Introduction
Surgery is considered the primary therapeutic option
for the treatment of patients with early stage non-small cell lung
cancer (NSCLC) (1–3). Although reported recurrence rates after
definitive surgery vary between 28 and 60%, poor post-recurrence
survival rates remain a challenge to the long-term survival of
patients with NSCLC (4–7).
Standard treatment the for post-operative recurrence
of NSCLC remains controversial. It is commonly systemic therapy
with cytotoxic agents and/or molecular targeted agents as for
metastatic stage IV disease (8,9).
However, certain patients with loco-regional (only) recurrence or
oligo-recurrences, that is, the state with a limited number of
recurrent lesions and controlled primary lesions, a condition
termed oligo-recurrence (10–13), are
expected to achieve long-term survival and even cure with intensive
local therapy alone (14–19).
Loco-regional recurrence of NSCLC is said to occur
in 20–45% of patients during follow-up (5,14,20). If
oligo-recurrence is included, >50% of patients with recurrence
may be suitable for localized curative therapy (21,22).
Although salvage surgery is considered to be the most promising
current treatment, the majority of candidate patients do not
undergo surgery because of post-operative comorbidities or poor
baseline pulmonary function. Additionally, most of these patients
are unable to tolerate chemotherapy, highlighting the importance of
radiotherapy (23–25).
Stereotactic body radiotherapy (SBRT) is an
important therapeutic option for patients with medically inoperable
early-stage NSCLC or oligometastatic lung tumors (26–28).
Even for patients in which operative treatment would be viable,
SBRT has previously achieved results similar to those of surgery
(29–31), and an increasing number of studies
have reported the expansion of factors that may indicate the
selection of SBRT, including large tumors and advanced-stage NSCLC
(32,33). Nevertheless, few large-scale studies
have reported the effect of SBRT on post-operative oligo-recurrence
of NSCLC (34,35).
Therefore, the present study aimed to
retrospectively assess the efficacy and safety of salvage SBRT for
post-operative oligo-recurrence following primary curative lung
resection in patients with NSCLC.
Materials and methods
Case eligibility
Following Institutional Review Board approval from
the Ethics Committee of the University of Tokyo (Tokyo, Japan), a
retrospective review was conducted of patients treated with SBRT,
admitted to University of Tokyo Hospital between September 2010 and
November 2016. The patients selected had previously received
pulmonary resection for a primary NSCLC, and later developed
nodular lesions in the thorax, which were determined to be
post-operative oligo-recurrences. The median age of patients was 74
years, ranging from 50 years to 86 years. There were 38 males and
14 females. Seven patients rejected surgery at their own
discretion. Written informed consent was obtained from all patients
prior to treatment initiation.
Inclusion and exclusion criteria
The present study included patients who met the
following inclusion criteria: i) Initial resection of NSCLC with
curative intent; ii) clinical diagnosis of post-operative
recurrence (approved by the Tumor Board for Lung Cancer of
University of Tokyo Hospital based on biopsy or image findings and
clinical data); iii) recurrent disease within the thorax, including
mediastinal and hilar lymph nodes; iv) absence of metastases to
solid organs, or pleural seeding; and v) <10 years between
initial surgery and SBRT.
The exclusion criteria were as follow: i) <6
months of follow-up completed (excluding mortality as the reason);
ii) no CT confirmation of recurrence after completion of SBRT; and
iii) history of adjuvant radiotherapy. SBRT was not conducted in
patients with apparent interstitial pneumonitis or pulmonary
fibrosis on the chest film, due to the absence of established
policy regarding performance status or respiratory function in
these patients.
Clinical data collected from each patient included
age, sex, interval between surgery and recurrence, recurrence sites
and smoking index. Staging of the primary tumor was conducted in
accordance with the 7th edition of the Union for International
Cancer Control staging system for lung cancer (36).
Procedure
The decision to conduct appropriated salvage
therapies was approved by the Tumor Board for Lung Cancer when
post-operative oligo-recurrence was suspected. SBRT was often
selected as the therapeutic approach for locoregional or
intrathoracic oligometastases, where it could safely be applied. In
other cases, chemoradiotherapy, chemotherapy, immunotherapy,
palliative therapy and best supportive care were considered.
Although cases that administered chemotherapy before and/or after
SBRT were not excluded, there were no cases included in which
chemotherapy was performed synchronously with SBRT.
Prior to the initiation of treatment, patients were
immobilized in a stereotactic body frame and underwent a
four-dimensional (4D) CT scan (2-mm slice thickness). Scans were
performed using an external respiratory monitoring system (AZ-733
V®; Anzai Medical Co, Ltd.) under free breathing or with
abdominal compression in cases where tumor excursion was >1
cm.
Mechanistically, 4D-CT planning divides the
respiratory cycle into 10 sections. Respiratory phase data were
transferred to a treatment planning system (TPS;
Pinnacle3® version 9.1; Philips Healthcare). Gross tumor
volume (GTV) was delineated in each respiratory phase using the
lung window (window, 1,600 HU; level, −300 HU). The 10 GTVs were
fused to form the internal target volume. A uniform 5-mm margin was
then added to create the planning target volume (PTV) (37–39). The
main organs at risk (OARs), namely the heart, lungs, esophagus,
spinal cord, proximal tracheobronchial tree and brachial plexus,
were contoured according to the guidelines outlined in the
Radiation Therapy Oncology Group (RTOG) 0236 trial (40).
Patients admitted between September 2010 and March
2013 were treated using a conventional SBRT plan using 6–12 beams,
whereas patients admitted from April 2013 onwards were treated
using volumetric modulated arc therapy (VMAT)-SBRT with 6 or 10 MV
beams using an Elekta-synergy system (Elekta Instrument AB). There
was no significant difference in treatment outcome between the two
methods (41). VMAT plans were
designed using a single partial arc with angle ranges of −40° to
180° (left lung) or −180° to 40° (right lung), as previously
detailed (37,38,41,42).
Dosimetric planning and plan analysis were performed using
Pinnacle3. The collapsed cone convolution method
(comparable to the superposition method) in the TPS was used
(42,43). All final calculations were performed
using a grid size of 2.0 mm. Dose distributions were calculated
using CT data obtained at peak exhalation. Treatment was performed
with 48–55 Gy in 4 fractions for peripheral tumors (39 lesions) or
56 Gy in 7 fractions for central tumors (17 cases) to cover 95% of
the PTV (D95%).
The central lesion was defined as a tumor <2 cm
from the proximal bronchial tree, according to the RTOG 0236
guidelines, or <2 cm in any direction from a critical
mediastinal structure, including the bronchial tree, esophagus,
heart, brachial plexus, major vessels, spinal cord, phrenic nerve
and recurrent laryngeal nerve, as in most recent studies (44,45). For
certain hilar or mediastinal tumors, or tumors invading the trachea
or bronchi, a regimen with increased number of fractions (such as
50 Gy in 10 fractions) was used (46,47). The
dose constraints of OARs were defined based on the protocol of the
Japan Clinical Oncology Group (JCOG) 0403 trial (48). Regarding the pulmonary dose, adding
to the constraints described in JCOG 0403 (V20, V15, mean lung
dose, V10 (<40%) and V5 (<70%) were also restricted to reduce
the volume of low dose area, which tends to be large in VMAT plans
(49,50) (Table
I). Furthermore, dosage deviations to organs other than those
listed in Table I were permitted
according to clinical benefit (51).
 | Table I.Dose constraints of organs at
risk. |
Table I.
