Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare type
of non-small cell lung cancer (NSCLC) composed of poorly
differentiated cells and sarcoma or sarcomatoid components (spindle
and/or giant cells) (1). PSC
accounts for 0.3–1.0% of all lung cancer cases (2,3).
Systematic reports of PSC are uncommon due to the inherent rarity
of the disease. Therefore, decisions regarding treatment strategies
are based on clinicopathological descriptions of small case series
or single case reports (4,5).
Previously, Yendamuri et al (2) identified 1,921 patients with PSC from
the Surveillance, Epidemiology and End Results (SEER) database
(1973–2008). Another study conducted by Steuer et al
(6) identified 7,965 patients with
PSC from the National Cancer Database (1998–2011). The
aforementioned studies confirmed that PSC is a relatively rare
histological type of lung cancer with lower overall survival (OS)
rates compared with other NSCLC types. Complete resection is an
essential treatment in the absence of metastasis in patients with
PSC. However, the role of adjuvant chemotherapy remains unclear
(7,8). To the best of our knowledge, few
studies (6,9) have focused on the effect of
radiotherapy on the survival of patients with PSC. An unfavorable
prognosis is anticipated due to the pathological features of poorly
differentiated tumors. The different pathological types of PSC are
also poorly studied.
To date, the PSC treatment strategy that is selected
depends on the disease stage (10).
Thus, similar to other types of NSCLC, clinicians need to pay
attention to early-stage resectable PSC. Clinicians must achieve a
rapid preoperative diagnosis and minimize the delay before surgery
to avoid tumor progression. Radical surgical resection (11) is recommended for operable patients
and also facilitates accurate diagnosis. Patients with PSC
significantly benefit from cancer-directed surgery (12,13).
In the present study, five specific types of
sarcomatoid carcinoma were used to address the aforementioned
unresolved questions using the World Health Organization (WHO)
classification system (2015). The five specific types of
sarcomatoid carcinoma studied were pure spindle cell, pure giant
cell, carcinosarcoma, pulmonary blastoma and pleomorphic, which
includes tumors with spindle and giant cells (14). The Surveillance, Epidemiology, and
End Results (SEER) program is supported by the National Cancer
Institute. The program collects a variety of details, including
cancer incidence and survival, from 18 population-based cancer
registries throughout the USA, covering 27.8% of the USA population
(15). A retrospective analysis of
patients with PSC registered in the SEER database between 1988 and
2016 was performed to determine the prognostic impact of
clinicopathological characteristics and treatment modalities.
Materials and methods
Patients
All patients with histologically confirmed PSC
between 1988 and 2016 were extracted from the SEER database
(https://seer.cancer.gov/) using SEER*Stat 8.3.5
software (https://seer.cancer.gov/seerstat/). The age range of
the patients was 18–94 years (mean age, 65.9 years), and there was
a slightly higher percentage of males (59.9%; n=1,032) compared
with females (40.1%; n=691). Patients with primary PSC (n=1,723) as
their only primary cancer were included. The inclusion criteria
were: i) Pathologically confirmed PSC between 1988 and 2016; and
ii) the site recode ICD-O-3/WHO 2008 definition (International
Classification of Diseases for Oncology, Third Edition) (16) was ‘Lung and Bronchus’. The exclusion
criteria included: i) Multiple primary tumors; ii) no prognostic
data; and iii) lack of clinicopathological data.
The demographic features and clinicopathological
characteristics of these patients were collected and are presented
in Table I. Of the 1,723 patients,
776 underwent surgery. Of the 776 patients, 264 had stage I, 145
had stage II, 211 had stage III, 44 had stage IV PSC and 112 had no
data regarding stage. The OS time was the measured primary outcome
and was defined as the interval from diagnosis to death from any
cause or the last follow-up in the SEER database.
Tumor-Node-Metastasis (TNM) staging was performed according to the
American Joint Committee on Cancer (AJCC) 3rd Edition (1988–2003)
(https://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/3rd.html),
6th Edition (2004–2009) and 7th Edition (2010–2016) (https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx).
Several variables, such as age at diagnosis, sex, laterality,
primary site, pathological type, differentiation, TNM and the use
of surgery and radiotherapy were collected.
 | Table I.Demographic and clinical
characteristics patients with pulmonary sarcomatoid carcinoma. |
Table I.
