Introduction
Colorectal cancer is the 3rd most common and fatal
type of cancer worldwide (1); ~1.4
million cases are diagnosed annually, and mortality occurs in half
these cases each year, globally (2).
Chemotherapeutic treatment is considered the primary strategy for
colorectal cancer; however, chemoresistance frequently develops in
response to first-line chemotherapeutic agents, including
5-fluorouracil (5-FU) and doxorubicin (DOX), which is a major
barrier for achieving effective therapy (3). Despite numerous studies examining the
potential mechanism underlying the induction of chemoresistance,
including investigations into activated NF-κB (4), induction of the transcription factor
activator protein 1 (5) and
activation of multiple drug resistance protein 1 signaling
(6), the mechanisms remain largely
unknown.
In different cancer types, including colon cancer, a
subpopulation of cancer stem-like cells (CSCs) has been identified
(7,8). This subpopulation is characterized by
cells presenting with stem-like properties, including self-renewal
and differentiation, resulting in the production of tumor cells
that have a long-term ability for tumor renewal (9,10). It
has also been revealed that CSCs may contribute to malignant
features, such as tumorigenesis, progression, maintenance and
recurrence in several types of cancer (11–13).
Seymour et al (14) reported
that in glioblastoma, the existence of glioma stem-like cells led
to recurrence and metastasis via upregulation of the pluripotency
gene, Sox2. Moreover, CSCs have been successfully enriched from
several cancer cell lines using novel medium (15,16) and
being identified to increase resistance of cells to several
anticancer drugs (17). In
pancreatic cancer-derived CSCs, it has been revealed that enriched
cells presented with increased chemoresistance via regulation of
epithelial-mesenchymal transition (EMT) and increased surveillance
(18).
Growth-arrest-specific transcript 5 (GAS5), which
was originally isolated from mouse NIH3T3 cells and is found on
chromosome 1q25 (19), is one of the
most common long non-coding RNAs (lncRNAs). A previous study
demonstrated that GAS5 may act as a key regulator of tumor
proliferation, migration and EMT in cancer cells (20). Furthermore, it has been reported that
in cervical cancer, GAS5 may act as a tumor suppressor by sponging
microRNA (miR)-21, which induced regulation of cisplatin resistance
(21). In pancreatic cancer, GAS5
reversed EMT and induced gemcitabine sensitivity by targeting the
miR-221/suppressor of cytokine signaling 3 pathway (22). It has also been reported that in
clear cell renal cell carcinoma, GAS5 functioned as a competitive
endogenous RNA to regulate solute carrier family 39 member 1
(SLC39A1, also termed hZIP1) expression via sponging miR-223, which
resulted in chemosensitivity to cisplatin (23). Furthermore, lncRNAs are reported to
be involved in the regulation of physiological processes in CSCs,
including liver (24,25) and pancreatic CSCs (24,25).
However, the potential role of GAS5 in CSCs derived from colorectal
cancer is not fully understood.
In the present study, CSCs were successfully
enriched from HCT116 cells, and their potential to maintain
self-renewal capacity and to regulate the malignant features of
CSCs (including proliferation, tumor formation, migration and
chemoresistance) were identified. It was revealed that GAS5 exerted
its protective roles via nodal growth differentiation factor
(NODAL) signaling, resulting in the maintenance of CSC stemness and
induction of chemoresistance. Moreover, it was demonstrated that
GAS5 exerted opposing effects on CSCs derived from HCT116 cells
compared with parental HCT116 cells. Collectively, the present
study identified a novel role of GAS5 in regulating physiological
processes in CSCs, which suggests that GAS5 may be a potential
therapeutic target for colorectal cancer.
Materials and methods
Cell culture and enrichment of CSCs
from HCT116 cells
Human colorectal cancer HCT116 cells were purchased
from the American Type Culture Collection and stored in liquid
nitrogen. Cells were cultured in DMEM (Thermo Fisher Scientific,
Inc.), supplemented with 10% heat-inactivated FBS (Gibco; Thermo
Fisher Scientific, Inc.) in a 5% CO2 incubator at 37°C.
The medium was refreshed every 2 days, and when the confluence
reached 80–90%, cells were harvested with 0.25% trypsin (Gibco;
Thermo Fisher Scientific, Inc.) and passaged.