Dose constraints of organs at
risk.
| Organ at risk | Dose
constraints | Dose effort
targets |
|---|
| Lung |
|
|
| MLD
<18.0 Gy | MLD <18.0
Gy |
|
| V20
<20% | V20 <20% |
|
| V15
<25% | V15 <25% |
|
| V10
<40% | V10 <40% |
|
| V5
<70% | V5
<70% |
|
| Spinal cord | 25 Gy/4–10
fractions (0 cm3) | 40 Gy/4–10
fractions (0cm3) |
| Heart (mean) | 25 Gy/4–10
fractions (0 cm3) |
|
| Heart (max) | 55 Gy/4–10
fractions (0 cm3) |
|
| Liver | 30 Gy/4–10
fractions (<10 cm3) |
|
| Esophagus |
| 40 Gy/4–10
fractions (0 cm3) |
| pulmonary
artery |
| 40 Gy/4–10
fractions (0 cm3) |
|
Trachea/bronchus |
| 40 Gy/4–10
fractions (0 cm3) |
| rib, chest
wall |
| 40 Gy/4–10
fractions (0 cm3) |
Follow-up and chart review
Follow-up commenced on the first day of radiotherapy
initiation and ended on May 1, 2018. Patients underwent a CT scan
of the chest and abdomen every 3 months for 2 years, and received
≥1 scan every 6 months thereafter.
Tumor recurrence was diagnosed as progressive and
increasing by CT scan abnormalities, and confirmed by progressive
and incremental increases in the standardized uptake value of a
lesion, following serial PET imaging (with or without biopsy).
Furthermore, locoregional recurrence was defined as disease
recurrence at the surgical margin, ipsilateral hemi-thorax or
regional lymph nodes. Distant metastasis was defined as metastasis
to the contralateral lung and to outside of the hemi-thorax or
mediastinum.
Overall survival (OS) was defined as the period from
the first day of SBRT initiation to the date of mortality from any
cause, or to the last follow-up visit or telephone contact prior to
May 1, 2018. Progression-free survival (PFS) and local control (LC)
were defined as the interval from the first day of SBRT to
documented disease progression and locoregional recurrence (or
mortality/follow-out), respectively. If SBRT was performed twice
after surgery to treat recurrence, PFS for the first SBRT was
defined as the interval between the two SBRTs.
Evaluation of toxicity
Toxicity was evaluated and graded according to the
Common Terminology Criteria for Adverse Events v4.0 (52). Radiation pneumonitis (RP) was
diagnosed according to clinical symptoms, including cough,
shortness of breath, fever and radiologic findings in the absence
of any other likely cause. All uncertain cases were discussed by
the tumor board and either verified via biopsy or by consensus of
the board. All hospital records, follow-up notes and images were
reviewed, including all patient data regarding tumor and treatment
characteristics.
Statistical analysis
The 1- and 3-year OS, RFS and LC rate were
calculated using the Kaplan-Meier method; the log rank test was
used for group comparisons. Survival was calculated from the end of
SBRT. Cox proportional hazards models were used to assess factors
associated with survival. All statistical analyses were performed
using EZR version 1.36 which is a graphical interface for R (The R
Foundation for Statistical Computing) (53), and the significance of univariate and
multivariate analyses was set at P<0.05. All statistical tests
were two-sided. The biologically effective dose (BED) was
calculated using the BED10 linear-quadratic equation
with an α/β value of 10 for tumors (54).
Results
Patient and treatment
characteristics
A total of 61 patients (with 70 lesions among them)
received chest SBRT for post-operative oligo-recurrence of NSCLC.
Following exclusion of 9 patients in whom the time from surgery to
SBRT was >10 years, a total of 52 patients, with 59 lesions
among them, were evaluated. Patient characteristics are summarized
in Table II.
 | Table II.Clinicopathological
characteristics. |
Table II.
Clinicopathological
characteristics.
|
Characteristics | Value |
|---|
| Patient
characteristics (n=52) |
|
| Median
age at recurrence, years (range) | 74 (50–86) |
| Sex
(male:female), n | 38:14 |
| KPS
(≥90:<90), n | 47:5 |
| Smoking
history (yes:no), n | 30:22 |
| Median
Brinkman index, n (range) | 580 (0–3,000) |
|
Operability of recurrent tumor
(operable:inoperable) | 7:45 |
| Tumor
characteristics (n=59) |
|
| Median
SUVmax, n (range) | 4.65
(0.87–19.5) |
|
Histological type at primary
surgery (Ad:Sq), n | 45:14 |
| pT
classification (pT1:pT≥2) | 27:32 |
| pN
classification (pN0:pN≥1) | 50:9 |
| pM
classification (pM0:pM≥1) | 59:0 |
| Type of
initial surgery (lobectomy or pneumonectomy:sublobular resection),
n | 41:18 |
| Median
disease-free interval prior to SBRT, months | 30.5 |
|
Disease-free interval prior to
SBRT (<1:≥1 years), n | 12:47 |
|
Disease-free interval prior to
SBRT (<5:≥5 years), n | 47:12 |
| SBRT for the
recurrence tumor (n=59) | Value |
| Median
tumor size, cm (range) | 1.7 (0.1–5.6) |
| Tumor
size (≤2:>2 cm), n | 34:25 |
|
Recurrent site
(central:peripheral), n | 20:39 |
| Dose
prescription |
|
| Median
BED10, Gy (range) | 112.5
(75–130.6) |
| 55 Gy
in 4 fractions (BED10 130.6 Gy)), n (%) | 21 (35.6%) |
| 50 Gy
in 4 fractions (BED10 112.5 Gy), n (%) | 18 (30.5%) |
| 56 Gy
in 7 fractions (BED10 100.8 Gy), n (%) | 18 (30.5%) |
| 50 Gy
in 10 fractions (BED10 75 Gy), n (%) | 2 (3.4%) |
Seven patients rejected surgery at their own
discretion. Decreased respiratory function was the most common
parameter used for selection of SBRT instead of surgery (55,56). The
initial pulmonary resection was standard surgery (lobectomy with
mediastinal lymph node dissection) or more in 35/52 patients
(67.3%) with 41/59 lesions (69.5%), one case of which was
pneumonectomy, whereas limited resection was performed for the
remaining 17/52 cases (32.7%) with 18/59 lesions (30.5%). The
majority of patients were unsuitable for chemotherapy, and only
8/52 (15.4%) patients received chemotherapy each before and after
SBRT.
Pathological diagnosis at primary surgery was
adenocarcinoma in 39/52 patients (75.0%) and squamous carcinoma in
13/52 (25.0%). Further, 9/52 patients (17.3%) exhibited
pathological lymph node metastases. The epidermal growth factor
receptor mutation status was detected in 4/11 of the patients in
which it was tested for.
The median interval between initial resection and
salvage SBRT for recurrence was 30.5 months (range, 2.0–99.0
months). A total of 20/59 lesions (33.9%) were located in the
central area, which is a greater proportion than that in the whole
SBRT cohort of University of Tokyo Hospital during the same period
(41).
