Introduction
It has been demonstrated that inflammation serves
important roles in the development, growth and/or invasion of
various types of cancer (1–3). For example, Helicobacter pylori
(H. pylori) infection causes chronic inflammation in the
gastric mucosa and is subsequently involved in the development of
gastric cancers (GCs) (4,5), although the precise mechanism remains
unclear. Proinflammatory cytokines function not only in the
gastrointestinal immune system, but also in cell growth and/or
apoptosis in the gastric mucosa, resulting in the development and
progression of GCs (6,7). Downstream of cytokine signaling,
various activated transcription factors, such as signal transducer
and activator of transcriptions (STATs), NF-κB and AP-1, serve a
role in the regulation of target genes that are involved in gastric
carcinogenesis (7). Among these
cytokine-associated transcription factors, STAT3 has been
highlighted in inflammation-associated carcinogenesis in various
organs, such as lung, pancreas and liver (8–12).
Notably, mice possessing STAT3 hyperactivation, which lack the
negative feedback by SHP2/SOCS3 binding onto gp130, develop gastric
tumors accompanied by chronic gastritis (13,14);
however, the clinical significance of overactivated STAT3 and its
function in human gastric carcinogenesis remains to be clarified.
STAT3 is constitutively activated in numerous types of cancer, for
example lung and pancreatic cancer and hepatocellular carcinoma
(15–17), and serves a role in cell
proliferation, migration and in anti-apoptosis by activating target
genes, including cyclin D1, matrix metalloproteases or
Bcl-xL (18,19). It has also been shown that Ki67 is a
well-known marker to evaluate the ability of cell proliferation
(20). Hence, the present study
aimed to investigate the correlation between phosphorylated
(p-)STAT3 and Ki67 expression levels in patients with early GC.
H. pylori infection over two decades causes a
sequence of histological changes in the non-neoplastic gastric
mucosa (non-NGM), referred to as Correa's hypothesis (4,5), along
with simultaneous accumulation of genetic and epigenetic
alterations, for example microsatellite instability or p53
and E-cadherin mutations (21,22).
Cytokine signaling activates cytokine receptor-associated Janus
kinase (JAK) (23,24), which in turn phosphorylates STAT3,
rendering it functional (23,24). On
the other hand, the suppressor of cytokine signaling 3
(SOCS3) can bind to cytokine receptors and JAK to inhibit
JAK/STAT3 signaling, acting as a tumor suppressor in a negative
feedback loop (25,26). In this regard, alteration of
SOCS3 appears to be a crucial step of carcinogenesis in
various organs, including the head and neck, pancreas, liver, blood
and brain (27–31). The present study investigated
SOCS3 methylation and p-STAT3 expression levels in the
non-NGM of patients with early GC in relation to non-NGM cell
proliferative ability that may impact GC development.
Materials and methods
Patients and biopsies
A total of fifty-one patients with early GC (39 male
and 12 female; median age 72; age range 48–87) and 22 patients with
gastritis without GC (12 males and 10 females; median age 64; age
range 30–81) were enrolled into the present study between January
2011 and March 2013 at the Hyogo College of Medicine Hospital
(Hyogo, Japan). Patients with early GC were diagnosed by previous
endoscopic examination with biopsy at the Hyogo College of Medicine
Hospital. The exclusion criteria were as follows: i) Patients with
malignancy in other organs; ii) patients with an allergy to drugs
used for H. pylori eradication; iii) patients regularly
taking a nonsteroidal anti-inflammatory drug, including aspirin;
iv) patients with a history of esophagectomy or gastrectomy; and v)
patients who were determined by their physicians to be unqualified
for any other reason, for example severe pneumonia. Biopsy
specimens were routinely obtained from the non-NGM of all patients
at the greater curvature of the mid corpus of the stomach (at least
3 cm far from the lesion), where biopsy was possible before and
after treatment with endoscopic submucosal resection (ESD). All
patients with early GC underwent ESD and were followed up using
endoscopic examination 1 year later. Among them, 13 patients
received H. pylori eradication after ESD treatment and
biopsy specimens were obtained for a second time from the same
location at the greater curvature of the stomach when undergoing
follow-up endoscopic examinations 1 year after ESD. The severity of
gastric atrophy was classified by endoscopic examination according
to the criteria of Kimura and Takemoto, as reported previously
(32,33). The serum was isolated from blood
samples from the patients before ESD treatment. The serum H.
pylori immunoglobulin G (IgG) antibody titer was analyzed using
an ELISA kit (E plate test; Eiken Chemical Co., Ltd.). Written
informed consent was provided by all the patients and the present
study was approved by The Ethics Committee of Hyogo College of
Medicine.
DNA extraction and bisulfite
treatment
DNA was isolated from biopsy specimens using a
QIAamp DNA Micro kit (Qiagen GmbH). The DNA (500 ng) was modified
with sodium bisulfite using an EpiTect Bisulfite kit (Qiagen GmbH),
as recommended in the manufacturer's protocol (34). Sodium bisulfite converts unmethylated
cytosine to uracil, whereas methylated cytosines are resistant
(35). DNA samples were subsequently
purified using the Wizard DNA Clean-Up System (Promega
Biotechnologies, Inc.) and precipitated in 16 µl water, as
previously reported (34).
Qualitative methylation-specific PCR
(MSP) for SOCS3 gene
Bisulfite-treated genomic DNA was amplified using
either methylated or unmethylated specific primer sets, using the
sequences as follows: Methylated specific forward,
5′-TATATATTCGCGAGCGCGGTTT-3′, and reverse, 5′-CGCTGCGCCCAGATGTT-3′;
unmethylated specific forward,
5′-TGTGGTGGTTGTTTATATATTTGTGAGTGTGGTT-3′, and reverse,
5′-CAACCAACAATAACCCACACTACACCCA-3′ (36). The amplifications were performed in a
total reaction volume of 50 µl containing 20 pmol of each set of
primers, 1.25 U EpiTaq HS DNA polymerase, PCR buffer with
MgCl2 (both Takara Bio, Inc) and 0.3 mM each dNTP. The
PCR was conducted as follows: Initial denaturation at 95°C for 5
min; 30 cycles at 98°C for 10 sec; 64°C for 30 sec; 72°C for 30
sec; final extension at 72°C for 7 min. The PCR products were
electrophoresed using 2% agarose gel and then visualized using
ethidium bromide staining under UV illumination.
