Introduction
Ovarian cancer is one of the most common
gynecological tumors (1). Although
surgical resection combined with platinum and taxane-based
chemotherapy inhibits the development of tumors and improves short
and medium-term survival, the 5-year survival rate remains <30%
due to drug resistance and recrudescence worldwide (2). Therefore, exploring the underlying
mechanisms of ovarian cancer development and metastasis may aid the
identification of a novel therapeutic target.
N6-methyladenosine (m6A) is a highly conserved
functional modification of RNA widely distributed in all eukaryotic
cells (3). The m6A modification
functions in a number of biological processes, such as mRNA
post-transcriptional processing, location and translation; m6A is
important in human diseases, including obesity and liver cancer
development (4,5). Catalyzation of the m6A modification is
mediated by a methyltransferase complex consisting of three
proteins: Methyltransferase-like 3 (METTL3), methyltransferase-like
14 and Wilms tumor 1-associating protein (6). Functional studies have demonstrated
that METTL3 is closely associated with the development of various
tumors, and its functions (pro or anti-tumor) vary between
different tumor types. For example, Lin et al (7) have reported that METTL3 functions as a
translational promotor for multiple oncogenes, including epidermal
growth factor receptor (EGFR) and tafazzin (TAZ), in lung cancer,
contributing to tumor proliferation, survival and invasion. Reduced
METTL3 expression levels in human myeloid leukemia cell lines
induces cell differentiation and apoptosis, delaying leukemia
progression in recipient mice in vivo (8). Li et al (9) have demonstrated that METTL3 functions
as a tumor suppressor in the development of renal cell carcinoma by
inhibiting proliferation and invasion. However, the function of
METTL3 in human ovarian cancer remains unclear.
The present study aimed to investigate METTL3
expression levels in different ovarian cancer tissue histotypes and
analyze the functional effect of METTL3 knockdown in the human
ovarian cancer cell lines SKOV3 and OVCAR3.
Materials and methods
Patient tissue samples
The present study was approved by The Ethics
Committee in Shandong Provincial Hospital (Jinan, China) and all
patients provided informed written consent. A total of 52 ovarian
cancer tissue and adjacent normal tissue specimens were collected
from patients diagnosed with ovarian cancer and treated at Shandong
Provincial Hospital between February 2018 and March 2019. All
patients were aged between 35 and 67 years and had only undergone
surgery, receiving no other treatment. The clinicopathological data
of patients obtained included age, tumor size, tumor site, lymph
node metastasis and clinical stages (Table I).
 | Table I.METTL3 expression levels associated
with the clinicopathological parameters in ovarian cancer
tissues. |
Table I.
METTL3 expression levels associated
with the clinicopathological parameters in ovarian cancer
tissues.
|
|
| METTL3 expression
level |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | n | High, n (%) | Low, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
|
<50 | 38 | 28 (73.7) | 10 (26.3) | 0.863 |
| ≥50 | 14 | 10 (71.4) | 4 (28.6) |
|
| Tumor diameter,
cm |
|
|
|
|
|
<3 | 23 | 13 (56.5) | 10 (43.5) | 0.019a |
| ≥3 | 29 | 25 (86.2) | 4 (13.8) |
|
| Lymph node
metastasis |
|
|
|
|
| Yes | 34 | 29 (85.3) | 5 (14.7) | 0.016a |
| No | 18 | 9 (50) | 9 (50) |
|
| Pathological
grading |
|
|
|
|
| I–II | 13 | 6 (12.9) | 7 (87.1) | 0.030a |
|
III–IV | 39 | 32 (56.5) | 7 (43.5) |
|
| Histotype |
|
|
|
|
| Serous
adenocarcinoma | 17 | 14 (82.4) | 3 (17.6) | 0.613 |
| Mucinous
adenocarcinoma | 16 | 11 (68.8) | 5 (31.2) |
|
|
Endometrioid
adenocarcinoma | 12 | 8 (66.7) | 4 (33.3) |
|
| Clear
cell carcinoma | 7 | 5 (71.43) | 2 (28.57) |
|
Immunohistochemistry
The ovarian cancer tissues and adjacent paracancer
tissues were stained using an EliVision™ Plus kit (Fuzhou Maixin
Biotech Co., Ltd.) according to the manufacturers protocol. Images
were obtained using an upright light microscope system (Nikon
Corporation; magnifications, ×100 and ×400). The METTL3
immunostaining score was the sum of the staining intensity score
and the positive staining cell rate score. The staining intensity
was scored as follows: no staining, 0; weak staining, 1; moderate
staining, 2; and strong staining, 3. The positive staining cell
rate was scored as follows: 0 to 5%, 0; 5 to 25%, 1; 26 to 50%, 2;
51 to 75%, 3; and >75%, 4. A score <2 points was considered
low METTL3 expression, whereas >3 points was considered high
METTL3 expression.