To enrich CSCs, HCT116 cells were maintained in
DMEM/Ham Nutrient Mixture F-12 (1:1) supplemented with epidermal
growth factor (EGF; 20 ng/ml), human fibroblast growth factor basic
(hFGFb; 10 ng/ml) and 2% B27. All reagents were bought from Thermo
Fisher Scientific, Inc. Every 3 days the medium was half
replaced.
Serial replating assay
To detect self-renewal capacity, cells were replated
at a clonal density of 1,000 cells/well in 6-well plate and
cultured in DMEM/Ham Nutrient Mixture F-12 (1:1) supplemented with
2% B27, 10 ng/ml EGF and 20 ng/ml hFGFb. Every 2 days, the medium
was half replaced, and after 14 days, spheres >40 µm in diameter
were counted under a X71 (U-RFL-T) fluorescence microscope (Olympus
Corporation; magnification, ×40). This process was repeated three
times.
Western blotting
Cells were suspended in lysis buffer containing 50
mM Tris-HCl, 150 mM NaCl, 0.02% NaN3, 100 µg/ml PMSF, 1
µg/ml aprotinin, 1 µg/ml pepstatin A and 1% Triton X-100. A
SoniConvert® homogenizer (DocSense Biotech) was used to
lyse the cells. After centrifugation at 12,000 × g for 10 min at
4°C, 20 µg total protein quantitatively measured using the
bicinchoninic acid kit (Sigma-Aldrich; Merck KGaA) was resolved
using SDS-PAGE on 10% gels, and was then transferred to a
nitrocellulose membrane. After transferring, the membrane was
blocked with 5% BSA (Beyotime Institute of Biotechnology) in PBS
for 30 min at room temperature. The primary antibodies used were as
follows: Rabbit monoclonal anti-Oct4 antibody (1:2,000; cat. no.
ab181557), rabbit monoclonal anti-Sox2 antibody (1:2,000; cat. no.
ab93689) and rabbit monoclonal anti-β-actin antibody (1:5,000; cat.
no. ab179467). In addition, goat anti-rabbit IgG H&L antibody
(horseradish peroxidase labeled; 1:10,000; cat. no. ab7090) was
used as the secondary antibody. All antibodies were purchased from
Abcam and incubated with the membrane at room temperature for 1 h,
followed by three washes with PBS supplemented with 0.1% Tween-20.
Blot bands were semi-quantified via densitometry with ImageJ
software (version 1.52r; National Institutes of Health). β-actin
was used as an internal reference. The signal was detected with ECL
plus western blotting detection reagents (Pierce; Thermo Fisher
Scientific, Inc.) and imaged using X-ray film.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Subsequently, 1 µg total RNA underwent RT using a RT
kit (Thermo Fisher Scientific, Inc.) at 42°C for 1 h. After RT,
cDNA was used as a template for qPCR by using SYBR™
Green PCR master mix (Thermo Fisher Scientific, Inc.), which was
conducted under the following conditions: 95°C for 5 min; 35 cycles
of 95°C for 10 sec; and a final extension at 60°C for 1 min. An ABI
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
was used for PCR. The primers used for qPCR were as follows: GAS5,
forward 5′-CGACTCCTGTGAGGTATGGTG-3′ and reverse
5′-ATCCTTCCTTGGGGACACAAC-3′; and β-actin, forward
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse
5′-CTCCTTAATGTCACGCACGAT-3′. The qPCR results were analyzed and
expressed relative to the threshold cycle (Cq) values, and were
then converted to fold changes; all data was analyzed using
2−ΔΔCq method. A 2.0-fold change was considered to be
significant (26). In total, three
repeats were conducted for each sample.
Transfection with small interfering
(si)RNA or GAS5 coding sequence
siRNA was used for knocking down the expression of
GAS5 in CSCs derived from HCT116 cells. In total, two 21-nucleotide
siRNAs were used for silencing: siGAS5-1,
5′-GCAAGCCTAACTCAAGCCA-3′; siGAS5-2, 5′-GGACCAGCTTAATGGTTCT-3′.
si-negative control (siNC): CCTGAGACCAAGCCATAAC was employed as a
NC. Briefly, siRNA (50 nmol) was chemically synthesized by
Guangzhou RiboBio Co., Ltd. and transfected into ~1×106
cells with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the siRNA manufacturer's
protocol (Guangzhou RiboBio Co., Ltd.). After 48 h, cells were used
for subsequent experimentation.