Immunohistochemistry
The biopsy specimens were fixed in 10% formalin
solution at room temperature overnight and embedded in paraffin.
Immunohistochemical staining for Ki67 and p-STAT3 was performed
using an Envision kit (Dako; Agilent Technologies) as previously
described (37,38), using the primary antibodies anti-Ki67
antibody (1:50; cat no. IR626; Dako; Agilent Technologies) and
anti-phospho-specific STAT3 (Tyr705) antibody (1:15; cat no. 9131;
Cell Signaling Technology). In brief, 4-µm-thick sections were
placed on slides, deparaffinized in xylene and rehydrated through a
descending series of ethanol (100, 90, 80 and 70%). The slides were
then placed in Dako REAL Target Retrieval Solution (Dako; Agilent
Technologies) and treated by microwave heating (MI-77; Azumaya) at
400 W and 95°C for 10 min to facilitate antigen retrieval, followed
by pretreatment with 0.3% H2O2 in methanol
for 20 min at room temperature to quench endogenous peroxidase
activity. The sections were then washed 3 times by
phosphate-buffered saline and followed by the treated with blocking
buffer (Protein Block Serum-Free; Dako Agilent Technologies) for 30
min at room temperature. Thereafter, the sections were incubated
with the primary antibodies for 60 min at room temperature, washed
3 times in phosphate-buffered saline and incubated with anti-mouse
(ready to use; cat. no. K4001) or anti-rabbit IgG antibody (ready
to use; cat. no. K4003) (both Dako; Agilent Technologies, Inc.) for
30 min at room temperature and washed 3 times in phosphate-buffered
saline. Finally, the sections were incubated in
3,3′-diaminobenzidine tetrahydrochloride with 0.05% hydrogen
peroxide for 3 min at room temperature and then counterstained with
Mayer's hematoxylin for 1 min at room temperature.
To evaluate the immunoreactivity of Ki67 and
p-STAT3, 100 epithelial cells were counted in 5 different visual
fields for each section under light microscope (magnification,
×400). The labeling index was calculated as the percentage of
positive cells.
Statistical analysis
All values were expressed as the mean ± standard
error of the mean. The significance of differences between two
unpaired groups was assessed using a Student's t-test or
Mann-Whitney U-test. Clinicopathological parameters including sex,
age, anti-H. pylori antibody, gastric atrophy and
SOCS3 methylation positivity, were assessed using
χ2 analyses. The correlation between p-STAT3 and Ki67
labeling index was assessed using linear regression analysis. For
multiple comparisons, the paired data before and after eradication
were analyzed using two-way repeated measures ANOVA followed by
Bonferroni's correction. P<0.05 was considered to indicate a
statistically significant difference.
Results
Association between the
characteristics of patients and SOCS3 methylation in the non-NGM of
patients with or without early GC
Representative electrophoresis gels of MSP products
for SOCS3 are shown in Fig.
1. The clinical and endoscopic features of the patients with or
without early GC are presented in Table
I. A total of 17 out of the 51 patients with early GC (33.3%)
had SOCS3 methylation. Sex, age, anti-H. pylori
antibody and gastric atrophy were not significantly associated with
SOCS3 methylation positivity in the non-NGM of patients with
early GC. The positivity of SOCS3 methylation in the non-NGM
was significantly higher in patients with early GC compared with
those without (P=0.020) (Table I).
Parameters including age (P=0.0003), anti-H. pylori antibody
(P=0.0001) and gastric atrophy (P=0.0005) were significantly
different between patients with early GC and those without
(Table I). Regarding anti-H.
pylori-IgG level, 19/51 patients with early GC were negative;
however, 14 (74%) of these 19 patients showed an open-type gastric
atrophy. Overall, 6/51 early GC patients was negative for
anti-H. pylori antibody due to past eradication therapy.
SOCS3 methylation was detected in 3/6 of these patients with
early GC. SOCS3 methylation positivity was detected in 4/10
(40%) patients with early GC with closed-type atrophy and in 13/41
(32%) of patients with open-type atrophy.
 | Table I.Characteristics of patients with
(n=51) and without (n=22) early gastric cancer. |
Table I.
Characteristics of patients with
(n=51) and without (n=22) early gastric cancer.
|
| Without early
GC | With early GC |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Without early
GC | P-value in ‘Without
GC group’ | With early GC | P-value in ‘With GC
group’ | P-value, with vs.
without |
|---|
| Sex, n (n;
%)a |
| NS |
| NS | NS |
|
Male | 12 (0; 0.0) |
| 39 (12;
30.8) |
|
|
|
Female | 10 (2;
20.0) |
| 12 (5; 41.7) |
|
|
| Mean age ± SEM
(range), years | 61.1±2.9
(30–81) | NS | 71.1±1.2
(48–87) | NS |
0.0003 |
| <65
years, n (n; %)a | 11 (0; 0.0) |
| 13 (4; 30.8) |
| 0.041 |
| ≥65
years, n (n; %)a | 11 (2;
18.2) |
| 38 (13;
34.2) |
|
|
| Anti-H.