Ovarian cancer cell culture and
transfection
Human ovarian cancer cell lines SKOV3 and OVCAR3
were purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences. The cells were cultured in Dulbeccos
Modified Eagles Medium (DMEM; HyClone; GE Healthcare Life Sciences)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in 5% CO2. Short hairpin (sh)RNAs (pSUPER)
shRNA1, shRNA2 and shRNA3 targeting METTL3 and a control shRNA were
designed and synthesized by Shanghai GenePharma Co., Ltd. Ovarian
cancer cells were seeded into a 6-well plate and cultured to
logarithmic phase for 24 h at 37°C. shRNA transfection was
performed using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Following transfection for 48 h, METTL3
mRNA expression levels were determined using reverse
transcription-quantitative (RT-q) PCR.
RT-qPCR
Total RNA of human ovarian cancer cells was prepared
using an Ultrapure RNA Extraction kit (CWBio) according to the
manufacturers protocol. A total of 1 µg RNA was reverse-transcribed
into cDNA with random primers using a HiFiScript cDNA Synthesis kit
(CWBio) according to the manufacturers protocol. The reverse
transcription reaction conditions were as follows: incubation at
42°C for 50 min, followed by incubation at 85°C for 5 min to
terminate the reaction. METTL3 mRNA expression levels were
determined by fluorescence qPCR using UltraSYBR mixture (CoWin
Biosciences) according to the manufacturers protocol and an Applied
Biosystems 7500 FAST Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 30
sec; 40 cycles of 95°C for 5 sec, 60°C for 30 sec and one cycle of
melting curve at 95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec
and 50°C for 30 sec. Quantification of METTL3 mRNA expression
levels was performed using the 2−ΔΔCt method (10) with β-actin as an internal control.
The sequences of primers were as follows: METTL3 forward,
5′-ACCCTGACAGATGATGAGATGC-3′ and reverse,
5′-CGTTCATACCCCCAGAGGTTTAG-3′; β-actin forward,
5′-TCCTCCCTGGAGAAGAGCTAC-3′ and reverse,
5′-TCCTGCTTGCTGATCCACAT-3′.
CCK-8 assay
Cells were seeded into a 96-well plate (3,000
cells/well) and cultured in DMEM at 37°C for 0, 24, 48 or 72 h, and
10 µl CCK-8 solution (Beyotime Institute of Biotechnology) was
added into each well according to the manufacturers protocols.
After incubation for 2 h at 37°C and 5% CO2, the
absorbance of cells at 450 nm was detected using a microplate
reader.
Colony formation assay
SKOV3 and OVCAR3 cells were seeded in a 6-cm petri
dish at the density of 500 cells/well and normally cultured in DMEM
at 37°C for 14 days. Subsequently, the colonies were fixed with 4%
methanol and stained with 0.1% crystal violet at 37°C for 30 min
(Sigma-Aldrich; Merck KGaA). Images of visible colonies were
captured with a HP Scanjet G4010 scanner and counted manually.
Flow cytometry detection of cell
apoptosis
Transfected SKOV3 and OVCAR3 cells were stained
using an Annexin V-FITC Apoptosis Detection kit I (BD Biosciences)
according to the manufacturers protocols. Cells were analyzed using
the BD FACS Canto II system (BD Biosciences) and analyzed using
FlowJo software version 4.5 (Tree Star, Inc.). Viable cells were
negative for both PI and Annexin V, while apoptotic cells were
positive for Annexin V and negative for PI. Late apoptotic dead
cells showed both Annexin V and PI positivity.
Transwell invasion assay
Cell invasion was evaluated using Matrigel-coated
Transwell chambers at 37°C for 24 h (BD Biosciences). A total of
1×105 ovarian cancer cells in 200 µl serum-free DMEM
were added into the upper chamber. A total of 500 µl DMEM with 10%
FBS was added to the lower chamber, and the cells were incubated
for 24 h. The non-invasive cells remaining in the upper chamber of
the Transwell plate were scraped off with a cotton swab. Invaded
cells on the lower surface of the chamber were stained with 0.1%
crystal violet at 25°C for 10 min. The cell number was counted as
the average of five random fields under a light microscope (Nikon
TE2000; Nikon Corporation).