Demographic and clinical
characteristics patients with pulmonary sarcomatoid carcinoma.
|
Demographic/clinical characteristic | Cases, n (%) |
|---|
| Age at diagnosis,
years |
|
|
<76 | 1,309 (75.97) |
|
≥76 | 414 (24.03) |
| Sex |
|
|
Male | 1,032 (59.90) |
|
Female | 691 (40.10) |
| Ethnicity |
|
|
White | 1,375 (79.80) |
|
Black | 239 (13.87) |
|
Others | 109 (6.33) |
| Marital status |
|
|
Married | 777 (45.10) |
|
Unmarried | 946 (54.90) |
| Laterality |
|
|
Left-origin of primary | 739 (42.89) |
|
Right-origin of primary | 964 (55.95) |
|
Bilateral, single primary | 20 (1.16) |
| Primary site |
|
| Main
bronchus | 53 (3.08) |
| Upper
lobe | 972 (56.41) |
| Middle
lobe | 89 (5.17) |
| Lower
lobe | 469 (27.22) |
|
Overlapping lesion | 42 (2.44) |
| Lung,
NOS | 98 (5.69) |
| Pathological
Type |
|
| Giant
cell carcinoma | 535 (31.05) |
| Spindle
cell carcinoma | 445 (25.83) |
|
Pleomorphic carcinoma | 403 (23.39) |
|
Carcinosarcoma | 288 (16.72) |
|
Pulmonary blastoma | 52 (3.02) |
| Differentiation,
grade |
|
| I | 20 (1.16) |
| II | 14 (0.81) |
|
III | 627 (36.39) |
| IV | 332 (19.27) |
| ND | 730 (42.37) |
| Tumor size, mm |
|
|
<56 | 923 (53.57) |
|
≥56 | 800 (46.43) |
| TNM |
|
| I | 318 (18.46) |
| II | 175 (10.16) |
|
III | 427 (24.78) |
| IV | 665 (38.60) |
| ND | 138 (8.01) |
| Surgery |
|
| No | 940 (54.56) |
|
Yes | 776 (45.04) |
| ND | 7 (0.41) |
| Radiation |
|
| No | 1,038 (60.24) |
|
Yes | 685 (39.76) |
| Chemotherapy |
|
| No | 1,080 (62.68) |
|
Yes | 643 (37.32) |
Statistical analysis
The optimal cut-off value of age and tumor size for
OS rate was determined using X-tile (Yale School of Medicine)
software (version 3.6.1). The Kaplan-Meier method was used to
generate survival curves. Differences in OS rate stratified by each
covariate were analyzed by the log-rank test. Univariate and
multivariate cox proportional hazard models were used to assess the
associations between each covariate and OS rate. The results were
presented as hazard ratios and 95% confidence intervals (CIs).
Propensity matching was performed in R v.3.5.2 (https://www.r-project.org/) using the nearest neighbor
matching and a caliper width of 0.02. A nomogram was developed and
modeled using the rms package of R software (version 5.1–4;
http://cran.r-project.org/web/packages/rms/index.html).
Statistical analysis was conducted using SPSS 20 (IBM Corp.).
P<0.05 (two sided) was considered to indicate a statistically
significant difference.
Results
Baseline characteristics of patients
with PSC
A total of 3,897 patients initially diagnosed with
PSC between 1975 and 2016 were identified in the SEER database. Of
these, 1,723 eligible patients were included in the study. The
study workflow and selection process are presented in Fig. 1. Patient demographic and
clinicopathological characteristics are presented in Table I.
Survival analyses
The median survival time was 8 months (range, 0–337
months). The 1-, 2-, 3- and 5-year survival rates were 38.6, 26.3,
22.1 and 18.1%, respectively. The optimal cut-off values of age and
tumor size at diagnosis were 76.0 years and 56 mm, respectively, as
set by X-tile analysis. The OS rates of patients with
clinicopathological and demographic characteristics, such as male
(P=0.004; Fig. 2B), aged ≥76 years
(P<0.0001; Fig. 2A), unmarried
(P=0.0123; Fig. 2D), poor
tumor-differentiated grade (P<0.0001; Fig. 3E) and large tumor size ≥56 mm
(P<0.0001; Fig. 3B), were
significantly worse compared with other patients. The analyses
stratified by stage demonstrated that stage I patients with PSC
demonstrated improved OS outcomes compared with stage II–IV
patients (P<0.0001; Fig. 3A). The
5-year OS rates for patients with PSC were as follows: Stage I,
40.3; stage II, 23.8; stage III, 16.0 and stage IV, 2.3%. Patients
with pulmonary blastoma demonstrated improved OS rates compared
with other pathological types (P<0.0001; Fig. 3F). Other factors, including
laterality (Fig. 3C), ethnicity
(Fig. 2C) and primary site (Fig. 3D), did not significantly affect OS
rate.