The most common fractionation schemes were 55 Gy in
4 fractions (BED10=130.6 Gy; 21 lesions, 35.6%),
followed by 50 Gy in 4 fractions (BED10=112.5 Gy; 18
lesions, 30.5%), and 56 Gy in 7 fractions (BED10=100.8
Gy; 18 lesions, 30.5%), resulting in a median BED10 of
112.5 Gy (Table II).
Treatment outcomes and failure
patterns
The median follow-up time for all patients was 25
months (range, 3–63 months), and for surviving patients it was 35
months (range, 10–71 months). During follow-up, 18 (34.6%) patients
experienced disease progression. Among these, locoregional
recurrence was observed in only 4 (7.7%) patients, all of whom
suffered from intrapulmonary metastasis and/or pleural
dissemination, other than local recurrence. In 8 (44.4%) patients
with relapse, ≥2 lesions were found at first recurrence. The most
common site of recurrent lesions was the contralateral lung (8
cases; 44.4%), followed by lymph node (6 cases; 33.3%), local and
bone (4 cases; 22.2%) and brain and pleural dissemination (3 cases;
16.7%).
Survival and prognostic factors
A total of 19 patients (36.5%) died during
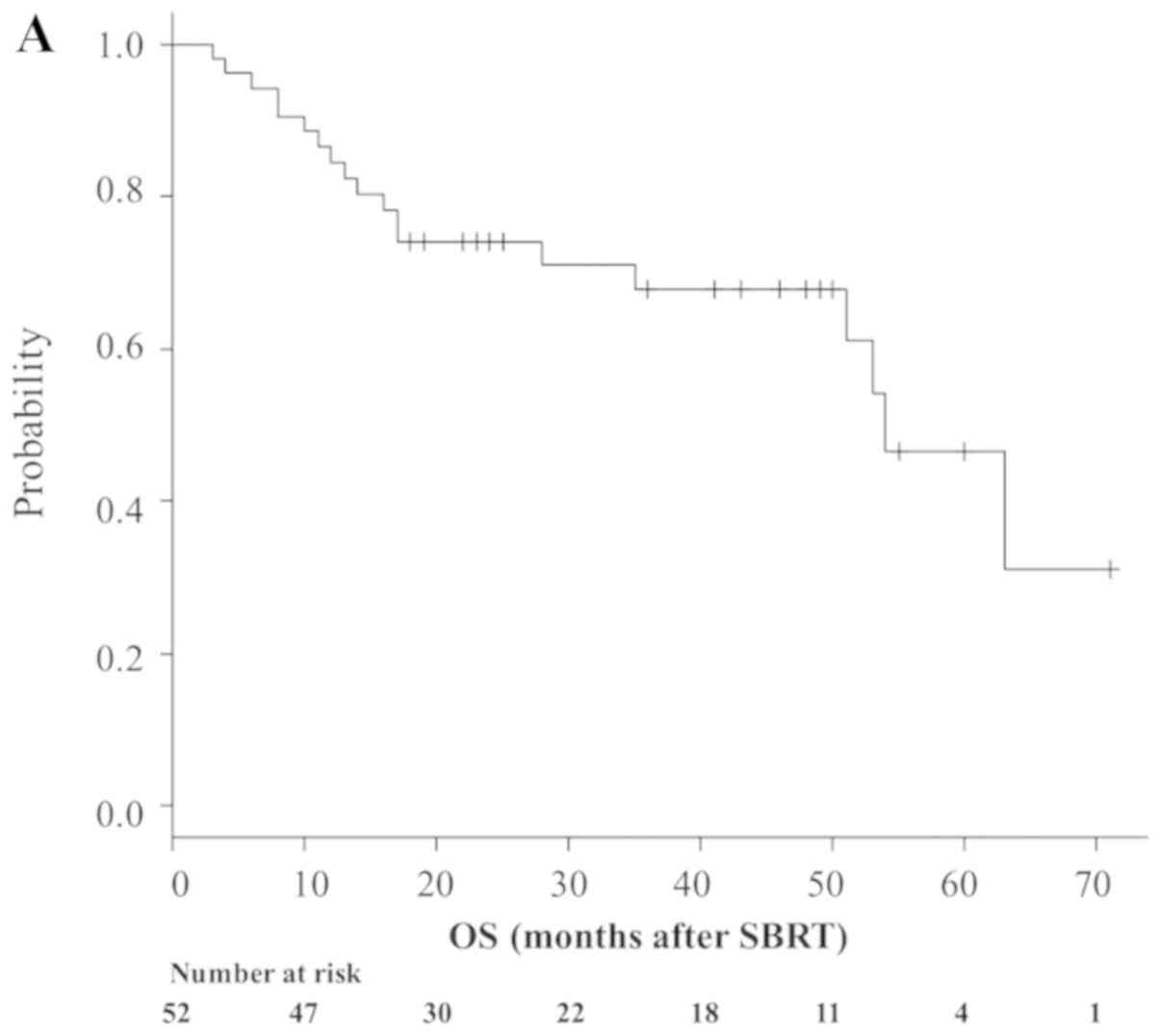
follow-up. Median OS time was 54 months (95% CI, 51-NA; Fig. 1A), while 1- and 3-year OS rates were
84.4% (95% CI, 71.3–91.9%) and 67.8% (95% CI, 51.8–79.5%),
respectively (Fig. 1A). A total of
33 patients (63.5%) remained alive at the last follow-up, while 25
(48.1%) were both alive and progression-free. Median PFS time was
51 months (95% CI, 28–60), while 1- and 3-year PFS rates were 80.8%
(95% CI, 67.2–89.2) and 58.7% (95% CI, 43.2–71.3), respectively
(Fig. 1B). A total of 4 patients
(7.7%) developed local failure. Median LC was 71 months (range, 60
months-NA). The 1- and 3-year Kaplan-Meier-estimated LC rate was
97.9% (95% CI, 85.8–99.7) and 94.9% (95% CI, 80.8–98.7),
respectively (Fig. 1C).