Western blotting
Following transfection for 48 h, cell lysates were
prepared using RIPA lysis buffer and protease cocktail inhibitor I
(Merck KGaA). The protein was separated using 10% SDS-PAGE and
subsequently transferred to a PVDF membrane. After blocking in a 5%
non-fat milk for 1 h, the membrane was washed with TBS + 0.1%
Tween-20. Membranes were incubated with primary antibodies against
METTL3 (1:1,000; cat. no. GTX105037; GeneTex, Inc.), Bcl-2
(1:2,000; cat. no. 60178-1-Ig; ProteinTech Group, Inc.); Bax
(1:1,000; cat. no. 50599-2-Ig; ProteinTech Group, Inc.); active
caspase3 (1:1,000; rabbit polyclonal antibody; cat. no. 19677-1-AP;
ProteinTech Group, Inc.); p-AKT (1:1,000; cat. no. 66444-1-Ig;
ProteinTech Group, Inc.); AKT (1:500; cat. no. 9272; Cell Signaling
Technology, Inc.); p70S6K (1:1,000; cat. no. GTX107562; GeneTex,
Inc.); Cyclin D1 (1:1,000; cat. no. GTX108624; GeneTex, Inc.) and
tubulin (1:1,000; cat. no. GTX76511; GeneTex, Inc.) at 4°C
overnight, followed by incubation with anti-rabbit IgG (1:2,000;
cat. no. GTX300119; GeneTex, Inc.) or anti-mouse IgG (1:2,000; cat.
no. GTX300120; GeneTex, Inc.) secondary antibodies for 1 h at room
temperature. Protein bands were visualized using Pierce ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). Protein band
intensity was analyzed using Image J software, v1.41 (National
Institutes of Health).
Statistical analysis
Data analysis was performed using GraphPad Prism 7
(GraphPad Software, Inc.), and all experiments were performed in
triplicate. Differences between two groups were evaluated using
Students t-test; one-way ANOVA was used to analyze multiple groups,
followed by Tukeys post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
METTL3 expression levels are
associated with clinical parameters in patients with ovarian
cancer
METTL3 expression levels were significantly highly
in serous adenocarcinoma, mucinous adenocarcinoma, endometrioid
adenocarcinoma and clear cell carcinoma tissues compared with
corresponding paracancerous tissues. METTL3 expression levels were
not associated with different histotypes of ovarian cancer tissues.
High METTL3 expression levels were associated with large tumor size
(P=0.0188), lymph node metastasis (P=0.0163) and high pathological
grade (P=0.0303). The pathological grade was staged according to
the International Union against Cancer/American Joint Committee on
Cancer system (11). These data
indicated that METTL3 expression levels may be associated with the
tumor growth and metastasis of ovarian cancer (Fig. 1 and Table
II).
 | Table II.METTL3 expression levels in ovarian
cancer and para-carcinoma tissue. |
Table II.
METTL3 expression levels in ovarian
cancer and para-carcinoma tissue.
|
|
| METTL3 expression
level |
|
|---|
|
|
|
|
|
|---|
| Tissue | n | Low, n (%) | High, n (%) | P-value |
|---|
| Ovarian cancer | 52 | 14 (18.3) | 38 (81.7) |
<0.001a |
| Para-carcinoma | 52 | 40 (87.1) | 12 (12.9) |
<0.001a |
Downregulation of METTL3 inhibits
proliferation and colony formation in human ovarian cancer
tissues
To investigate the biological function of METTL3 in
human ovarian cancer, loss-of-function assays were performed. The
interference efficiency results demonstrated that shRNA1, shRNA2
and shRNA3 targeting METTL3 significantly downregulated METTL3 mRNA
expression levels in SKOV3 and OVCAR3 cells compared with negative
control group (P<0.05; Fig. 2A and
B). shRNA3 in SKOV3 cells and shRNA1 in OVCAR3 cells (shMETTL3)
were selected for further experiments due to the interference
efficiencies, and knockdown efficacy was validated at the protein
level (Fig. 2C and D). The cell
proliferation assay results indicated that METTL3 knockdown
significantly inhibited the proliferation of SKOV3 and OVCAR3 cells
(Fig. 3A and B; P<0.05). In
addition, cell colony number was also significantly decreased when
METTL3 expression was silenced (Fig. 3C
and D).