Surgery and radiotherapy effects on OS
of patients with PSC
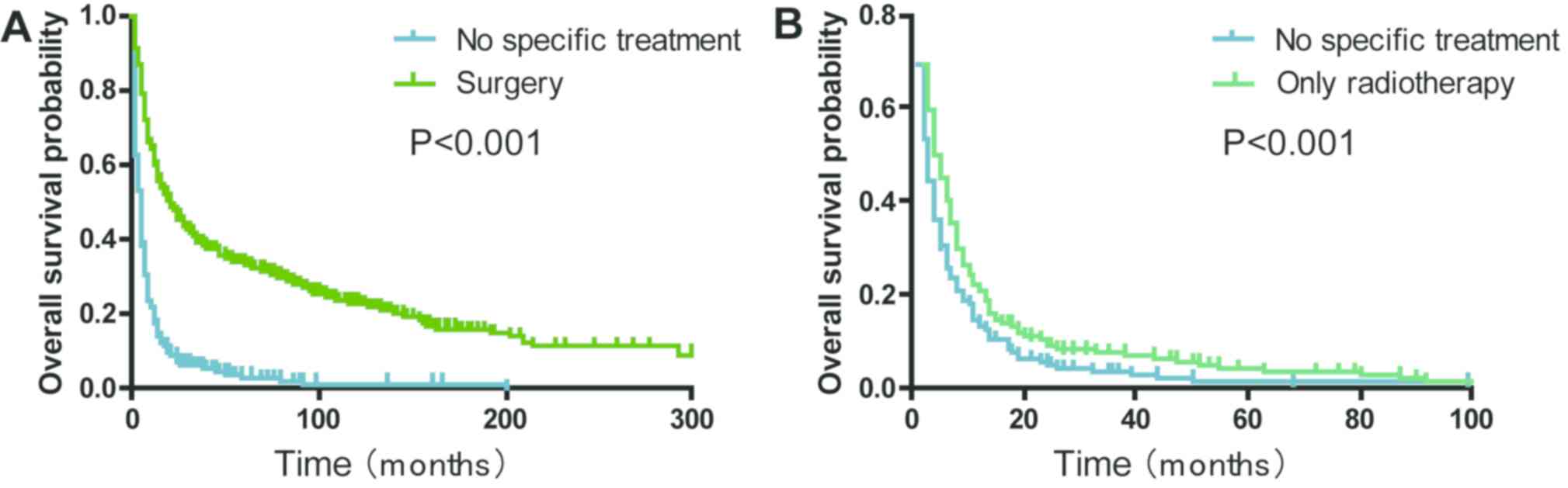
Patients with a history of surgical treatment at the
primary site exhibited a significantly improved OS compared with
those that received no specific treatment (median survival, 21.0
vs. 4.0 months; P<0.001; Fig.
4A). The 2-year OS rate of the ‘only radiotherapy’ group was
significantly higher compared with the ‘no specific treatment’
group (9.1 vs. 5.4%; P<0.001; Fig.
4B). The analyses stratified by stage demonstrated that
radiotherapy significantly improved the OS rate in stage I–III
patients with PSC without surgical resection (P<0.001; Fig. 5A) and stage IV patients with PSC
without surgical resection (P=0.1764, Fig. 5B). Subgroup analyses for AJCC 3rd,
6th and 7th groups demonstrated that similar trends still existed
(all P<0.05; Fig. S1).
Furthermore, the patients that received only surgery
demonstrated improved progress compared with those who received
surgery combined with radiotherapy (P<0.001; Fig. 6A). After matching the propensity
scores, the ‘surgery plus radiotherapy group’ consisted of 156 and
‘only surgery group’ of 247 patients with PSC (1:2). However, no
significant differences in the prognosis were found between the two
subgroups (P=0.052; Fig. 6B).
Independent prognostic risk factors
that affect OS of patients with PSC
In the present study, univariate and multivariate
Cox proportional hazard analyses of the variables potentially
influencing OS of patients with PSC were investigated. Univariate
analysis revealed that older age, male, unmarried, giant cell
carcinoma, worse pathological grade, larger tumor size, later TNM
stages and no special treatments were associated with a worse OS
rate (all P<0.05; Table II).