The coding sequence of GAS5 was amplified from CSCs
cDNA using the followed primers: Forward,
5′-ATAGGGCTAGCTTTCGAGGTAGGAGTCGACT-3′ and reverse,
5′-ATAGGCGGCCGCGGATTGCAAAAATTTATTAA-3′. The PCR product was
amplified using PlatinumTM Green Hot Start PCR Master mix (2X;
Thermo Fisher Scientific, Inc.) under the following conditions:
95°C for 5 min; 35 cycles of 95°C for 10 sec, extension at 60°C for
3 min. The PCR product and pEGFP-N3 plasmid (Beijing Solarbio
Science & Technology Co., Ltd.) were digested using NheI
and NotI restriction enzymes (Takara Bio, Inc.).
Subsequently, the digested PCR product was inserted into the
pEGFP-N3 plasmid using the Quick Ligation kit (cat. no. M2200S; New
England Biolabs, Inc.) and successful insertion was confirmed by
sequencing which was provided by Sangon Biotech Co., Ltd. In total,
1.6 µg plasmid was transfected into ~1×106 cells with
Lipofectamine® 2000, according to the manufacturer's
protocol. Also, an empty vector was transfected into cells, and
this was considered as the vector group. After 48 h, cells were
used for further analysis.
Cell Counting Kit-8 (CCK-8) assay for
cell viability
Cells (2×105/well) were seeded into
96-well plates. 0.1, 1, 3, 5 and 10 µg/ml of 5-FU, or 0.1, 0.3,
0.5, 0.7 and 1.0 µg/ml DOX was added into medium for 24-h
culturing. Next, 10 µl CCK-8 solution (Beyotime Institute of
Biotechnology) was added to the cell culture for a 2 h
co-incubation at 37°C. Absorbance was measured at 450 nm and cell
viability was expressed as optical density.
Cells (2×105/well) were seeded into
96-well plates. After 1, 2, 3 or 4-day culturing with or without
SB431542, 10 µl CCK-8 solution (Beyotime Institute of
Biotechnology) was added to the cell culture for a 2 h
co-incubation at 37°C. The mock group was treated with the same
volume of DMSO. Absorbance was measured at 450 nm and cell
viability was expressed as optical density. Each assay was repeated
three times.
Cell cycle analysis
The percentage of cells in each cell cycle phase was
analyzed by propidium iodide (PI) staining and flow cytometry.
Cells were washed three times with ice-cold PBS and a final
concentration of 75% ethanol was added for fixation at 4°C for 4 h.
Subsequently, 100 µg/ml Rnase (Beyotime Institute of Biotechnology)
was added to each sample and incubated at 37°C for 30 min, and
cells were stained with 50 µg/ml PI for 10 min at room temperature
and analyzed on a FACS LSRII flow cytometer (BD Biosciences). Then
data were analyzed using FlowJo software (FlowJo LLC, version
9.7.4)
Tumor formation in soft agar
Cells (2×103) were suspended in
serum-free DMEM/F-12 medium and dissolved in 0.3% low-melting
agarose (Sigma-Aldrich; Merck KGaA) supplemented with 20 ng/ml EGF,
10 ng/ml hFGFb, 2% B27 and 1% antibiotic-antimycotic mixture. Cells
were allowed to grow for 14 days and colonies with >50 cells
were counted under a X71 (U-RFL-T) fluorescence microscope (Olympus
Corporation; magnification, ×40).
Transwell assay
To analyze migratory ability, 2×103 cells
were plated in the upper chambers of semi-permeable Transwell
inserts filled with 200 µl of SFM and 600 µl DMEM/Ham Nutrient
Mixture F-12 (1:1), supplemented with 20 ng/ml EGF, 10 ng/ml hFGFb,
2% B27 and 1% antibiotic-antimycotic mixture was added to the lower
chambers. After incubation at 37°C for 24 h, cells were fixed using
4% paraformaldehyde at room temperature for 10 min, and stained
with 0.5% crystal violet at room temperature for 10 min. After 2–3
washes with ice-cold PBS, images were captured under a X71
(U-RFL-T) fluorescence microscope (Olympus Corporation;
magnification, ×40).