 | Figure 2.METTL3 knockdown in SKOV3 and OVCAR3
ovarian cancer cells mediated by shRNA transfection. All
experiments were performed in triplicate. shRNA1, shRNA2, shRNA3,
shMETTL3 or a negative control shRNA was introduced into SKOV3 and
OVCAR3 cells. METLL3 mRNA expression levels in (A) SKOV3 and (B)
OVCAR3 cells treated with shRNA1, shRNA2, shRNA3 or NC. (C) METTL3
protein expression levels in SKOV3 and OCVAR3 cells treated with
shRNA3 and shRNA1, respectively. (D) Quantified METTL3 protein
expression levels using ImageJ software in SKOV3 and OVCAR3 cells.
*P<0.05 vs. NC. METTL3, methyltransferase-like 3; sh, short
hairpin; NC, negative control; shMETTL3, shRNA3 in SHOV3 cells or
shRNA1 in OVCAR3 cells. |
METTL3 knockdown induces apoptosis and
may lead to the activation of the mitochondrial apoptosis pathway
in ovarian cancer cells
Apoptosis analysis results suggested that the
percentage of SKOV3 and OVCAR3 cells undergoing apoptosis was
significantly increased when METTL3 expression levels were knocked
down compared with the control at 48 h and 72 h (Fig. 4A-D). METTL3 knockdown also led to a
significant upregulation of the pro-apoptotic protein Bax and
downstream effector Caspase 3 expression levels, whereas the
expression of the anti-apoptotic protein Bcl2 was downregulated
(Fig. 4E and F). These data
suggested that METTL3 knockdown activated the mitochondrial
apoptosis pathway in SKOC3 and OVCAR3 cells.
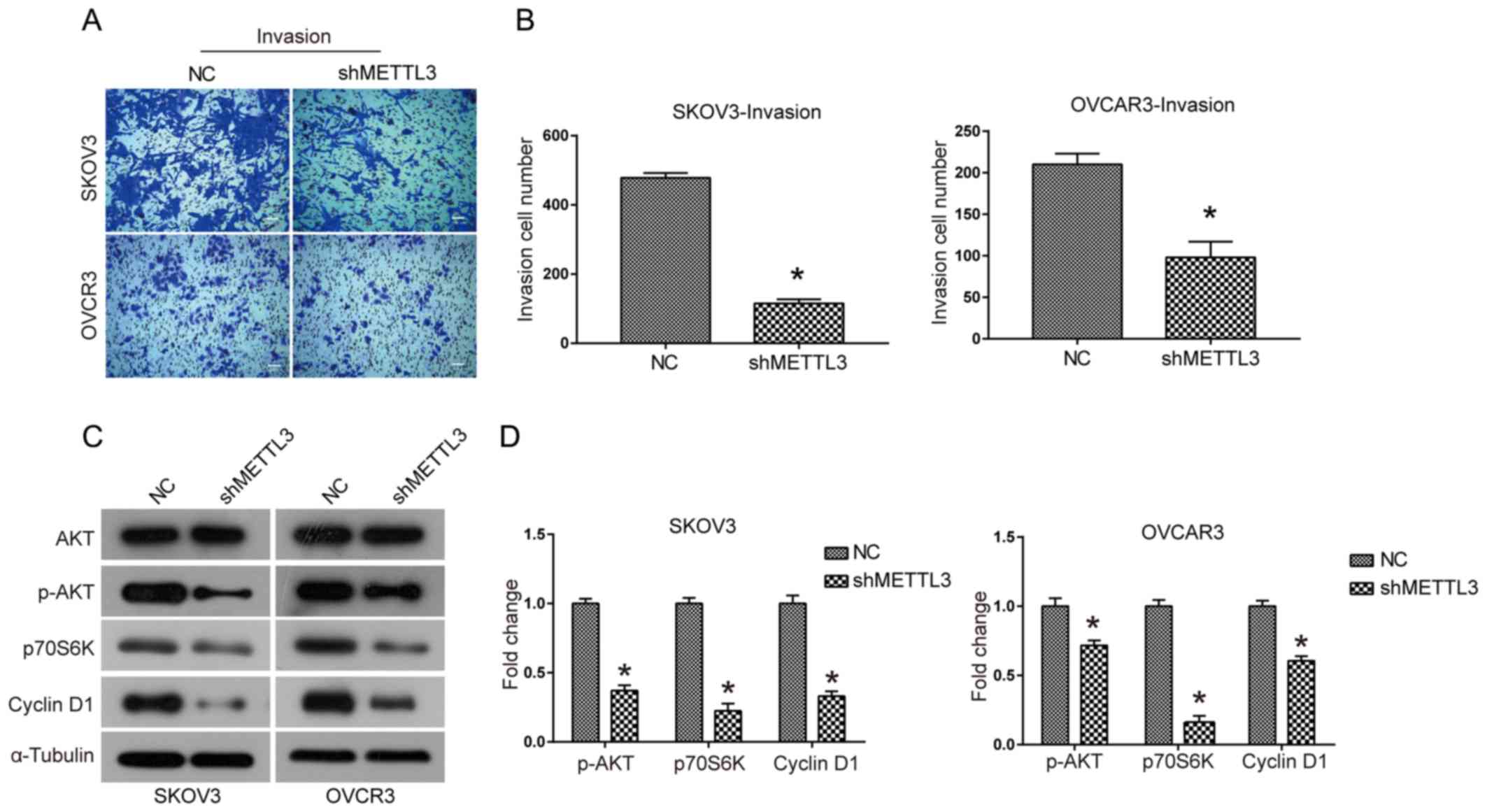
METTL3 knockdown inhibits cell
invasion and may reduce the activation of the AKT signaling
pathway
The effect of METTL3 knockdown on the invasive
ability of SKOV3 and OVCAR3 cells was investigated using Transwell
assays. The invasive ability of SKOV3 and OVCAR3 cells was
significantly reduced when METTL3 expression levels were knocked
down compared with the control (P<0.05; Fig. 5A and B). It was hypothesized that thr
AKT signaling pathway may be associated with the biological
function of METTL3. METTL3 knockdown led to decreased expression
levels of phosphorylated AKT and its downstream effectors p70S6K
and Cyclin D1 (Fig. 5C and D). These