Using multivariate analysis, all factors except for differentiation
(P=0.190, Table II) were still
identified as independent risk factors of prognosis for patients
with PSC.
 | Table II.Univariate and multivariate Cox
proportional hazard analyses of the clinical characteristics for
overall survival of patients with pulmonary sarcomatoid
carcinoma. |
Table II.
Univariate and multivariate Cox
proportional hazard analyses of the clinical characteristics for
overall survival of patients with pulmonary sarcomatoid
carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age at diagnosis,
years |
|
|
|
|
|
|
|
<76 | 1 (Ref.) |
| – | 1 (Ref.) |
| – |
|
≥76 | 1.531 | 1.351–1.735 | <0.001 | 1.440 | 1.265–1.639 | <0.001 |
| Sex |
|
|
|
|
|
|
|
Male | 1 (Ref.) |
| – | 1 (Ref.) |
| – |
|
Female | 0.821 | 0.734–0.919 |
0.001 | 0.863 | 0.768–0.968 |
0.012 |
| Marital status |
|
|
|
|
|
|
|
Unmarried | 1 (Ref.) |
| – | 1 (Ref.) |
| – |
|
Married | 0.850 | 0.762–0.949 |
0.004 | 0.869 | 0.776–0.974 |
0.016 |
| Pathological
Type |
|
|
|
|
|
|
|
Pulmonary blastoma | 1 (Ref.) |
| – | 1 (Ref.) |
| – |
|
Pleomorphic carcinoma | 2.317 | 1.451–3.699 | <0.001 | 2.043 | 1.269–3.291 |
0.003 |
| Spindle
cell carcinoma | 3.315 | 2.083–5.275 | <0.001 | 2.210 | 1.373–3.557 |
0.001 |
|
Carcinosarcoma | 2.823 | 1.752–4.547 | <0.001 | 2.634 | 1.623–4.276 | <0.001 |
| Giant
cell carcinoma | 3.638 | 2.291–5.777 | <0.001 | 2.704 | 1.684–4.341 | <0.001 |
|
Differentiation |
|
|
|
|
|
0.190 |
| I | 1 (Ref.) |
| – |
|
|
|
| II | 2.776 | 1.127–6.836 |
0.026 |
|
|
|
|
III | 3.038 | 1.569–5.884 |
0.001 |
|
|
|
| IV | 3.621 | 1.861–7.046 | <0.001 |
|
|
|
|
Unknown | 3.785 | 1.956–7.324 | <0.001 |
|
|
|
| Tumor size, mm |
|
|
|
|
|
|
|
<56 | 1 (Ref.) |
| – | 1 (Ref) |
| – |
|
≥56 | 1.574 | 1.409–1.757 | <0.001 | 1.292 | 1.152–1.449 | <0.001 |
| TNM |
|
|
|
|
|
|
| I | 1 (Ref.) |
| – | 1 (Ref.) |
| – |
| II | 1.416 | 1.137–1.764 |
0.002 | 1.335 | 1.067–1.670 |
0.011 |
|
III | 2.044 | 1.729–2.417 | <0.001 | 1.593 | 1.320–1.923 | <0.001 |
| IV | 4.874 | 4.140–5.739 | <0.001 | 2.947 | 2.413–3.600 | <0.001 |
| Treatment
modality |
|
|
|
|
|
|
| Only
Surgery | 0.203 | 0.174–0.237 | <0.001 | 0.407 | 0.336–0.494 | <0.001 |
| Surgery
plus RT | 0.292 | 0.242–0.353 | <0.001 | 0.406 | 0.332–0.497 | <0.001 |
| Only
RT | 0.644 | 0.558–0.743 | <0.001 | 0.672 | 0.580–0.778 | <0.001 |
| No
special treatment | 1 (Ref.) |
| – | 1 (Ref.) |
| – |
Predicting OS of patients with PSC
using a nonogram model
Age and tumor size were stratified using X-tile
software. This revealed that the optimal cut-off points for age for
predicting progression were 63 and 79 years old. The optimal
cut-off points for tumor size for predicting progression were 50
and 75 mm. Subsequently, patients with PSC were divided into three
groups according to these optimal cut-off values. These groups
included the following: i) Young group (<63 years); ii) middle
aged group (63-79 years); and iii) old group (>79 years). In
addition patients were grouped according to tumor size: i) <50
mm; ii) 50–75 mm; and iii) >75 mm. Significant differences were
observed between the Kaplan-Meier curves for OS among the three age
and tumor size groups (all P<0.05; data not shown).