pylori antibody, n (n; %)a |
| NS |
| NS |
0.0001 |
|
Negative | 5 (0;
0.0) |
| 19 (6; 31.6) |
|
|
|
Positive | 15 (2;
13.3) |
| 26 (8; 30.8) |
|
|
|
Era-negative | 2 (0;
0.0) |
| 6 (3;
50.0) |
|
|
| Gastric atrophy, n
(n; %)a |
| NS |
| NS |
0.0005 |
|
None | 5 (0;
0.0) |
| 0 (0; 0.0) |
|
|
|
Closed | 7 (1;
14.3) |
| 10 (4; 40.0) |
|
|
|
Open | 10 (1;
10.0) |
| 41 (13;
31.7) |
|
|
| SOCS3
methylation positive, n (%) |
|
|
|
| 0.020 |
|
Positive | 2 (9.1) |
| 17 (33.3) |
|
|
|
Negative | 20 (90.9) |
| 34 (66.7) |
|
|
A total of 22 patients had chronic gastritis but no
cancerous lesions. Among them, 15 patients were positive for both
anti-H. pylori antibody and gastric atrophy and 2 (13.3%)
were also positive for SOCS3 methylation. A total of 5
patients were negative for both anti-H. pylori antibody and
gastric atrophy and had no SOCS3 methylation (Table II). The remaining two patients were
negative for anti-H. pylori antibody after eradication but
positive for gastric atrophy. These patients had no SOCS3
methylation. When the 15 patients with anti-H. pylori
antibody-positivity were compared with 5 patients with anti-H.
pylori antibody negativity, the presence of gastric atrophy was
significantly associated with H. pylori infection
(P<0.0001) (Table II).
 | Table II.Characteristics in patients without
early gastric cancer, with or without H. pylori
infection. |
Table II.
Characteristics in patients without
early gastric cancer, with or without H. pylori
infection.
| Characteristic | H.
pylori-negative (n=5) | H.
pylori-positive (n=15) | P-value |
|---|
| Sex, n (n;
%)a |
|
| NS |
|
Male | 2 (0; 0.0) | 10 (0; 0.0) |
|
|
Female | 3 (0; 0.0) | 5 (2;
40.0) |
|
| Mean age ± SEM
(range), years | 66.8±2.3
(62–74) | 58.1±3.9
(30–81) | NS |
| <65
years, n (n; %)a | 2 (0;
0.0%) | 8 (0;
0.0) | NS |
| ≥65
years, n (n; %)a | 3 (0; 0%) | 7 (2;
28.6) |
|
| Gastric atrophy,
years, n (n; %)a |
|
| <0.0001 |
|
None | 5 (0; 0.0) | 0 (0;
0.0) |
|
|
Closed | 0 (0; 0.0) | 7 (1;
14.3) |
|
|
Open | 0 (0; 0.0) | 8 (1;
12.5) |
|
| SOCS3
methylation positive | 0 (0.0) | 2 (13.3) | NS |
The group of patients without early GC contained
patients positive and negative for H. pylori infection. The
15 patients who were positive for both anti-H. pylori