Predictive factors for OS
Table III provides
the results of univariate and multivariate analyses for OS. Sex,
possibility of re-operation, disease-free interval between initial
surgery and local recurrence (≥1 vs. <1 years) and location
(central or peripheral) and dose prescription were revealed to be
significant prognostic factors for OS, following univariate
analysis. By contrast, multivariate analysis indicated that
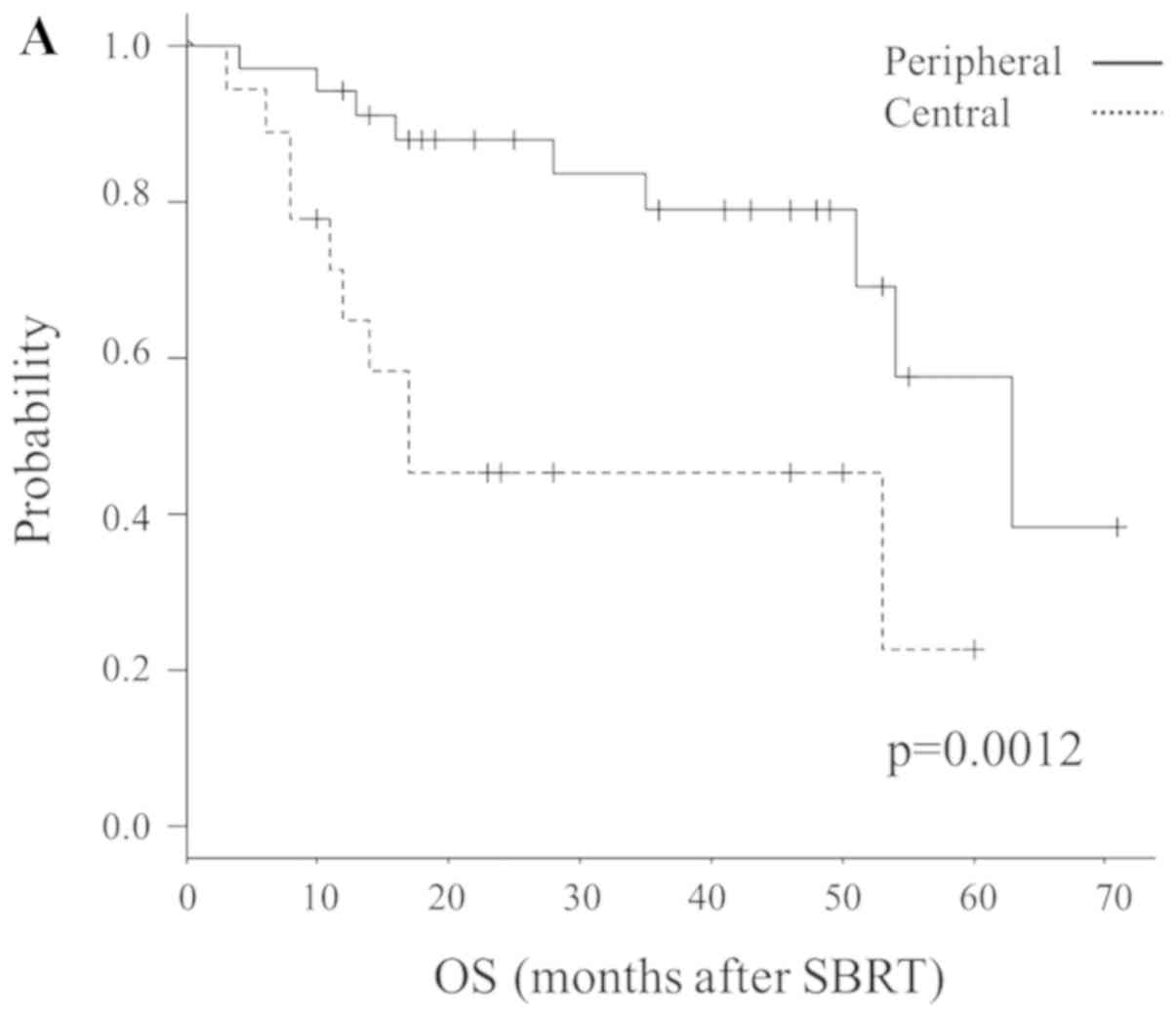
location (central vs. peripheral; P=0.0012) and possibility of
re-operation (impossible vs. possible; P=0.00092) were significant
prognostic factors for OS. Fig. 2
illustrates the Kaplan-Meier curves according to these factors.
 | Table III.Analysis of clinical and dosimetric
variables associated with OS (patients, n=52; tumors, n=59). |
Table III.
Analysis of clinical and dosimetric
variables associated with OS (patients, n=52; tumors, n=59).
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Patient |
|
|
|
|
| Age at
recurrences, years (≤75 vs. >75) | 1.81
(0.22–1.48) | 0.74 |
|
|
| Sex
(male vs. female) | 1.72
(4.31–6.90) | 0.0062 | 3.94
(0.80–19.37) | 0.091 |
| Smoking
history (yes vs. no) | 1.11
(0.01–1.05) | 0.55 |
|
|
| Initial surgery for
primary NSCLC |
|
|
|
|
|
Histology (adenocarcinoma vs.
alternative subtypes) | 1.19
(0.0038–3.73) | 0.23 |
|
|
| Extent
of pulmonary resection (sublobular resection vs. lobectomy or
pneumonectomy) | 4.16
(0.048–3.61) | 0.43 |
|
|
| T
status (pT2 vs. pT1) | 6.38
(0.10–3.93) | 0.63 |
|
|
| Lymphatic invasion
(present vs. absent) | 3.51
(0.023–5.32) | 0.45 |
|
|
| Lymph node
metastasis (pN≥1 vs. pN0) | 1.37
(0.73–2.56) | 0.080 |
|
|
| Disease-free
interval, years (≥1 vs. <1) | 4.76
(1.99–1.14) | 0.017 | 0.92
(4.26–19.52) | 0.062 |
| Disease-free
interval, years (≥5 vs. <5) | 2.50
(0.24–2.55) | 0.44 |
|
|
| SBRT for recurrent
tumors |
|
|
|
|
|
Possibility of re-operation
(impossible vs. possible) | 2.46
(29.07–2.08) | <0.0010 | 9.53
(2.51–36.15) | <0.001 |
| Tumor
size (≤2 cm vs. >2 cm) | 6.05
(0.043–8.52) | 0.71 |
|
|
| Tumor
size (≤3 cm vs. >3 cm) | 2.38
(0.0058–9.77) | 0.45 |
|
|
| SUVmax
(≥5.0 vs. <5.0) | 1.57
(0.17–1.43) | 0.69 |
|
|
| Location (lower or
mediastinum vs. upper or middle) | 1.18
(0.29–4.86) | 0.82 |
|
|
| Central lesion
(central vs. peripheral) | 1.22
(0.37–4.050) | 0.011 | 5.51
(1.96–15.49) | 0.0012 |
| Dose prescription
(55 Gy 4 Fr vs. the others (50 Gy, 4 Fr; | 2.52
(2.10–3.00) | 0.011 | 0.03
(0.27–2.31) | 0.23 |
| 56 Gy, 7 Fr; 50 Gy,
10 Fr) |
|
|
|
|
| Chemotherapy after
SBRT (present vs. absent) | 7.97
(0.035–1.82) | 0.89 |
|
|
Regarding PFS, in addition to these factors, age
(≥75 years; P=0.037), type of prior surgery (limited surgery;
P=0.0046), diameter of the primary tumor (pT ≥2; P=0.014) and
recurrent lesion (≥2 cm; P=0.039) were also significant prognostic
factors.
Toxicity
In total, 9 patients (17.3%) developed grade 2
adverse events (AEs), and 4 patients (7.7%) developed grade 3 or
greater AEs. Of these, two patients (3.8%) developed grade 5 AEs,
meaning mortality due to toxicity. One grade 5 case was radiation
pneumonitis and the second was hemoptysis. The prescribed dose for
both patients was 56 Gy in 7 fractions. Further details of the two
aforementioned cases have been reported by the present authors in a
previous study (33).
The doses for OARs in these two cases were
summarized in Table IV. In both
cases, dose-restricted organs were not irradiated above the limit,
but some unrestricted OARs were being given higher doses than the
effort target (in other words, the indicators to achieve if
possible). The other common features of the two cases were having
recurrence following lung lobectomy, central lesions and presence
of a smoking history.
 | Table IV.Irradiated dose for organs at risk of
two patients who exhibited grade 5 AEs. |
Table IV.