Annexin V-FITC/PI double staining
Annexin V-FITC/PI double staining was performed with
the Annexin V-FITC/PI apoptosis detection kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Cells (1×106) were suspended from 6-well plate and
washed with ice-cold PBS. Then cells were co-incubated with 5 µg/ml
5-FU, 0.7 µg/ml DOX or 10 µM of SB431542 for 24 h. For staining,
cells were resuspended in binding buffer containing 5 µl Annexin
V-FITC for 10 min in the dark at room temperature, and then 5 µl PI
was added for 5 min staining at room temperature. Then, 400 µl
binding buffer was added to each sample and flow cytometric
analysis was performed on a FACS LSRII flow cytometer (BD
Biosciences). Data were analyzed using FlowJo software (version
10.6.1; FlowJo LLC).
Statistical analysis
Data are presented as the mean ± SD, and all
experiments were repeated three times independently. One-way ANOVA
followed by Bonferroni's multiple comparison tests was used for
comparisons among experimental groups, and was conducted using
GraphPad Prism 5 software (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Identifying and enriching CSCs from
HCT116 cells and detection of GAS5
For CSC enrichment, parental HCT116 cells were
cultured in serum-free medium supplemented with EGF, hFGFb and B27,
as described previously (27). Cells
were passaged every 10 days, and it was observed that spheres
formed in every passage (Fig. 1A).
When counting spheres >40 µm in diameter, no significant
decrease in sphere formation ability was observed at the different
passages; these data indicate the existence of a self-renewal
capacity (Fig. 1B), which is a
characteristic of CSCs (28).
To further characterize the stemness of enriched
cells, quantitative analyses were performed on Oct4 and Sox2, two
stem cell factors that are important for the self-renewal
properties of CSCs (29). An
increase in the protein expression levels of Oct4 and Sox2 was
identified in three passages of enriched cells compared with
parental HCT116 cells (Fig. 1C).
Subsequently, all three passages of CSCs were used to assess GAS5
mRNA expression; it was revealed that GAS5 was significantly
upregulated in CSCs compared with parental HCT116 cells, regardless
of the passage number (Fig. 1D).
GAS5 is essential for maintaining
stemness in CSCs derived from HCT116 cells
Xu et al (30)
reported that in human embryonic stem cells, the presence of GAS5
was critical for controlling self-renewal capacity; therefore, the
present study assessed whether GAS5 was involved in regulating
stemness in CSCs derived from HCT116 cells. The efficiency of GAS5
knockdown using GAS5-targeting siRNA at different sites (siGAS5-1
and siGAS5-2; Fig. 2A) was assessed
by transfection at the early passage. Western blotting demonstrated
that knockdown of GAS5 did not affect Oct4 and Sox2 protein
expression levels, which indicated that GAS5 may not affect the
stemness of CSCs (Fig. 2B). However,
the serial replating assay suggested that knockdown of GAS5
abolished sphere formation ability (Fig.
2C). Therefore, these two controversial results indicate that
GAS5 may affect self-renewal capacity by regulating other
physiological processes, such as proliferation, but not stem cell
factors. Thus, cell viability was detected from day 1–4 after GASG5
knockdown; it was identified that proliferative capacity was
significantly decreased by GAS5 knockdown (Fig. 2D). The cell phase distribution had a
consistent tendency, which demonstrated that knockdown of GAS5
significantly increased the proportion of cells in
G1/G0 phase and decrease the proportion of
cells phase in S and G2/M, thus indicating that the cell
cycle was arrested at this point. Collectively, these results
suggest that knockdown of GAS5 primarily decreases cellular
proliferation, which as a result inhibits sphere formation in
CSCs.
The effects of GAS5 on other malignant features were
also detected, including tumor formation in soft agar and cell
migration. It was revealed that knockdown of GAS5 inhibited these
two malignant features (Fig. 2F and
G), which suggested its important role in regulating
physiological processes in CSCs.