antibody were isolated, then gastric atrophy and the associations
between characteristics of patients and SOCS3 methylation in
the non-NGM of patients with or without early gastric cancer were
re-analyzed. Subsequently, age, gastric atrophy and the positivity
of SOCS3 methylation in the non-NGM was significantly higher
in patients with early GC compared with that in patients without
early GC (P=0.047, P=0.0002 and P=0.046, respectively; Table III).
 | Table III.Characteristics in patients with
early gastric cancer with H. pylori infection and patients
with early gastric cancer without H. pylori infection. |
Table III.
Characteristics in patients with
early gastric cancer with H. pylori infection and patients
with early gastric cancer without H. pylori infection.
|
Characteristics | Without early GC
H. pylori-positive, n=15 | With early GC,
n=51 | P-value, with vs.
without |
|---|
| Sex, n (n;
%)a |
|
| NS |
|
Male | 10 (0;
0.0%) | 39 (12;
30.8) |
|
|
Female | 5 (2;
40.0%) | 12 (5; 41.7) |
|
| Mean age ± SEM
(range), years | 58.1±3.9
(30–81) | 71.1±1.2
(48–87) | 0.0002 |
| <65
years, n (n; %)a | 8 (0; 0.0) | 13 (4; 30.8) | 0.047b |
| ≥65
years, n (n; %)a | 7 (2;
28.6) | 38 (13;
34.2) |
|
| Gastric atrophy, n
(n; %)a |
|
|
0.0002b |
|
None | 0 (0; 0.0) | 0 (0; 0.0) |
|
|
Closed | 7 (1;
14.3) | 10 (4; 40.0) |
|
|
Open | 8 (1;
12.5) | 41 (13;
31.7) |
|
| SOCS3
methylation positive | 2 (13.3) | 17 (33.3) | 0.046b |
Correlation between SOCS3 methylation
and p-STAT3 and Ki67 expression levels in the non-NGM in patients
with early GC
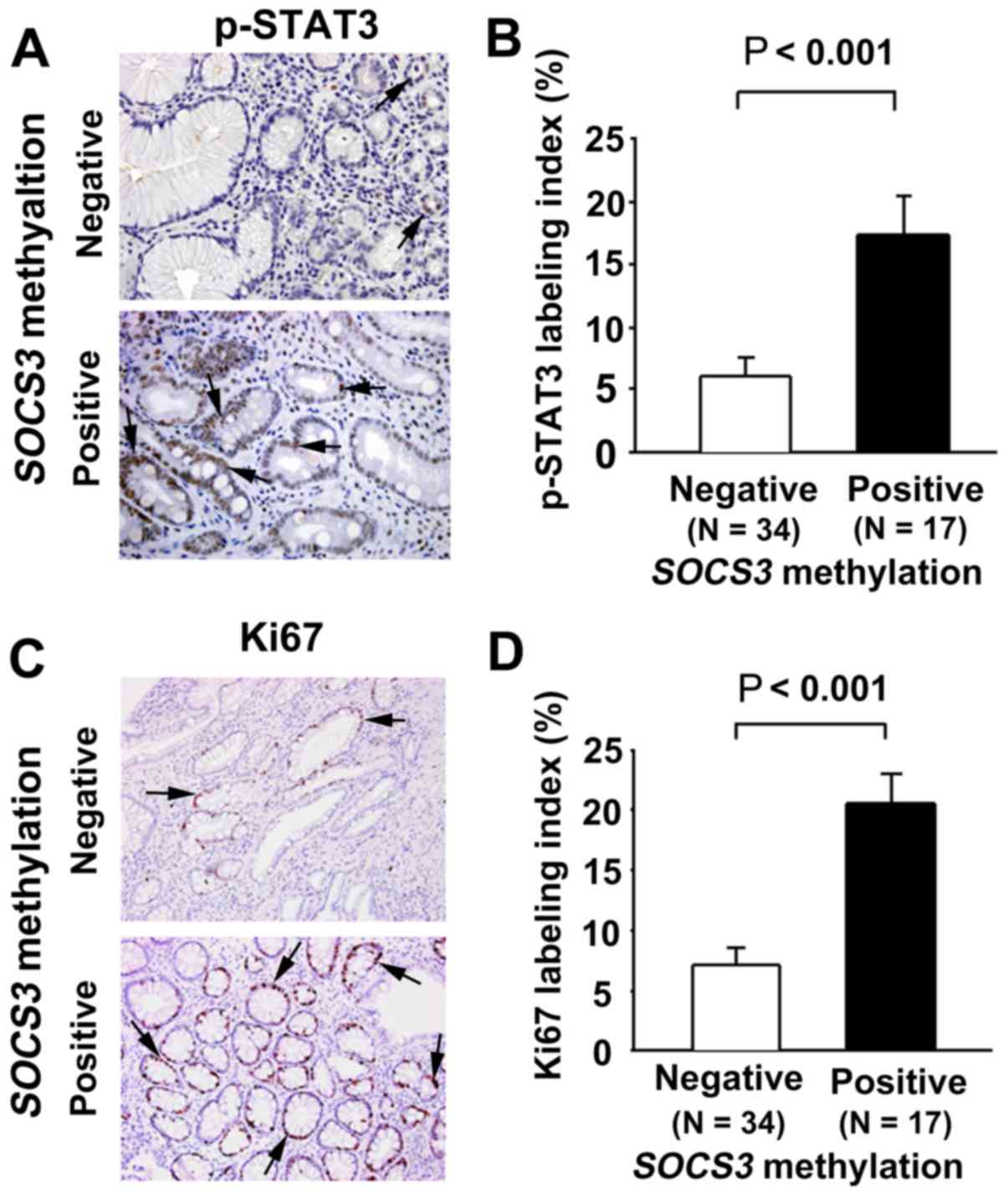
p-STAT3 immunoreactivity was observed in the nuclei
of the non-neoplastic epithelial cells in the gastric mucosa
(Fig. 2A). The p-STAT3 labeling
index in the non-NGM was significantly higher in early GC patients
with SOCS3 methylation compared with those without (P<0.001;
Fig. 2B). Ki67 immunoreactivity (as
a cell proliferation marker) was also observed in the nuclei of the
non-neoplastic epithelial cells in the gastric mucosa (Fig. 2C). The Ki67 labeling index in the
non-NGM was significantly higher in patients with early GC with
SOCS3 methylation compared with those without (P<0.001;
Fig. 2D).
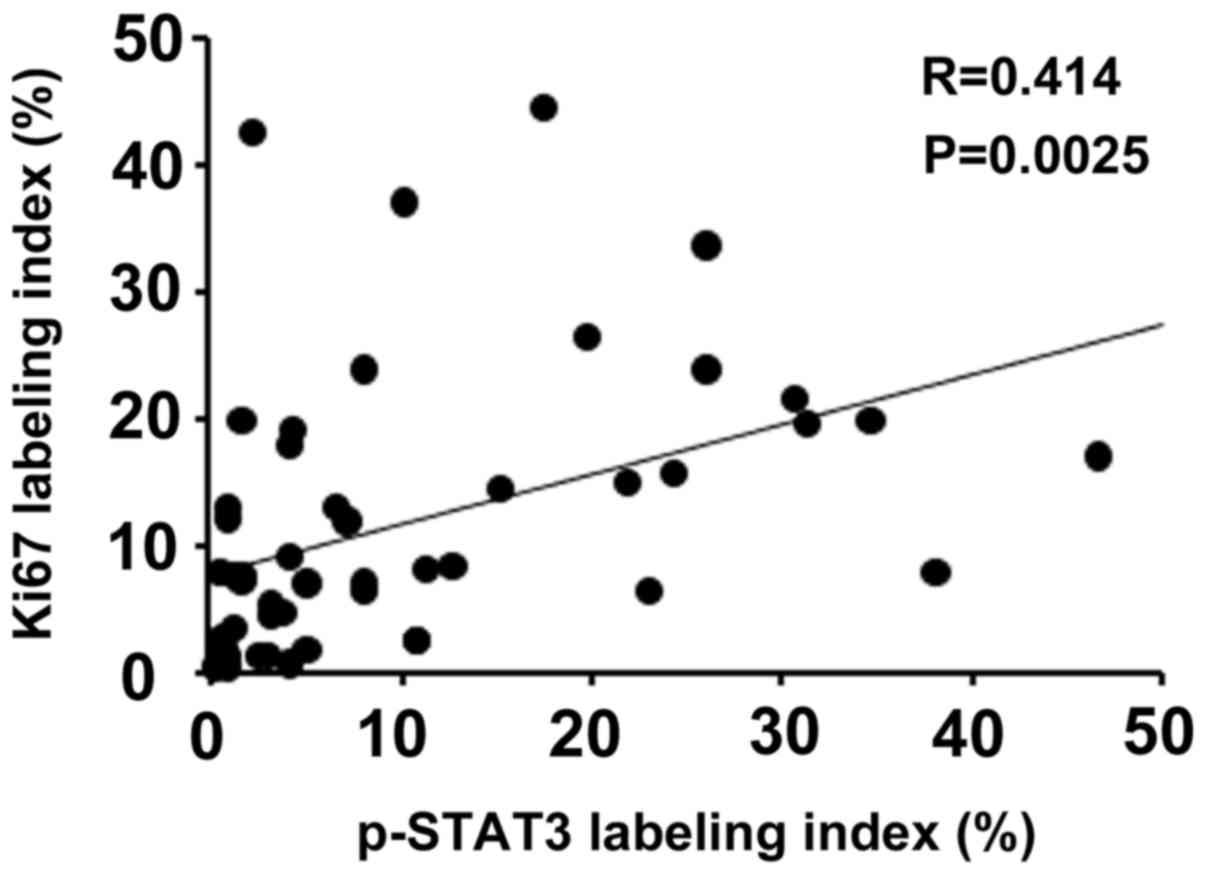
It is known that activated STAT3 plays a pivotal
role in cell proliferation (11,12).