Irradiated dose for organs at risk of
two patients who exhibited grade 5 AEs.
| OAR | Patient 1 | Patient 2 |
|---|
| ITV,
cm2 | 5.0 | 9.6 |
| PTV,
cm2 | 19.4 | 33.0 |
| Lung | 5.0 | 9.6 |
| V5
(%) | 16.6 | 31.2 |
| V10
(%) | 6.1 | 22.8 |
| V20
(%) | 3.8 | 11.3 |
| Mean
(cGy) | 355.8 | 703.9 |
| Trachea |
|
|
| Max
(cGy; point) | 628.7 | 162.4 |
| Max
(cGy; 5cc) | 226.1 | 122.3 |
| Carina |
|
|
| Max
(cGy; point) | 6,109.3 | 4,644.6 |
| Max
(cGy; 5cc) | – | 489.3 |
| Esophagus |
|
|
| Max
(cGy; point) | 3,402.0 | 5,364.6 |
| Max
(cGy; 5cc) | 1,809.0 | 1,223.2 |
| Pulmonary
artery |
|
|
| Max
(cGy; point) | 5,583.2 | 4,654.9 |
| Max
(cGy; 5cc) | 226.1 | 1,467.8 |
| Pulmonary
veins |
|
|
| Max
(cGy; point) | 2,412.2 | 5,610.4 |
| Max
(cGy; 5cc) | – | – |
| Aorta |
|
|
| Max
(cGy; point) | 2,818.5 | 3,834.4 |
| Max
(cGy; 5cc) | 2,366.8 | 2477 |
| Superior vena
cava |
|
|
| Max
(cGy; point) | 3,641.2 | 5,770.8 |
| Max
(cGy; 5cc) | 2,788.9 | 3,914.3 |
| Heart |
|
|
| Mean
(cGy) | 274.3 | 879 |
|
V30 (%) | 1.3 | 5.7 |
| Spine |
|
|
| Max
(cGy; point) | 1,387.5 | 2,034.8 |
| Chest wall |
|
|
| Max
(cGy; point) | – | 4,084.6 |
Discussion
Post-operative recurrence of NSCLC is commonly
treated using a multifaceted treatment program, including systemic
therapy, as with metastatic stage IV disease (8,9).
However, certain recurrent cases with local lesions alone or a
limited number of metastatic lesions (termed oligo-recurrence), may
occasionally be cured using localized therapy alone (11–14).
A number of studies have evaluated second resection
for local recurrence or intrathoracic oligo-recurrence in patients
who received surgery as initial treatment (6,57–59).
Hung et al (6) reported 1-
and 2-year post-recurrence OS rates of 48.7 and 17.6%,
respectively, in their study of 74 patients with local recurrence.
Notably, Kim et al (57)
reported a 5-year OS rate after second resection of 33.4% with an
operative mortality for the second resection of 5.8%. Previously,
Yukiue et al (58) achieved
2- and 5-year OS rates after second resection of 87.8 and 62.9%,
respectively. However, 9 patients (23%) exhibited serious
post-operative complications and 1 (2.6%) died during surgery,
raising concerns regarding the safety of the operation (58). Alternative reports on post-operative
recurrence have also indicated that re-operation may represent an
effective treatment for post-operative lung cancer recurrence, in
certain patients in which the oncological benefits outweigh the
surgical risk (14,59).
By contrast, SBRT has been recently recognized as an
alternative therapy for patients with inoperable early-stage NSCLC
or those who refuse surgery (26,28,31).
Regarding post-operative oligo-recurrence, SBRT is not an
established standard therapy and no large prospective clinical
trials have been conducted. Takeda et al (60) analyzed the outcomes of SBRT in 23
patients with isolated post-surgical local recurrence. LC and OS
rates were revealed to be 94.7 and 86.8% at 1 year and 84.0 and
76.4% at 2 years, respectively. Regarding AEs, 3 patients (13.0%)
suffered from RP grade ≥3 and the authors concluded that SBRT, when
used to treat isolated postsurgical local recurrence, achieves high
LC with limited toxicity (60).
Nishiyama et al (61)
subsequently investigated 41 patients with medically inoperable
diseases who underwent SBRT for second pulmonary nodules arising
from different types of cancer and reported that grade 2 RP AEs
occurred in five patients and one succumbed to grade 5 RP.
In the present study, the 3-year OS, PFS and LC
rates of all included patients were 67.8, 58.7 and 94.9%,
respectively, and these results are comparable not only to previous
studies on SBRT (4,16,17), but
also on re-operation. Considering that More than two thirds of
patients (≥67.3%) in the present analysis were regarded as
ineligible for surgery or chemotherapy, the survival outcomes for
salvage SBRT as a therapeutic technique are promising.
Additionally, it was revealed that the major failure
pattern after radical radiotherapy was distant metastasis. This
finding is consistent with the results of a study by Kelsey et
al (62), in which 50% of
patients developed distant metastases following salvage
radiotherapy. SBRT, which can achieve good local control, may be
expected to improve recurrence-free survival by combination with
recently developed immunotherapy.
Several retrospective studies have investigated
prognostic factors for OS time in patients with local recurrence
(62–64); the reported factors associated with
prolonged OS time were female, young age, long disease-free
interval between initial surgery and local recurrence (5,28,62–65),
early stage of primary tumor (5,66) and
high prescribed radiation dose (67,68). In
the present study, female and disease-free interval were
significantly associated with OS time only in univariate analysis.
In multivariate analysis, central lesions and re-operative lesions
(operative refusal by patients) were significantly associated with
poor survival prognosis.
Similar to previous findings (47–49,69–72),
central lesions exhibited a worse prognosis than peripheral lesions
in the present study. One possible reason for this may be an
increase in the number of AEs, which are associated with central
SBRT (44,46). Among the included patients, all 4
cases of AEs graded ≥3 (including a grade 5 case) occurred in
patients with central lesions.
Additionally, relatively low doses for central
tumors may also contribute to the inferior outcomes; higher
radiation doses have been reported to be associated with prolonged
OS time, even in patients with post-operative recurrence (67,68),
although there was no indication of a survival difference between
high and low BED (above and below BED10 ≤130.6), in the
present study. It was concluded that the cause was that most
patients treated at University of Tokyo Hospital have been treated
with BED10 100 Gy or higher. Notably, in a study by Kim
et al (4), it was suggested
that determining whether increasing radiation alone improves
survival may be difficult in a situation where high doses were
administered and irradiation technology was developed (4).
In the present study, patients who underwent
sublobular resection exhibited an improved prognosis compared with
those who received lobectomy or pneumonectomy. The prognosis of
initial surgery itself is considered to be improved with lobectomy
compared with sublobular resection (73,74),
indicating that the results are reversed in cases of post-operative
oligo-recurrence. The current findings may be a result of the
limited number of cases that were considered as appropriate (based
on invasion characteristics) reduce the ablation range, or even the
small population size, especially in the operable group.
In the present study, the irradiated dose for the
OARs of two patients with grade 5 AEs were reviewed. As described
in the results section, the dose delivered to restricted OARs in
these two cases did not exceed the constraints, but certain
unrestricted OARs were being treated with a higher dosage than the
effort target (48). The present
results indicated dose restrictions on certain OARs, such as blood
vessels and trachea, which have not currently been restricted.
In addition, patient factors, such as smoking
history (75) and interstitial lung
disease (76,77), have been reported as risk factors
too. The occurrence of severe AEs may be associated with the
clinicopathological factors of patients and tumors as well as the
radiation dose. All these factors act synergistically and it is
difficult to accurately quantify the relative contribution of each
factor. Although a conclusion was not reached in the present study,
risk stratification combining both patient and radiation factors
should be performed in future research. Collecting and analyzing
data of serious AEs is difficult for a single institution; thus,
risk analyses will require multi-center, long-term data
accumulation to improve their statistical power.