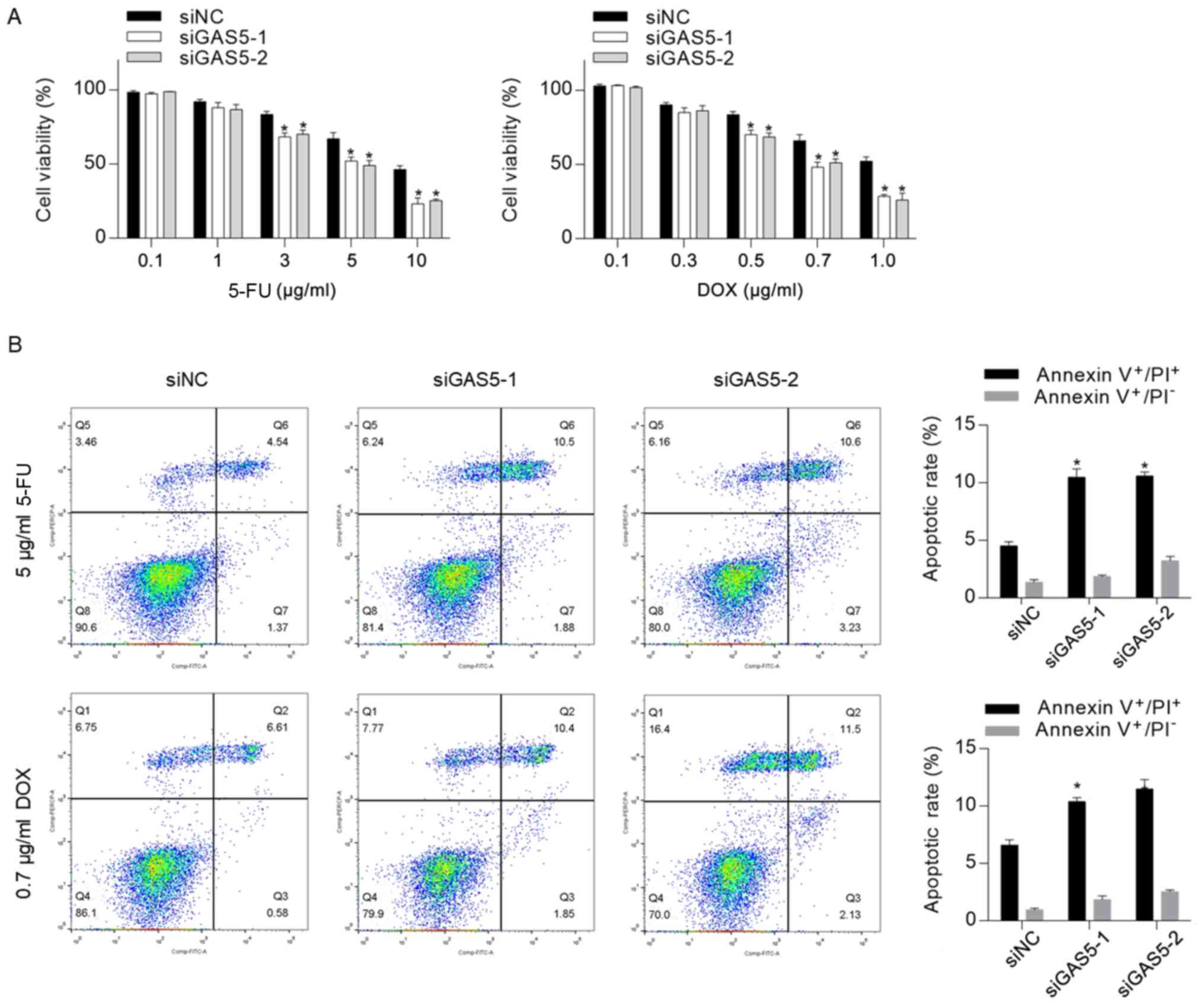
GAS5 may desensitize CSCs to
chemotherapeutic agents
Previous studies have revealed that GAS5, which acts
as a tumor suppressor, increased chemosensitivity in several types
of cancer, including cervical cancer (21), ovarian cancer (31), pancreatic cancer (22) and clear cell renal cell carcinoma
(23). To assess whether knockdown
of GAS5 exerted a regulatory role in CSC chemosensitivity, the
cytotoxicity of 5-FU and DOX to CSCs was measured, and it was
identified that knockdown of GAS5 significantly sensitized CSCs to
5-FU and DOX (Fig. 3A). Moreover, 5
µg/ml 5-FU or 0.7 µg/ml DOX were supplemented into medium for 24-h
treatment followed by apoptosis analysis. In line with the
cytotoxicity results, it was revealed that GAS5 knockdown
significantly increased chemotherapeutic treatment-induced
apoptosis (Fig. 3B).