results suggested that knockdown of METTL3 expression led to
reduced activation of the AKT signaling pathway in ovarian cancer
cells.
Discussion
In the present study, METTL3 expression levels in
ovarian cancer tissues were investigated; the results demonstrated
that knockdown of METTL3 significantly inhibited proliferation,
colony formation and invasion of ovarian cancer cells. In addition,
the apoptotic rate was increased when METTL3 expression levels were
knocked down. Downregulation of METTL3 increased the expression
levels of the pro-apoptotic Bax and Caspase 3, whereas the
expression levels of the anti-apoptotic Bcl2 were decreased. During
apoptosis, Bax binds to the mitochondrial outer membrane and
promotes its permeability, causing the release of cytochrome c into
the cytoplasm, which induces the activation of Caspase 3 (12). By contrast, Bcl2 functions as an
apoptosis inhibitor and blocks the promotion of mitochondrial
permeability (13). To the best of
our knowledge, the present study demonstrated the oncogenic
function of METTL3 in the biological process of ovarian cancer
cells for the first time.
An increasing number of studies have demonstrated
the function of METTL3 in tumor formation and progression. For
example, Lin et al (7) have
reported that METTL3 promotes the translation of epidermal growth
factor receptor and the Hippo pathway effector TAZ in human lung
cancer cells. In addition, reduced METTL3 expression levels inhibit
tumor growth and metastasis to the lung of hepatocellular carcinoma
(HCC) in vitro and in vivo (14). Du et al (15) have demonstrated that microRNA-33a
functions as a tumor suppressor in non-small-cell lung carcinoma
cells, suppressing the translation of METTL3 mRNA. However, another
study identified METTL3 as a tumor suppressor in renal cell
carcinoma, inhibiting tumor proliferation, migration and cell cycle
progression (9). These results from
previous studies suggest that the functions of METTL3 can vary
between different tumor types, and a possible explanation for this
may be the high degree of tumor heterogeneity or gene
mutation-induced function alteration.