The present study had several limitations.
Primarily, it was conducted at a single institution and using a
retrospective design. Therefore, a degree of intrinsic bias may
remain, and information regarding clinical examinations
(respiratory function, PET and status of gene expression) was
insufficient in some cases, so that it was not possible to examine
the associations between treatment outcomes. Additionally, the
number of patients was low, which may have limited the statistical
confidence of the results. Further research is necessary, including
prospective studies with a large sample size, in order to support
the conclusions of the present study. Finally, it is difficult to
distinguish between post-operative recurrence and multiple primary
lung cancers, even when pathological examinations are
performed.
Furthermore, it is difficult to compare AE risk in
cases of different prescriptions, because dose division for each
dose restriction has not yet been established. This is an issue to
be clarified in future research.
The present study suggested that salvage SBRT
represents a promising treatment for patients with NSCLC exhibiting
post-operative locoregional or intrathoracic oligo-recurrence,
particularly in LC. Independent risk factors associated with a
decreased OS were a central lesion and the possibility of
re-operation. The AEs were also considered as tolerable. However,
further research is required on the selection of subjects and
stratification by risk factors.
Acknowledgements
The authors would like to thank Dr Libby Cone for
editing the drafts of this manuscript.
Funding
The present study was supported by a Grant-in-Aid
from Japan Society for the Promotion of Science, KAKENHI JP
Scientific Research (C) (grant no. 18K07667).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SA, HY, WT, JNa, MS, OA and KeN participated in
research design. Acquisition of the data was performed by SA, TI,
SO and TK. Evaluation of the images was conducted by SA, KaN, TO
and YN. Interpretation of the data was conducted by SA, MA and JNi.
The manuscript was prepared by SA, HY and WT, and written by SA and
HY. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee, University of Tokyo Hospital [Tokyo, Japan;
3372-(3)/2016]. Written informed
consent for data collection and analysis was obtained from the
respective patients.
Patient consent for publication
Patients provided written consent for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese lung cancer registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goya T, Asamura H, Yoshimura H, Kato H,
Shimokata K, Tsuchiya R, Sohara Y, Miya T and Miyaoka E; Japanese
Joint Committee of Lung Cancer Registry, : Prognosis of 6644
resected non-small cell lung cancers in Japan: A Japanese lung
cancer registry study. Lung Cancer. 50:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sawabata N, Miyaoka E, Asamura H,
Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M and
Yokoi K; Japanese Joint Committee for Lung Cancer Registration, :
Japanese lung cancer registry study of 11,663 surgical cases in
2004: Demographic and prognosis changes over decade. J Thorac
Oncol. 6:1229–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim E, Song C, Kim MY and Kim JS:
Long-term outcomes after salvage radiotherapy for postoperative
locoregionally recurrent non-small-cell lung cancer. Radiat Oncol
J. 35:55–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugimura H, Nichols FC, Yang P, Allen MS,
Cassivi SD, Deschamps C, Williams BA and Pairolero PC: Survival
after recurrent nonsmall-cell lung cancer after complete pulmonary
resection. Ann Thorac Surg. 83:409–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hung JJ, Hsu WH, Hsieh CC, Huang BS, Huang
MH, Liu JS and Wu YC: Post-recurrence survival in completely
resected stage I non-small cell lung cancer with local recurrence.
Thorax. 64:192–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Endo C, Sakurada A, Notsuda H, Noda M,
Hoshikawa Y, Okada Y and Kondo T: Results of long-term follow-up of
patients with completely resected non-small cell lung cancer. Ann
Thorac Surg. 93:1061–1068. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Socinski MA, Evans T, Gettinger S, Hensing
TA, VanDam Sequist L, Ireland B and Stinchcombe TE: Treatment of
stage IV non-small cell lung cancer: Diagnosis and management of
lung cancer, 3rd ed: American college of chest physicians
evidence-based clinical practice guidelines. Chest. 143 (Suppl
5):e341S–e368S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mok TS, Lee K and Leung L: Targeting
epidermal growth factor receptor in the management of lung cancer.
Semin Oncol. 41:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niibe Y and Hayakawa K: Oligometastases
and oligo-recurrence: The new era of cancer therapy. Jpn J Clin
Oncol. 40:107–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niibe Y and Chang JY: Novel insights of
oligometastases and oligo-recurrence and review of the literature.
Pulm Med. 2012:2610962012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niibe Y, Chang JY, Onishi H, Salama J,
Hiraki T and Yamashita H: Oligometastases/Oligo-recurrence of lung
cancer. Pulm Med. 2013:4382362013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niibe Y, Jingu K and Onishi H:
Oligo-recurrence and Sync-oligometastases. J Thorac Oncol.
13:e59–e60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hishida T, Yoshida J, Aokage K, Nagai K
and Tsuboi M: Postoperative oligo-recurrence of non-small-cell lung
cancer: Clinical features and survival†. Eur J Cardiothorac Surg.
49:847–853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ashworth A, Rodrigues G, Boldt G and Palma
D: Is there an oligometastatic state in non-small cell lung cancer?
A systematic review of the literature. Lung Cancer. 82:197–203.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niibe Y, Jingu K and Onishi H: Long-term
outcome of surgery or stereotactic radiotherapy for lung
oligo-recurrence. J Thorac Oncol. 12:e1912017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamashita H, Niibe Y, Yamamoto T, Katsui
K, Jingu K, Kanazawa S, Terahara A and Nakagawa K: Lung
stereotactic radiotherapy for oligometastases: Comparison of
oligo-recurrence and sync-oligometastases. Jpn J Clin Oncol.
46:687–691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sekihara K, Hishida T, Yoshida J, Oki T,
Omori T, Katsumata S, Ueda T, Miyoshi T, Goto M, Nakasone S, et al:
Long-term survival outcome after postoperative recurrence of
non-small-cell lung cancer: Who is ‘cured’ from postoperative
recurrence? Eur J Cardiothorac Surg. 52:522–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brooks ED, Verma V, Senan S, De Baere T,
Lu S, Brunelli A and Chang JY; International Association for the
Study of Lung Cancer Advanced Radiation Technology Committee, :
Salvage therapy for locoregional recurrence after stereotactic
ablative radiotherapy for early-stage NSCLC. J Thorac Onco. Nov
9–2019.(Epub ahead of print).
|
|
20
|
Taylor MD, Nagji AS, Bhamidipati CM,
Theodosakis N, Kozower BD, Lau CL and Jones DR: Tumor recurrence
after complete resection for non-small cell lung cancer. Ann Thorac
Surg. 93:1813–1821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Voltolini L, Paladini P, Luzzi L,
Ghiribelli C, Di Bisceglie M and Gotti G: Iterative surgical
resections for local recurrent and second primary bronchogenic
carcinoma. Eur J Cardiothorac Surg. 18:529–534. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Battafarano RJ, Force SD, Meyers BF, Bell
J, Guthrie TJ, Cooper JD and Patterson GA: Benefits of resection
for metachronous lung cancer. J Thorac Cardiovasc Surg.