GAS5 exerts regulatory roles via
regulating NODAL
NODAL signaling has been reported to be regulated by
GAS5, helping to maintain stemness (30). Therefore, the present study aimed to
identify whether NODAL was involved in GAS5-mediated regulation of
CSCs. The serial replating assay demonstrated that GAS5 knockdown
and pretreatment with SB431542 significantly decreased self-renewal
capacity in CSCs, thus indicating that both of these are important
for maintaining stemness (Fig. 4A).
The cell viability assay also suggested that GAS5 knockdown and
inhibition of NODAL signaling via SB431542 decreased cell
proliferation (Fig. 4B). To further
assess whether NODAL signaling contributed to GAS5
knockdown-induced chemosensitivity, the apoptotic rate after 5-FU
treatment was measured, and it was revealed that inhibition of
NODAL signaling sensitized CSCs to chemotherapeutic treatment
(Fig. 4C). However, this finding was
inconsistent with those from previous studies, which reported the
anti-tumor effects of GAS5 (21–23,31). To
assess whether GAS5 exerts different effects on HCT116 cells, GAS5
was efficiently overexpressed in HCT116 cells (Fig. 4D) and apoptotic cell death was
detected (Fig. 4E). Flow cytometry
results indicated that, in HCT116 cells, overexpression of GAS5
sensitized cells to chemotherapy, thus suggesting that GAS5
potentially exerts opposite effects on chemoresistance in HCT116
cells and CSCs derived from HCT116.
Discussion
The present study detected the expression of GAS5 in
CSCs derived from a human colorectal cancer cell line, and
investigated its roles in regulating malignant features and
maintaining the stemness of CSCs. While GAS5 has been reported to
act as a tumor suppressor in several types of cancer (19–21), the
present results suggested that GAS5 may be essential for
maintaining stemness, and that it exerted critical roles in
promoting the malignant features of CSCs, including proliferation,
tumor formation, migration and chemoresistance.
By culturing HCT116 cells in serum-free medium
supplemented with EGF, hFGFb and B27, CSCs presented a stem cell
morphological phenotype and exhibited high expression levels of
stem cell factors, including Oct4 and Sox2; the cells were enriched
and identified by assessing their self-renewal capacity. Moreover,
future studies aim to isolate CSCs from colorectal tumor tissue and
investigate whether the potential role of GAS5 found in the HCT116
cell line also exists in primary cells. In the present study,
compared with parental HCT116 cells, the expression of GAS5 was
significantly higher in CSCs in different passages, thus indicating
its potential role in maintaining stemness. Considering the
relatively high expression of GAS5, its overexpression may have an
effect on the malignant features and stemness of CSCs. In the
present study, GAS5 expression was knocked down in CSCs, in order
to investigate its potential effects. While GAS5 knockdown
inhibited sphere formation of CSCs, the stem cell factors Oct4 and
Sox2 were not significantly affected, which suggested that GAS5
knockdown may affect sphere formation by modifying pluripotency.
Cellular proliferation and cell cycle distribution were
subsequently assessed, and it was revealed that GAS5 knockdown
inhibited cell proliferation via cell cycle arrest at the
G1/G0 phase. In addition, the inhibitory
effects of GAS5 knockdown on other malignant features were also
detected, including tumor formation and migration. GAS5 has been
widely reported to act as a tumor suppressor in several types of
cancer cells (19–21), which is the opposite of the effects
of siGAS5 on the malignant features of CSCs observed in the present
study. Although there is no direct evidence of the mechanisms
underlying how GAS5 modifies pluripotency, it is speculated that
modification of pluripotency is the primary mechanism by which GAS5
exerts its regulatory roles in CSCs.
GAS5 has also been studied for its beneficial role
in increasing chemosensitivity in several cancer cell types
(21–23). In the present study, 5-FU and DOX
were used to detect the effects of GAS5 knockdown on
chemoresistance in CSCs; it was revealed that GAS5 knockdown
increased the chemosensitivity and chemotherapeutic agent-induced
apoptosis of CSCs. NODAL signaling has an important role in
regulating the stem-like properties of CSCs (32,33), and
a previous study revealed a critical role for NODAL signaling in
the induction of chemoresistance in CSCs (33). Therefore, the present study examined
whether NODAL signaling was involved in the GAS5-mediated
regulation of CSCs. It was demonstrated that GAS5 knockdown and
NODAL inhibition with SB431542 pretreatment decreased the
self-renewal capacity and chemoresistance of cells, thus indicating
that GAS5 may exert tumor-promoting effects in a NODAL-dependent
manner. However, whilst the present study did not identify the
exact regulatory mechanism between GAS5 and NODAL signaling, the
results indicated that it may be beneficial to investigate the
regulatory mechanism between GAS5 and NODAL signaling in other
cancer cells. Moreover, a limitation of the present study was that
the effects of GAS5 on chemosensitivity were not assessed in
vivo, thus it is important to examine whether GAS5 is relevant
to the regulation of malignant features in vivo.