Therefore, the correlation between p-STAT3 and Ki67 expression
levels was investigated in the non-NGM of patients with early GC.
The labeling index of Ki67 was positively correlated with that of
p-STAT3 (r=0.414; P=0.0025; Fig.
3).
Effect of H. pylori eradication on
SOCS3 methylation and p-STAT3/Ki67 expression in the non-NGM in
patients with early GC
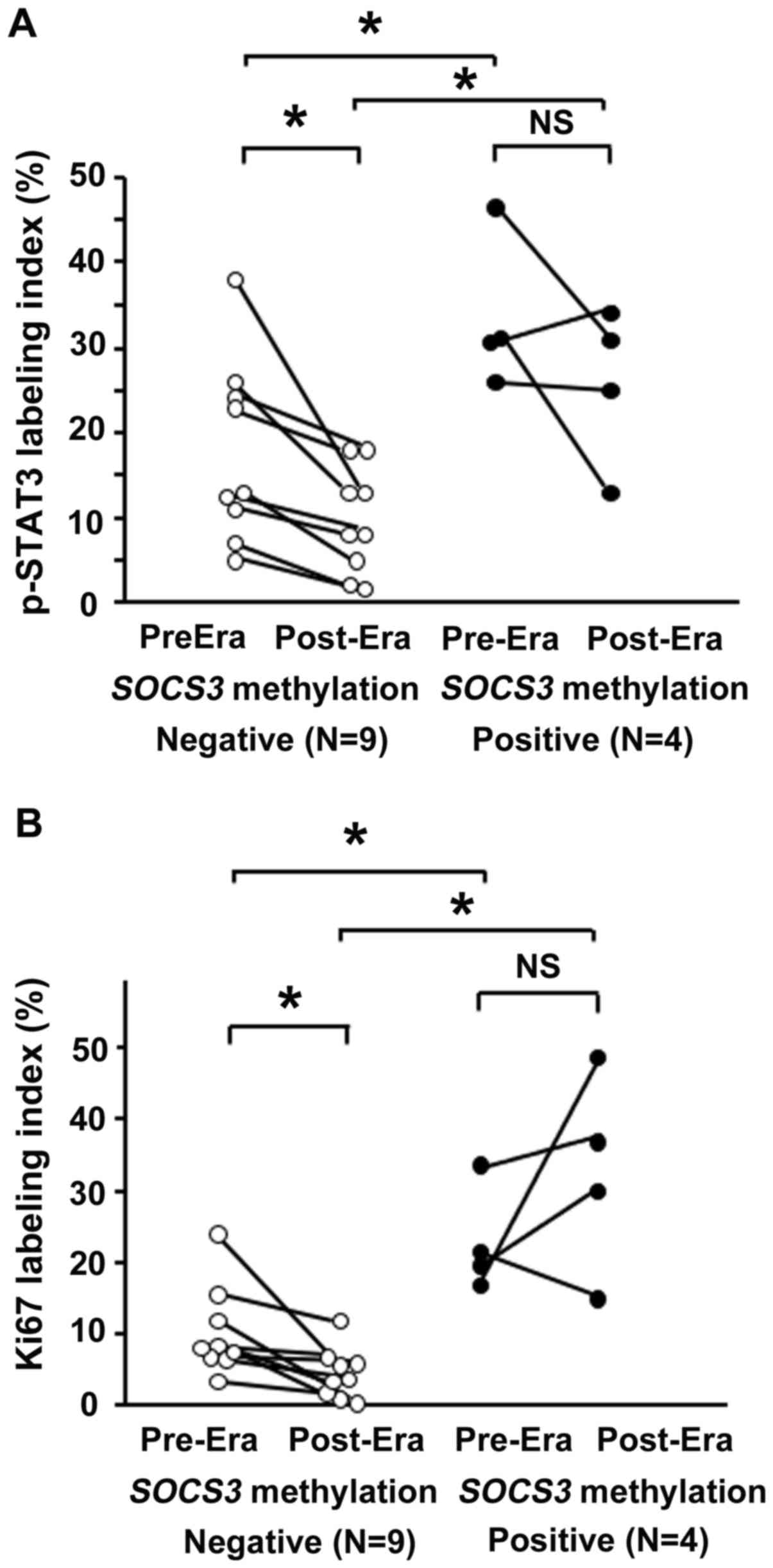
A total of 13 patients were investigated who
received H. pylori eradication therapy after ESD and in whom
gastric biopsy sampling had been performed prior to and one year
following eradication. A total of 4 patients were positive for
SOCS3 methylation, whereas 9 were negative (Fig. 4); although the small number examined
was a limitation in this study.
Before H. pylori eradication, the p-STAT3
labeling index in the non-NGM was significantly higher in the
SOCS3 methylation-positive group (33.6±4.9) than in negative
group (17.7±3.6) (P<0.05). After eradication, the p-STAT3
labeling index was significantly reduced in the SOCS3
methylation-negative group (9.6±2.1) (P<0.05) but remained
unchanged in the SOCS3 methylation-positive group. The
p-STAT3 labeling index remained significantly higher in the
SOCS3 methylation-positive group (25.8±4.7) compared with
that in the negative group (Fig.
4A).
Ki67 expression levels were also investigated in the
aforementioned 13 patients. Before H. pylori eradication,
the Ki67 labeling index in the non-NGM was significantly higher in
the SOCS3 methylation-positive group (23.0±3.7) compared
with that in the negative group (10.3±2.1) (P<0.05). This
difference was sustained even after eradication (P<0.05). In the
SOCS3 methylation-negative group, the Ki67 labeling index
was significantly reduced by eradication treatment (4.5±1.2),
whereas it was not significantly changed after eradication in the
SOCS3 methylation-positive group (32.7±7.0) (Fig. 4B).
