expression levels (20). p21 is one of the most important
cyclin-dependent kinases and regulates cell cycle transition from
the G1 to the S phase (28). A previous study by Zhang et al
(29) demonstrated that
downregulation of p21 was closely associated with poor prognosis in
patients with TSCC. Collectively, this indicates that the
inhibitory effect of lncRNA PCAT-1 knockdown in TSCC is, at least
in part associated with the induction of p21 expression.
The results of the present study indicated that
lncRNA PCAT-1 is overexpressed in TSCC specimens, and that its
inhibition impairs the growth, metastasis and invasiveness of TSCC,
whilst inducing apoptosis. Furthermore, the inhibitory effect of
lncRNA PCAT-1 knockdown on TSCC cells was associated with the
upregulation of p21, indicating an interaction between lncRNA
PCAT-1 and p21 signaling during the progression of TSCC. However,
the present results only provide a preliminary evaluation of the
mechanism by which lncRNA PCAT-1 acts in the genesis and
progression of TSCC. Due to the original experimental design, the
clinicopathological information of some patients was not collected,
thus analyzing the potential of lncRNA PCAT-1 in predicating the
progression of TSCC was not possible. To fully explain the role of
lncRNA PCAT-1 in the oncogenesis of tongue tissues, more
comprehensive studies with complete patient information and
modulation of the downstream effectors of lncRNA PCAT-1 are
required, which may aid the development of lncRNA PCAT-1-based
anti-TSCC therapies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed are available
from the corresponding author on reasonable request.
Authors' contributions
MY designed the experiments, performed the data
collection and drafted the manuscript. TZ designed the experiments
and drafted the manuscript. LF performed the data collection and
analyzed the data. RT performed the data collection and analyzed
the data. DL analyzed the data and YD analyzed the data and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The People's Hospital of Tongliang District, Chongqing
City. All patients provided written informed consent for the use of
their tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sanchez Calle A, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun H, Huang Z, Sheng W and Xu M: Emerging
roles of long non-coding RNAs in tumor metabolism. J Hematol Oncol.
11:1062018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huarte M and Rinn JL: Large non-coding
RNAs: Missing links in cancer? Hum Molr Genet. 19:152–161. 2010.
View Article : Google Scholar
|
|
7
|
Ewan AG, Carolyn JB and Wan LL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
John RP and Arul MC: The emergence of
lncRNAs in cancer biology. Cancer Discov. 1:391–407. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough H, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by non-coding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gabory A, Jammes H and Dandolo L: The H19
locus: Role of an imprinted non-coding RNA in growth and
development. Bioessays. 32:473–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panzitt K, Tschernatsch MM, Guelly C,
Moustafa T, Stradner M, Strohmaier HM, Buck CR, Denk H, Schroeder
R, Trauner M and Zatloukal K: Characterization of HULC, a novel
gene with striking up-regulation in hepatocellular carcinoma, as
noncoding RNA. Gastroenterology. 132:330–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rasool M, Malik A, Zahid S, Basit Ashraf
MA, Qazi MH, Asif M, Zaheer A, Arshad M, Raza A and Jamal MS:
Non-coding RNAs in cancer diagnosis and therapy. Noncoding RNA Res.
1:69–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prensner JR, Iyer MK, Balbin OA,
Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso
CS, Kominsky HD, et al: Transcriptome sequencing across a prostate
cancer cohort identifies PCAT-1, an unannotated lincRNA implicated
in disease progression. Nat Biotechnol. 29:742–749. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prensner JR, Iyer MK, Balbin OA,
Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani I, Grasso
CS, Kominsky HD, et al: Transcriptome sequencing identifies PCAT-1,
a novel lincRNA implicated in prostate cancer progression. Nat
Biotechnol. 29:742–749. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prensner JR, Wei C, Sumin H, Iyer MK, Cao
Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M, et al:
The long non-coding RNA PCAT-1 promotes prostate cancer cell
proliferation through cMyc. Neoplasia. 16:900–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi WH, Wu QQ, Li SQ, Yang TX, Liu ZH,
Tong YS, Tuo L, Wang S and Cao XF: Upregulation of the long
noncoding RNA PCAT-1 correlates with advanced clinical stage and
poor prognosis in esophageal squamous carcinoma. Tumour Biol.
36:2501–2507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiao L, Liu X, Tang Y, Zhao Z, Zhang J and
Liu H: Knockdown of long non-coding RNA prostate cancer-associated
ncRNA transcript 1 inhibits multidrug resistance and
c-Myc-dependent aggressiveness in colorectal cancer Caco-2 and
HT-29 cells. Mol Cell Biochem. 441:99–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Deng G, Liu T, Chen W and Zhou Y:
Long noncoding RNA PCAT-1 acts as an oncogene in osteosarcoma by
reducing p21 levels. Biochem Biophys Res Commun. 495:2622–2629.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang C, He W, Xu TY, Dai J, Xu L and Sun
F: Upregulation of lncRNA DGCR5 correlates with better prognosis
and inhibits bladder cancer progression via transcriptionally
facilitating P21 expression. J Cell Physiol. 234:6254–6262. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waldman T, Kinzler KW and Vogelstein B:
p21 is necessary for the p53-mediated G1 arrest in human cancer
cells. Cancer Res. 55:5187–5190. 1995.PubMed/NCBI
|
|
23
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okuyemi OT, Piccirillo JF and Spitznagel
E: TNM staging compared with a new clinicopathological model in
predicting oral tongue squamous cell carcinoma survival. Head Neck.
36:1481–1489. 2014.PubMed/NCBI
|
|
25
|
Gao W, Chan YW and Wong TS: Long
non-coding RNA deregulation in tongue squamous cell carcinoma.
Biomed Res Int. 2014:4058602014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varambally S, Dhanasekaran SM, Zhou M,
Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt
RG, Otte AP, et al: The polycomb group protein EZH2 is involved in
progression of prostate cancer. Nature. 419:624–629. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tedeschi A, Wutz G, Huet S, Jaritz M,
Wuensche A, Schirghuber E, Davidson IF, Tang W, Cisneros DA,
Bhaskara V, et al: Wapl is an essential regulator of chromatin
structure and chromosome segregation. Nature. 501:564–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Chen W, Fu X, Su X and Yang A:
CBX3 promotes tumor proliferation by regulating G1/S phase via p21
downregulation and associates with poor prognosis in tongue
squamous cell carcinoma. Gene. 654:49–56. 2018. View Article : Google Scholar : PubMed/NCBI
|