In conclusion, the present study identified GAS5 as
a factor that may promote colorectal CSC activity in cells derived
from HCT116 cells. The present study investigated the effects of
GAS5 on CSCs vs. parental HCT116 cells, and demonstrated that GAS5
exerted opposing effects on malignant features in CSCs compared
with parental cells. Furthermore, the involvement of NODAL
signaling was indicated to be essential for GAS5-mediated
modification in CSCs, which is a potential key reason for the
opposing effects of GAS5 in CSCs and parental HCT116 cells.
However, further investigation into the interaction of GAS5 with
NODAL signaling is required to understand the exact roles of GAS5
in regulating malignant features in colorectal CSCs.
Acknowledgements
The authors would like to thank Dr Huimin Shi
(Sichuan University) for language editing.
Funding
The present work was supported by Scholar supporting
program, Chongqing Scientific project (grant no. S20180827).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and DX designed and performed all experiments,
data collection and analysis. DX supervised the experiments, and
was involved in writing and revision of the manuscript. Both
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marmol I, Sanchez-de-Diego C, Pradilla DA,
Cerrada E and Rodriguez YM: Colorectal carcinoma: A general
overview and future perspectives in colorectal cancer. Int J Mol
Sci. 18:E1972017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papanastasopoulos P and Stebbing J:
Molecular basis of 5-fluorouracil-related toxicity: Lessons from
clinical practice. Anticancer Res. 34:1531–1535. 2014.PubMed/NCBI
|
|
4
|
Bharti AC and Aggarwal BB: Nuclear
factor-kappa B and cancer: Its role in prevention and therapy.
Biochem Pharmacol. 64:883–888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Chen Y, Li J, Zhang K, Chen J,
Chen D, Feng B, Song H, Feng J, Wang R and Chen L: Notch-1 confers
chemoresistance in lung adenocarcinoma to Taxanes through
AP-1/microRNA-451 mediated regulation of MDR-1. Mol Ther Nucleic
Acids. 5:e3752016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie Y and Zhong DW: AEG-1 is associated
with hypoxia-induced hepatocellular carcinoma chemoresistance via
regulating PI3K/AKT/HIF-1alpha/MDR-1 pathway. EXCLI J. 15:745–757.
2016.PubMed/NCBI
|
|
7
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vander Griend DJ, Karthaus WL, Dalrymple
S, Meeker A, DeMarzo AM and Isaacs JT: The role of CD133 in normal
human prostate stem cells and malignant cancer-initiating cells.
Cancer Res. 68:9703–9711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seymour T, Nowak A and Kakulas F:
Targeting aggressive cancer stem cells in glioblastoma. Front
Oncol. 5:1592015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe Y, Yoshimura K, Yoshikawa K,
Tsunedomi R, Shindo Y, Matsukuma S, Maeda N, Kanekiyo S, Suzuki N,
Kuramasu A, et al: A stem cell medium containing neural stimulating
factor induces a pancreatic cancer stem-like cell-enriched
population. Int J Oncol. 45:1857–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsukuma S, Yoshimura K, Ueno T, Oga A,
Inoue M, Watanabe Y, Kuramasu A, Fuse M, Tsunedomi R, Nagaoka S, et
al: Calreticulin is highly expressed in pancreatic cancer stem-like
cells. Cancer Sci. 107:1599–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hashimoto N, Tsunedomi R, Yoshimura K,
Watanabe Y, Hazama S and Oka M: Cancer stem-like sphere cells
induced from de-differentiated hepatocellular carcinoma-derived
cell lines possess the resistance to anti-cancer drugs. BMC Cancer.