Discussion
SOCS3 methylation frequently occurs in
various epithelial and non-epithelial malignancies, including head
and neck squamous cell carcinoma, pancreatic cancer, hepatocellular
carcinoma, multiple myeloma and glioma (27–31).
SOCS3 methylation is also detectable in various
inflammation-associated gastroenterological malignancies, including
hepatocellular carcinoma (39),
Barrett's adenocarcinoma (40) and
ulcerative colitis-associated types of colorectal cancer (41), suggesting the involvement of
SOCS3 methylation in different types of inflammatory gastric
cancer. In the present study, the status of SOCS3
methylation in the non-NGM, where GC arises, was investigated, and
it was revealed that SOCS3 methylation was detectable in
patients with early GC and in patients with non-GC gastritis. It is
still unclear whether SOCS3 methylation is specific for
H. pylori-related gastritis however, it is worth noting that
the patients with gastritis with positive SOCS3 methylation
had also been infected with H. pylori. The occurrence of
SOCS3 methylation in non-NGM of patients with early GC was
significantly higher compared with that in patients without GC
(P=0.020). However, as the group without GC included H.
pylori positive- and negative-patients, H.
pylori-positive patients without early GC and early GC patients
were further compared. As a result, the occurrence of SOCS3
methylation in non-NGM was still higher in patients with early GC
compared with patients without early GC (Table III), suggesting that SOCS3
methylation may occur in the development of H.
pylori-induced gastritis-carcinoma. However, as a limitation of
the present study, the number of patients with H.
pylori-infected gastritis was small. Therefore, the
aforementioned hypothesis requires further investigation. In
addition, it is well-known that the frequency of methylation
increases with age (42,43) and the grade of atrophy and age was
greater in patients with early GC compared with those without
(Table I). Thus, the aging factor
and its associated gastric atrophy may affect the frequency of
SOCS3 methylation when comparing the patients with early GC
and those without. However, when the patients with early GC where
analyzed alone, the occurrence of SOCS3 methylation in
non-NGM was not affected by age or gastric atrophy. This may
suggest that SOCS3 methylation in the non-NGM may not always
occur in older patients with high-grade gastric atrophy.
SOCS3 is a negative regulator of JAK/STAT signaling
and may act as a tumor suppressor (25,26).
Thus, dysfunction of SOCS3 resulting from methylation could lead to
continuous activation of STAT3 signaling and SOCS3
methylation has been reported to be associated with activation of
STAT3 phosphorylation in some types of carcinogenesis, such as
pancreatic cancer, ulcerative colitis-associated cancer and
cholangiocarcinoma (31,41,44). In
the present study, the association between SOCS3 methylation
status and p-STAT3 expression levels were investigated in
non-neoplastic epithelial cells in the gastric mucosa of patients
with early GC. It was revealed that p-STAT3 expression was higher
in patients positive for SOCS3 methylation. Activated STAT3
serves a role in cell proliferation in carcinogenesis (45,46) and
therefore the association between SOCS3 methylation status
and Ki67 expression levels were also investigated. The results from
the present study revealed that Ki67 expression levels were
enhanced in patients with early GC positive for SOCS3
methylation, consistent with a previous study which revealed that
enhanced Ki67 expression was associated with the suppression of
SOCS3 expression levels in hepatocellular carcinoma (47). Moreover, the expression levels of
p-STAT3 and Ki67 showed a positive association in the non-NGM of
patients with early GC in the present study, similar to a previous
report in which p-STAT3 expression and Ki67 expression levels were
associated in glioblastomas (48).
The findings of the present study suggest that the activated STAT3
signaling associated with SOCS3 methylation may accelerate
the proliferative ability of gastric epithelial cells in
individuals at risk of developing GC lesions. It is of concern that
the expression levels of p-STAT3 and Ki67 were compared
irrespective of H. pylori status using a serum anti-H.
pylori test, especially as anti-H. pylori-IgG expression
levels are often negative in patients with severe atrophic stomach
mucosa and/or widely spread intestinal metaplasia (49,50).
Indeed, in regardless of eradication, 19/51 patients with early GC
were negative for anti-H. pylori-IgG level in the present
study and 74% of such patients showed an open-type gastric atrophy.
It was a limitation in the present study that H. pylori
status was determined using anti-H. pylori-IgG expression
levels. However, it is notable that SOCS3 methylation often
occurs in patients with open- and closed-type gastric atrophy,
suggesting that SOCS3 methylation may occur in an early
phase of progression of gastric atrophy.
The effect of H. pylori eradication on
p-STAT3 and Ki67 expression levels in the non-NGM of patients with
early GC after ESD treatment were subsequently investigated.
p-STAT3 expression levels were significantly reduced following
eradication therapy in patients with early GC with negative
SOCS3 methylation, whereas no such effect was evident in
patients with early GC with positive SOCS3 methylation.
Similarly, eradication therapy significantly reduced the expression
levels of Ki67 in patients with early GC with negative SOCS3
methylation, but not significantly different in those with
SOCS3 methylation. It has been suggested that genetic
abnormalities, such as microsatellite instability or methylations
(51,52), that accumulate in the gastric mucosa
during H. pylori-induced chronic gastritis are difficult to
reverse using eradication therapy (52) and that GCs often occur in patients
after successful H. pylori eradication (53). In the present study, early GC
developed in 6 patients after eradication and SOCS3
methylation was detected in 3 of these patients. It was also
demonstrated that eradication had no effect on p-STAT3 and Ki67
expression levels in the non-NGM of patients with early GC with
positive SOCS3 methylation. The aforementioned findings from
the present study and previous research suggest that the non-NGM
retains a high propensity for cell proliferation in patients with
early GC with positive SOCS3 methylation. However, it is a
limitation of the present study that the number of patients
followed-up after eradication was small to divide the patients
according to SOCS3 methylation status. Thus, to verify the
results in the present study, large scale studies, with a large
number of patients during follow-up after eradication, will be
required.