The modes of METTL3 action can be divided into two
types: m6A-dependent and m6A-independent (14,16). For
example, METTL3 depletion can promote suppressor of cytokine
signaling 2 expression by decreasing m6A methylation-mediated
degradation, thus blocking the progression of HCC (14). Cai et al (16) demonstrated that METTL3-induced m6A
modification increased the expression levels of hepatitis B
X-interacting protein, thus promoting the proliferation of breast
cancer cells. METTL3 has also been identified to elevate
oncoprotein expression levels in tumor cells by functioning as a
transcription enhancer factor or promoting the assembly of mRNA
translation machinery (7). In the
present study, the mechanisms underlying the oncogenic function of
METTL3 in human ovarian cancer were investigated. A previous study
reported that the AKT signaling pathway regulates numerous
biological processes, such as promoting cell proliferation and
survival (17). The present study
demonstrated that METTL3 downregulation decreased the expression
levels of phosphorylated AKT and its downstream effectors,
including p70S6K and Cyclin D1, indicating reduced activation of
the AKT pathway. However, the present study was unable to identify
the direct target of METTL3 function in human ovarian cancer cell
lines due to the experimental conditions.
In conclusion, METTL3 knockdown inhibits
tumorigenesis and tumor progression of human ovarian cancer cells
in vitro, which may be mediated by reduced activation of the
AKT signaling pathway. These results may provide novel insight into
the potential targeting of METTL3 in ovarian cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81503298) and
Natural Science Foundation of Shandong Province (grant no.
ZR2014HM008).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors contributions
SL designed the study. SL, HG, XL, NL, FG and JL
performed experiments and analyzed data. SL wrote the manuscript.
All authors read and approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by The Ethics
Committee of Shandong Provincial Hospital (China). All patients
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
METTL3
|
methyltransferase-like 3
|
|
m6A
|
N6-methyladenosine
|
References
|
1
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scholz HS, Tasdemir H, Hunlich T, Turnwald
W, Both A and Egger H: Multivisceral cytoreductive surgery in FIGO
stages IIIC and IV epithelial ovarian cancer: Results and 5-year
follow-up. Gynecol Oncol. 106:591–595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei W, Ji X, Guo X and Ji S: Regulatory
role of N6 -methyladenosine (m6 A) methylation in RNA processing
and human diseases. J Cell Biochem. 118:2534–2543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han
D, Fu Y, Parisien M, Dai Q, Jia G, et al:
N-6-methyladenosine-dependent regulation of messenger RNA
stability. Nature. 505:117–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin S and Gregory RI: Methyltransferases
modulate RNA stability in embryonic stem cells. Nat Cell Biol.
16:129–131. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwartz S, Mumbach MR, Jovanovic M, Wang
T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N,
Cacchiarelli D, et al: Perturbation of m6A writers reveals two
distinct classes of mRNA methylation at internal and 5 sites. Cell
Rep. 8:284–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin S, Choe J, Du P, Triboulet R and
Gregory RI: The m(6)A methyltransferase METTL3 promotes translation
in human cancer cells. Mol Cell. 62:335–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vu LP, Pickering BF, Cheng Y, et al: The
N-6-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid
differentiation of normal hematopoietic and leukemia cells. Nat
Med. 23:1369–1376. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Tang J, Huang W, Wang F, Li P, Qin
C, Qin Z, Zou Q, Wei J, Hua L, et al: The M6A methyltransferase
METTL3: Acting as a tumor suppressor in renal cell carcinoma.
Oncotarget. 8:96103–96116. 2017.PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kroeger PT Jr and Drapkin R: Pathogenesis
and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol.
29:26–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi X, Yin XM and Dong Z: Inhibition of
Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation,
and Bax/Bak oligomerization suppressed. J Biol Chem.
278:16992–16999. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radha G and Raghavan SC: BCL2: A promising
cancer therapeutic target. Biochimica et Biophysica Acta (BBA). Rev
Can. 1868:309–314. 2017.
|
|
14
|
Chen M, Wei L, Law C-T, et al: RNA
N6-methyladenosine methyltransferase-like 3 promotes liver cancer
progression through YTHDF2-dependent posttranscriptional silencing
of SOCS2. Hepatology. 67:2254–2270. 2017. View Article : Google Scholar
|
|
15
|
Du M, Zhang Y, Mao Y, Mou J, Zhao J, Xue
Q, Wang D, Huang J, Gao S and Gao Y: MiR-33a suppresses
proliferation of NSCLC cells via targeting METTL3 mRNA. Biochem
Biophys Res Commun. 482:582–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang
Z, Liu Y, Zhang X, Zhang W and Ye L: HBXIP-elevated
methyltransferase METTL3 promotes the progression of breast cancer
via inhibiting tumor suppressor let-7g. Cancer Lett. 415:11–19.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|