127:836–842. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun B, Brooks ED, Komaki R, Liao Z, Jeter
M, McAleer M, Balter PA, Welsh JD, O'Reilly M, Gomez D, et al:
Long-term outcomes of salvage stereotactic ablative radiotherapy
for isolated lung recurrence of non-small cell lung cancer: A phase
II clinical trial. J Thorac Oncol. 12:983–992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saisho S, Yasuda K, Maeda A, Yukawa T,
Okita R, Hirami Y, Shimizu K and Nakata M: Post-recurrence survival
of patients with non-small-cell lung cancer after curative
resection with or without induction/adjuvant chemotherapy. Interact
Cardiovasc Thorac Surg. 16:166–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee K, Kim HR, Kim DK, Kim YH, Park SI,
Choi SH and Han J: Post-recurrence survival analysis of stage I
non-small-cell lung cancer. Asian Cardiovasc Thorac Ann.
25:623–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ricardi U, Badellino S and Filippi AR:
Stereotactic radiotherapy for early stage non-small cell lung
cancer. Radiat Oncol J. 33:57–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crabtree TD, Denlinger CE, Meyers BF, El
Naqa I, Zoole J, Krupnick AS, Kreisel D, Patterson GA and Bradley
JD: Stereotactic body radiation therapy versus surgical resection
for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg.
140:377–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura T, Nagata Y, Eba J, Ozawa S,
Ishikura S, Shibata T, Ito Y, Hiraoka M and Nishimura Y; Radiation
Oncology Study Group of the Japan Clinical Oncology Group, : A
randomized phase III trial of comparing two dose-fractionations
stereotactic body radiotherapy (SBRT) for medically inoperable
stage IA non-small cell lung cancer or small lung lesions
clinically diagnosed as primary lung cancer: Japan clinical
oncology group study JCOG1408 (J-SBRT trial). Jpn J Clin Oncol.
47:277–281. 2017.PubMed/NCBI
|
|
29
|
Rosen JE, Salazar MC, Wang Z, Yu JB,
Decker RH, Kim AW, Detterbeck FC and Boffa DJ: Lobectomy versus
stereotactic body radiotherapy in healthy patients with stage I
lung cancer. J Thorac Cardiovasc Surg. 152:44–54.e9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang JY, Liu YH, Zhu Z, Welsh JW, Gomez
DR, Komaki R, Roth JA and Swisher SG: Stereotactic ablative
radiotherapy: A potentially curable approach to early stage
multiple primary lung cancer. Cancer. 119:3402–3410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang JY, Senan S, Paul MA, Mehran RJ,
Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al:
Stereotactic ablative radiotherapy versus lobectomy for operable
stage I non-small-cell lung cancer: A pooled analysis of two
randomised trials. Lancet Oncol. 16:630–637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onimaru R, Shirato H, Shibata T, Hiraoka
M, Ishikura S, Karasawa K, Matsuo Y, Kokubo M, Shioyama Y,
Matsushita H, et al: Phase I study of stereotactic body radiation
therapy for peripheral T2N0M0 non-small cell lung cancer with
PTV<100 cc using a continual reassessment method (JCOG0702).
Radiother Oncol. 116:276–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Verma V, Shostrom VK, Zhen W, Zhang M,
Braunstein SE, Holland J, Hallemeier CL, Harkenrider MM, Iskhanian
A, Jabbour SK, et al: Influence of fractionation scheme and tumor
location on toxicities after stereotactic body radiation therapy
for large (≥5 cm) non-small cell lung cancer: A multi-institutional
analysis. Int J Radiat Oncol Biol Phys. 97:778–785. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iyengar P, Wardak Z, Gerber DE, Tumati V,
Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, et
al: Consolidative radiotherapy for limited metastatic
non-small-cell lung cancer: A phase 2 randomized clinical trial.
JAMA Oncol. 4:e1735012018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang HH, Zaorsky NG, Meng MB, Zeng XL,
Deng L, Song YC, Zhuang HQ, Li FT, Zhao LJ, Yuan ZY, et al:
Stereotactic radiation therapy for oligometastases or
oligorecurrence within mediastinal lymph nodes. Oncotarget.
7:18135–18145. 2016.PubMed/NCBI
|
|
36
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer (UICC): TNM classification of
malignant tumours. 7th. Wiley-Blackwell; Oxford: 2009
|
|
37
|
Yamashita H, Takahashi W, Haga A, Kida S,
Saotome N and Nakagawa K: Stereotactic body radiotherapy for small
lung tumors in the University of Tokyo hospital. Biomed Res Int.
2014:1365132014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakagawa K, Haga A, Kida S, Masutani Y,
Yamashita H, Takahashi W, Sakumi A, Saotome N, Shiraki T, Ohtomo K,
et al: 4D registration and 4D verification of lung tumor position
for stereotactic volumetric modulated arc therapy using
respiratory-correlated cone-beam CT. J Radiat Res. 54:152–156.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakagawa K, Haga A, Sakumi A, Yamashita H,
Igaki H, Shiraki T, Ohtomo K, Iwai Y and Yoda K: Impact of
flattening-filter-free techniques on delivery time for lung
stereotactic volumetric modulated arc therapy and image quality of
concurrent kilovoltage cone-beam computed tomography: A preliminary
phantom study. J Radiat Res. 55:200–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for medically
inoperable early stage lung cancer. JAMA. 303:1070–1076. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aoki S, Yamashita H, Haga A, Nawa K, Imae
T, Takahashi W, Abe O and Nakagawa K: Flattening filter-free
technique in volumetric modulated arc therapy for lung stereotactic
body radiotherapy: A clinical comparison with the flattening filter
technique. Oncol Lett. 15:3928–3936. 2018.PubMed/NCBI
|
|
42
|
Aoki S, Yamashita H, Haga A, Ota T,
Takahashi W, Ozaki S, Nawa K, Imae T, Abe O and Nakagawa K:
Stereotactic body radiotherapy for centrally-located lung tumors
with 56 Gy in seven fractions: A retrospective study. Oncol Lett.
16:4498–4506. 2018.PubMed/NCBI
|
|
43
|
Ahnesjö A: Collapsed cone convolution of
radiant energy for photon dose calculation in heterogeneous media.
Med Phys. 16:577–592. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roesch J, Panje C, Sterzing F, Mantel F,
Nestle U, Andratschke N and Guckenberger M: SBRT for centrally
localized NSCLC-What is too central? Radiat Oncol. 11:1572016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meng MB, Wang HH, Zaorsky NG, Sun BS, Zhu
L, Song YC, Li FT, Dong Y, Wang JS, Chen HM, et al: Risk-adapted
stereotactic body radiation therapy for central and ultra-central
early-stage inoperable non-small cell lung cancer. Cancer Sci.