In summary, it has been demonstrated that
SOCS3 methylation frequently occurs in the non-NGM of
patients with early GC. Moreover, it was shown that H. pylori
eradication does not affect p-STAT3 or Ki67 expression levels in
the non-NGM of patients with early GC with positive
SOCS3 methylation. The results from the preset study suggest
that SOCS3 methylation is associated with continuous p-STAT3
overexpression and enhancement of epithelial cell proliferation in
the non-NGM of patients with early GC, serving a role in the
development of GC. However, the present study had several
limitations including the lack of quantitative evaluation of
SOCS3 methylation and the suitability of sampling of biopsy
specimen. For instance, if biopsy specimens had been collected near
the cancerous lesions, the detection rate of SOCS3
methylation might be increased. In addition, quantitative
evaluation of SOCS3 methylation might clarify more
significant correlations among patients' characteristics, p-STAT3
and Ki67 expression levels in patients with early GC. Further
studies are required to investigate whether SOCS3
methylation could be a predictive marker for the development of
first and/or metachronous GC in a future large-scale studies.
Acknowledgements
The authors would like to thank Miss Chiyomi Ito and
Miss Mayumi Yamada (Hyogo College of Medicine) for their technical
assistance.
Funding
This study was supported in part by Grants-In-Aid
for Scientific Research (grant no. 17K0936) from the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HF, XZ and JW made substantial contributions to the
conception and design of the study. HF, XZ, JW, YR, TT, TO, SH and
HM contributed to the acquisition, analysis and interpretation of
data. HF, JW and HM were involved in drafting the manuscript and
revising it carefully for important intellectual content. All
authors have participated sufficiently in the work to take public
responsibility of appropriate portions of the content. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed involving human
participants were in accordance with the approval by The Ethics
Committee of Hyogo College of Medicine. Written informed consent
was provided by all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
SOCS3
|
suppressor of cytokine signaling 3
|
|
non-NGM
|
non-neoplastic gastric mucosa
|
|
GC
|
gastric cancer
|
References
|
1
|
Coussen LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walczak H: TNF and ubiquitin at the
crossroad of gene activation, cell death, inflammation, and cancer.
Immunol Rev. 244:9–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Merga YJ, O'Hara A, Burkitt MD, Duckworth
CA, Probert CS, Campbell BJ and Pritchard DM: Importance of the
alternative NF-κB activation pathway in inflammation-associated
gastrointestinal carcinogenesis. Am J Physiol Gastrointest Liver
Physiol. 310:G1081–G1090. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correa P: Helicobacter pylori and
gastric carcinogenesis. Am J Surg Pathol. 19 (Suppl 1):S37–S43.
1995.PubMed/NCBI
|
|
5
|
Danesh J: Helicobacter pylori
infection and gastric cancer: Systematic review of the
epidemiological studies. Aliment Pharmacol Ther. 13:851–856. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsujimoto H, Ono S, Ichikura T, Matsumoto
Y, Yamamoto J and Hase K: Roles of inflammatory cytokines in the
progression of gastric cancer: Friends or foes? Gastric Cancer.
13:212–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bockerstett KA and DiPaolo RJ: Regulation
of gastric carcinogenesis by inflammatory cytokines. Cell Mol
Gastroenterol Hepatol. 4:47–53. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B and Bromberg
JF: Mutations in the EGFR kinase domain mediate STAT3 activation
via IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rebouissou S, Amessou M, Couchy G, Poussin
K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P and
Zucman-Rossi J: Frequent in-frame somatic deletions activate gp130
in inflammatory hepatocellular tumours. Nature. 457:200–204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuda A, Wang SC, Morris JP IV, Folias
AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ and
Hebrok M: Stat3 and MMP7 contribute to pancreatic ductal
adenocarcinoma initiation and progression. Cancer Cell. 19:441–455.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Judd LM, Alderman BM, Howlett M, Shulkes
A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M and Giraud AS:
Gastric cancer development in mice lacking the SHP2 binding site on
the IL-6 family co-receptor gp130. Gastroenterology. 126:196–207.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Judd LM, Bredin K, Kalantzis A, Jenkins
BJ, Ernst M and Giraud AS: STAT3 activation regulates growth,
inflammation, and vascularization in a mouse model of gastric
tumorigenesis. Gastroenterology. 131:1073–1085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu P, Wu D, Zhao L, Huang L, Shen G, Huang
J and Chai Y: Prognostic role of STAT3 in solid tumors: A
systematic review and meta-analysis. Oncotarget. 7:19863–19883.
2016.PubMed/NCBI
|
|
16
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
18
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: Clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. Biomed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu CC, Woods AL and Levison DA: The
assessment of cellular proliferation by immunohistochemistry: A
review of currently available methods and their applications.
Histochem J. 24:121–131. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leung WK and Sung JJ: Intestinal
metaplasia and gastric carcinogenesis. Aliment Pharmacol Ther.
16:1209–1216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ushijima T and Sasako M: Focus on gastric
cancer. Cancer Cell. 5:121–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitsuyama K, Sata M and Rose-John S:
Interleukin-6 trans-signaling in inflammatory bowel disease.
Cytokine Growth Factor Rev. 17:451–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Y, Zhao H, Wang P, Wang J and Zou L:
The roles of SOCS3 and STAT3 in bacterial infection and
inflammatory diseases. Scand J Immunol. 88:e127272018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: New surprises in the Jak/Stat pathway.
Cell. 109 (Suppl):S121–S131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kubo M, Hanada T and Yoshimura A:
Suppressors of cytokine signaling and immunity. Nat Immunol.
4:1169–1176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weber A, Hengge UR, Bardenheuer W,
Tischoff I, Sommerer F, Markwarth A, Dietz A, Wittekind C and
Tannapfel A: SOCS-3 is frequently methylated in head and neck
squamous cell carcinoma and its precursor lesions and causes growth
inhibition. Oncogene. 24:6699–6708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang L, Hu B, Ni J, Wu J, Jiang W, Chen
C, Yang L, Zeng Y, Wan R, Hu G, et al: Transcriptional repression
of SOCS3 mediated by IL-6/STAT3 signaling via DNMT1 promotes
pancreatic cancer growth and metastasis. J Exp Clin Cancer Res.