14:7222014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Ma L, Zhang Z, Liu X, Gao H,
Zhuang Y, Yang P, Kornmann M, Tian X and Yang Y: Hedgehog signaling
regulates epithelial-mesenchymal transition in pancreatic cancer
stem-like cells. J Cancer. 7:408–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura Y, Takahashi N, Kakegawa E,
Yoshida K, Ito Y, Kayano H, Niitsu N, Jinnai I and Bessho M: The
GAS5 (growth arrest-specific transcript 5) gene fuses to BCL6 as a
result of t(1;3)(q25;q27) in a patient with B-cell lymphoma. Cancer
Genet Cytogenet. 182:144–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye K, Wang S, Zhang H, Han H, Ma B and Nan
W: Long noncoding RNA GAS5 suppresses cell growth and
epithelial-mesenchymal transition in osteosarcoma by regulating the
miR-221/ARHI pathway. J Cell Biochem. 118:4772–4781. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wen Q, Liu Y, Lyu H, Xu X, Wu Q, Liu N,
Yin Q, Li J and Sheng X: Long Noncoding RNA GAS5, which acts as a
tumor suppressor via microRNA 21, regulates cisplatin resistance
expression in cervical cancer. Int J Gynecol Cancer. 27:1096–1108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu B, Wu S, Ma J, Yan S, Xiao Z, Wan L,
Zhang F, Shang M and Mao A: lncRNA GAS5 reverses EMT and tumor stem
cell-mediated gemcitabine resistance and metastasis by targeting
miR-221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids.
13:472–482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong X, Kong C, Liu X, Bi J, Li Z, Li Z,
Zhu Y and Zhang Z: GAS5 functions as a ceRNA to regulate hZIP1
expression by sponging miR-223 in clear cell renal cell carcinoma.
Am J Cancer Res. 8:1414–1426. 2018.PubMed/NCBI
|
|
24
|
Zhao J, Fu Y, Wu J, Li J, Huang G and Qin
L: The diverse mechanisms of miRNAs and lncRNAs in the maintenance
of liver cancer stem cells. Biomed Res Int. 2018:86860272018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Dong P, Wang W, Huang M and Tian
B: Gemcitabine treatment causes resistance and malignancy of
pancreatic cancer stem-like cells via induction of lncRNA HOTAIR.
Exp Ther Med. 14:4773–4780. 2017.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noh KH, Kim BW, Song KH, Cho H, Lee YH,
Kim JH, Chung JY, Kim JH, Hewitt SM, Seong SY, et al: Nanog
signaling in cancer promotes stem-like phenotype and immune
evasion. J Clin Invest. 122:4077–4093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gkountela S and Aceto N: Stem-like
features of cancer cells on their way to metastasis. Biol Direct.
11:332016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh S, Trevino J, Bora-Singhal N,
Coppola D, Haura E, Altiok S and Chellappan SP: EGFR/Src/Akt
signaling modulates Sox2 expression and self-renewal of stem-like
side-population cells in non-small cell lung cancer. Mol Cancer.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu C, Zhang Y, Wang Q, Xu Z, Jiang J, Gao
Y, Gao M, Kang J, Wu M, Xiong J, et al: Long non-coding RNA GAS5
controls human embryonic stem cell self-renewal by maintaining
NODAL signalling. Nat Commun. 7:132872016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Long X, Song K, Hu H, Tian Q, Wang W, Dong
Q, Yin X and Di W: Long non-coding RNA GAS5 inhibits DDP-resistance
and tumor progression of epithelial ovarian cancer via
GAS5-E2F4-PARP1-MAPK axis. J Exp Clin Cancer Res. 38:3452019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu LL, Chen XH, Zhang G, Liu ZC, Wu N,
Wang H, Qi YF, Wang HS, Cai SH and Du J: CCL21 facilitates
chemoresistance and cancer stem cell-like Properties of colorectal
cancer cells through AKT/GSK-3β/Snail signals. Oxid Med Cell
Longev. 2016:58741272016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cioffi M, Trabulo SM, Sanchez-Ripoll Y,
Miranda-Lorenzo I, Lonardo E, Dorado J, Reis Vieira C, Ramirez JC,
Hidalgo M, Aicher A, et al: The miR-17-92 cluster counteracts
quiescence and chemoresistance in a distinct subpopulation of
pancreatic cancer stem cells. Gut. 64:1936–1948. 2015. View Article : Google Scholar : PubMed/NCBI
|