110:3553–3564. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang JH, Poon I, Erler D, Zhang L and
Cheung P: The safety and effectiveness of stereotactic body
radiotherapy for central versus ultracentral lung tumors. Radiother
Oncol. 129:277–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chaudhuri AA, Tang C, Binkley MS, Jin M,
Wynne JF, von Eyben R, Hara WY, Trakul N, Loo BW Jr and Diehn M:
Stereotactic ablative radiotherapy (SABR) for treatment of central
and ultra-central lung tumors. Lung Cancer. 89:50–56. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nagata Y, Hiraoka M, Shibata T, Onishi H,
Kokubo M, Karasawa K, Shioyama Y, Onimaru R, Kozuka T, Kunieda E,
et al: Prospective trial of stereotactic body radiation therapy for
both operable and inoperable T1N0M0 non-small cell lung cancer:
Japan clinical oncology group study JCOG0403. Int J Radiat Oncol
Biol Phys. 93:989–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sapkaroski D, Osborne C and Knight KA: A
review of stereotactic body radiotherapy-is volumetric modulated
arc therapy the answer? J Med Radiat Sci. 62:142–151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang X, Li T, Liu Y, Zhou L, Xu Y, Zhou X
and Gong Y: Planning analysis for locally advanced lung cancer:
Dosimetric and efficiency comparisons between intensity-modulated
radiotherapy (IMRT), single-arc/partial-arc volumetric modulated
arc therapy (SA/PA-VMAT). Radiat Oncol. 6:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yamashita H, Haga A, Takahashi W, Takenaka
R, Imae T, Takenaka S and Nakagawa K: Volumetric modulated arc
therapy for lung stereotactic radiation therapy can achieve high
local control rates. Radiat Oncol. 9:2432014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0 Published, (v4.03, June 14, 2010).
2009.
|
|
53
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dale RG: The application of the
linear-quadratic dose-effect equation to fractionated and
protracted radiotherapy. Br J Radiol. 58:515–528. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Puri V, Crabtree TD, Bell JM, Kreisel D,
Krupnick AS, Broderick S, Patterson GA and Meyers BF: National
cooperative group trials of ‘high-risk’ patients with lung cancer:
Are they truly ‘high-risk’? Ann Thorac Surg. 97:1678–1685. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferguson MK, Watson S, Johnson E and
Vigneswaran WT: Predicted postoperative lung function is associated
with all-cause long-term mortality after major lung resection for
cancer. Eur J Cardiothorac Surg. 45:660–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim GJ, Koshy M, Hanlon AL, Horiba MN,
Edelman MJ, Burrows WM, Battafarano RJ and Suntharalingam M: The
benefit of chemotherapy in esophageal cancer patients with residual
disease after trimodality therapy. Am J Clin Oncol. 39:136–141.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yukiue H, Tanahashi M, Haneda H, Suzuki E,
Yoshii N and Niwa H: Surgical treatment for recurrent and second
primary lung cancer. Kyobu Geka. 63:944–949. 2010.(In Japanese).
PubMed/NCBI
|
|
59
|
Subotic D, Molins L, Soldatovic I,
Moskovljevic D, Collado L and Hernández J: Completion
pneumonectomy: A valuable option for lung cancer recurrence or new
primaries. World J Surg Oncol. 16:982018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Takeda A, Sanuki N, Eriguchi T, Enomoto T,
Yokosuka T, Kaneko T, Handa H, Aoki Y, Oku Y and Kunieda E: Salvage
stereotactic ablative irradiation for isolated postsurgical local
recurrence of lung cancer. Ann Thorac Surg. 96:1776–1782. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nishiyama K, Kodama K, Teshima T and Tada
H: Stereotactic body radiotherapy for second pulmonary nodules
after operation for an initial lung cancer. Jpn J Clin Oncol.
45:947–952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kelsey CR, Clough RW and Marks LB: Local
recurrence following initial resection of NSCLC: Salvage is
possible with radiation therapy. Cancer J. 12:283–288. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gagliasso M, Migliaretti G and Ardissone
F: Assessing the prognostic impact of the international association
for the study of lung cancer proposed definitions of complete,
uncertain, and incomplete resection in non-small cell lung cancer
surgery. Lung Cancer. 111:124–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yoshino I, Yohena T, Kitajima M, Ushijima
C, Nishioka K, Ichinose Y and Sugimachi K: Survival of non-small
cell lung cancer patients with postoperative recurrence at distant
organs. Ann Thorac Cardiovasc Surg. 7:204–209. 2001.PubMed/NCBI
|
|
65
|
Sasaki H, Suzuki A, Tatematsu T, Shitara
M, Hikosaka Y, Okuda K, Moriyama S, Yano M and Fujii Y: Prognosis
of recurrent non-small cell lung cancer following complete
resection. Oncol Lett. 7:1300–1304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Walsh GL, O'Connor M, Willis KM, Milas M,
Wong RS, Nesbitt JC, Putnam JB Jr, Lee JJ and Roth JA: Is follow-up
of lung cancer patients after resection medically indicated and
cost-effective? Ann Thorac Surg. 60:1563–1570. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ichinose Y, Kato H, Koike T, Tsuchiya R,
Fujisawa T, Shimizu N, Watanabe Y, Mitsudomi T and Yoshimura M;
Japan Clinical Oncology Group, : Overall survival and local
recurrence of 406 completely resected stage IIIa-N2 non-small cell
lung cancer patients: Questionnaire survey of the Japan clinical
oncology group to plan for clinical trials. Lung Cancer. 34:29–36.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kagami Y, Nishio M, Narimatsu N, Mjoujin
M, Sakurai T, Hareyama M and Saito A: Radiotherapy for locoregional
recurrent tumors after resection of non-small cell lung cancer.
Lung Cancer. 20:31–35. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jeremic B and Bamberg M: External beam
radiation therapy for bronchial stump recurrence of non-small-cell
lung cancer after complete resection. Radiother Oncol. 64:251–257.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Timmerman R, McGarry R, Yiannoutsos C,
Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C,
Williams M and Fletcher J: Excessive toxicity when treating central
tumors in a phase II study of stereotactic body radiation therapy
for medically inoperable early-stage lung cancer. J Clin Oncol.
24:4833–4839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Oskan F: The quality of toxicity reporting
and the story of the lung SBRT ‘No-Fly Zone’. Int J Radiat Oncol
Biol Phys. 92:514–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Timmerman RD: The quality of toxicity
reporting and the story of the lung SBRT ‘No-Fly Zone’. In Regard
to Oskan. Int J Radiat Oncol Biol Phys. 93:726–727. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Song KJ and Flores RM: Is survival after
sublobar resection vs. lobectomy made equivalent by extent of
lymphadenectomy? Ann Transl Med. 7 (Suppl 6):S1912019.PubMed/NCBI
|
|
74
|
Hattori A, Matsunaga T, Takamochi K, Oh S
and Suzuki K: Locoregional recurrence after segmentectomy for
clinical-T1aN0M0 radiologically solid non-small-cell lung
carcinoma. Eur J Cardiothorac Surg. 51:518–525. 2017.PubMed/NCBI
|
|
75
|
Kim H, Pyo H, Noh JM, Lee W, Park B, Park
HY and Yoo H: Preliminary result of definitive radiotherapy in
patients with non-small cell lung cancer who have underlying
idiopathic pulmonary fibrosis: Comparison between X-ray and proton
therapy. Radiat Oncol. 14:192019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yamaguchi S, Ohguri T, Ide S, Aoki T,
Imada H, Yahara K, Narisada H and Korogi Y: Stereotactic body
radiotherapy for lung tumors in patients with subclinical
interstitial lung disease: The potential risk of extensive
radiation pneumonitis. Lung Cancer. 82:260–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Glick D, Lyen S, Kandel S, Shapera S, Le
LW, Lindsay P, Wong O, Bezjak A, Brade A, Cho BCJ, et al: Impact of
pretreatment interstitial lung disease on radiation pneumonitis and
survival in patients treated with lung stereotactic body radiation
therapy (SBRT). Clin Lung Cancer. 19:e219–e226. 2018. View Article : Google Scholar : PubMed/NCBI
|