35:272016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang BG, Wang N, Huang J, Yang Y, Sun LL,
Pan ZY and Zhou WP: Tumor SOCS3 methylation status predicts the
treatment response to TACE and prognosis in HCC patients.
Oncotarget. 8:28621–28627. 2017.PubMed/NCBI
|
|
30
|
Galm O, Yoshikawa H, Esteller M, Osieka R
and Herman JG: SOCS-1, a negative regulator of cytokine signaling,
is frequently silenced by methylation in multiple myeloma. Blood.
101:2784–2788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lindemann C, Hackmann O, Delic S, Schmidt
N, Reifenberger G and Riemenschneider MJ: SOCS3 promoter
methylation is mutually exclusive to EGFR amplification in gliomas
and promotes glioma cell invasion through STAT3 and FAK activation.
Acta Neuropathol. 122:241–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969. View Article : Google Scholar
|
|
33
|
Kitahara F, Kobayashi K, Sato T, Kojima Y,
Araki T and Fujino MA: Accuracy of screening for gastric cancer
using serum pepsinogen concentration. Gut. 44:693–697. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karibe T, Fukui H, Sekikawa A, Shiratori K
and Fujimori T: EXTL3 promoter methylation down-regulates EXTL3 and
heparan sulphate expression in mucinous colorectal cancers. J
Pathol. 216:32–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herman JG, Graff JR, Myohanen S, Nelkin BD
and Baylin SB: Methylation-specific PCR: A novel PCR assay for
methylation status of CpG islands. Proc Natl Acad Sci USA.
93:9821–9826. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He B, You L, Uematsu K, Zang K, Xu Z, Lee
AY, Costello JF, McCormick F and Jablons DM: SOCS-3 is frequently
silenced by hypermethylation and suppresses cell growth in human
lung cancer. Proc Natl Acad Sci USA. 100:14133–14138. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sekikawa A, Fukui H, Fujii S, Ichikawa K,
Tomita S, Imura J, Chiba T and Fujimori T: REG Ialpha protein
mediates an anti-apoptotic effect of STAT3 signaling in gastric
cancer cells. Carcinogenesis. 29:76–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fukui H, Sekikawa A, Tanaka H, Fujimori Y,
Katake Y, Fujii S, Ichikawa K, Tomita S, Imura J, Chiba T and
Fujimori T: DMBT1 is a novel gene induced by IL-22 in ulcerative
colitis. Inflamm Bowel Dis. 17:1177–1188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ogata H, Kobayashi T, Chinen T, Takaki H,
Sanada T, Minoda Y, Koga K, Takaesu G, Maehara Y, Iida M and
Yoshimura A: Deletion of the SOCS3 gene in liver parenchymal cells
promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology.
131:179–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tischoff I, Hengge UR, Vieth M, Ell C,
Stolte M, Weber A, Schmidt WE and Tannapfel A: Methylation of
SOCS-3 and SOCS-1 in the carcinogenesis of Barrett's
adenocarcinoma. Gut. 56:1047–1053. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, de Haar C, Chen M, Deuring J,
Gerrits MM, Smits R, Xia B, Kuipers EJ and van der Woude CJ:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Issa JP: CpG-island methylation in aging
and cancer. Curr Top Microbiol Immunol. 249:101–118.
2000.PubMed/NCBI
|
|
43
|
Ahuja N, Li Q, Mohan AL, Baylin SB and
Issa JP: Aging and DNA methylation in colorectal mucosa and cancer.
Cancer Res. 58:5489–5494. 1998.PubMed/NCBI
|
|
44
|
Ishimoto H: Epigenetic alterations in
cholangiocarcinoma-sustained IL-6/STAT3 signaling in
cholangio-carcinoma due to SOCS3 epigenetic silencing. Digestion.
79 (Suppl 1):S2–S8. 2009. View Article : Google Scholar
|
|
45
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rahaman SO, Harbor PC, Chernova O, Barnett
GH, Vogelbaum MA and Haque SJ: Inhibition of constitutively
activate Stat3 suppresses proliferation and induces apoptosis in
glioblastoma multiforme cells. Oncogene. 21:8404–8413. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu WY, Li J, Wu ZS, Zhang CL, Meng XL and
Lobie PE: Prognostic significance of phosphorylated signal
transducer and activator of transcription 3 and suppressor of
cytokine signaling 3 expression in hepatocellular carcinoma. Exp
Ther Med. 2:647–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chiba R, Akiya M, Hashimura M, Oguri Y,
Inukai M, Hara A and Saegusa M: ALK signaling cascade confers
multiple advantages to glioblastoma cells through
neovascularization and cell proliferation. PLoS One.
12:e01835162017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yoshida T, Kato J, Inoue I, Yoshimura N,
Deguchi H, Mukoubayashi C, Oka M, Watanabe M, Enomoto S, Niwa T, et
al: Cancer development based on chronic active gastritis and
resulting gastric atrophy as assessed by serum levels of pepsinogen
and Helicobacter pylori antibody titer. Int J Cancer.
134:1445–1457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adachi K, Mishiro T, Tanaka S and
Kinoshita Y: Analysis of negative result in serum anti-H.
pylori IgG antibody test in cases with gastric mucosal atrophy.
J Clin Biochem Nutr. 59:145–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Enomoto S, Maekita T, Ohata H, Yanaoka K,
Oka M and Ichinose M: Novel risk markers for gastric cancer
screening: Present status and future prospects. World J
Gastrointest Endosc. 2:381–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Watari J, Chen N, Amenta PS, Fukui H,
Oshima T, Tomita T, Miwa H, Lim KJ and Das KM: Helicobacter
pylori associated chronic gastritis, clinical syndromes,
precancerous lesions, and pathogenesis of gastric cancer
development. World J Gastroenterol. 20:5461–5473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kato M, Nishida T, Yamamoto K, Hayashi S,
Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, et
al: Scheduled endoscopic surveillance controls secondary cancer
after curative endoscopic resection for early gastric cancer: A
multicentre retrospective cohort study by Osaka University ESD
study group. Gut. 62:1425–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|