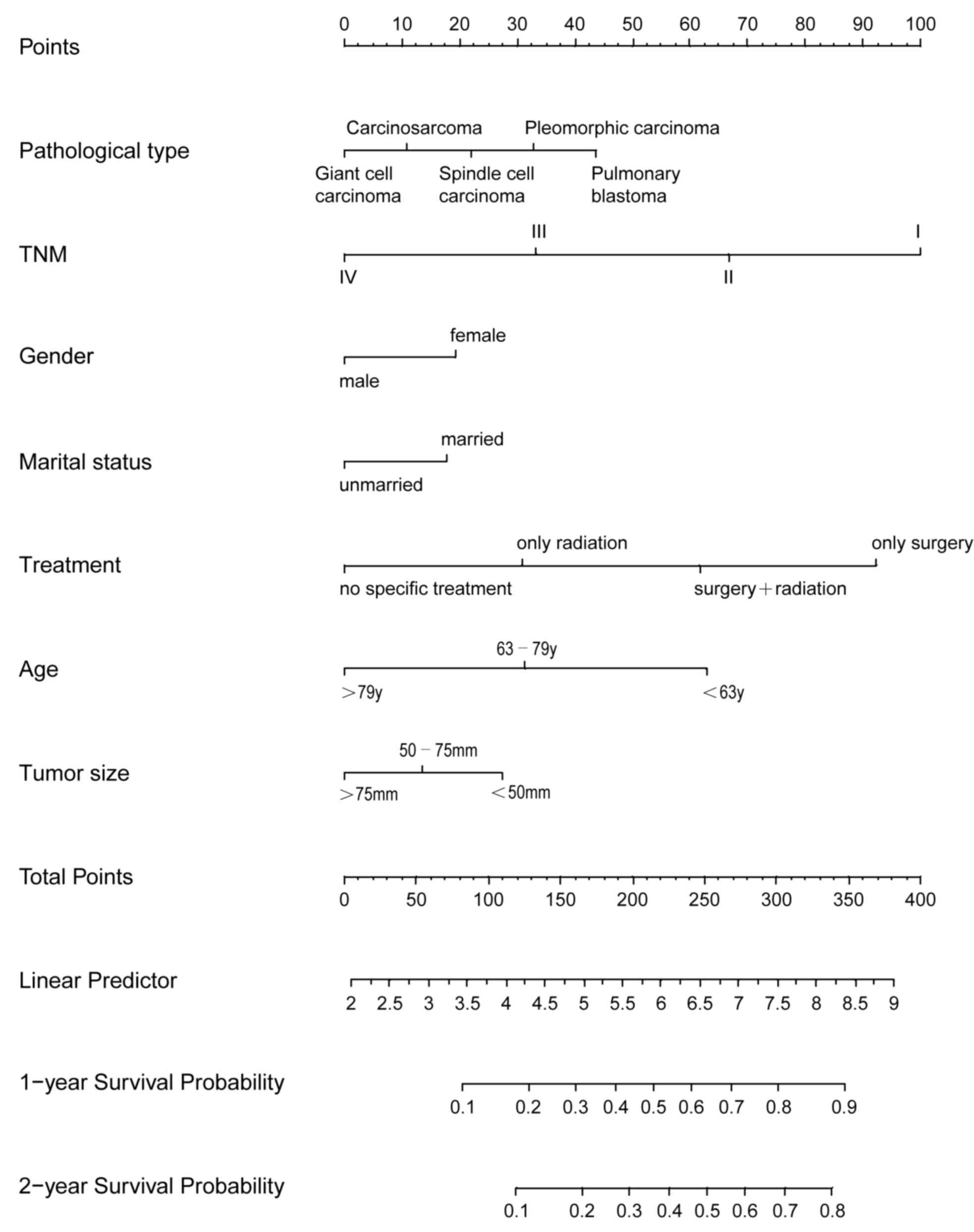
Next, a nomogram model was established to predict
the survival of patients with PSC. This model incorporated all the
independent prognostic factors found using multivariate analysis
(Fig. 7). In the model, a high score
was associated with good prognosis. TNM, treatment modality and age
contributed the most to prognosis, followed by pathological type
and tumor size. The C-index of the nomogram model for OS prediction
was 0.75 (95% CI, 0.74–0.76).
Discussion
The present study summarized the clinical
characteristics of patients with PSC and identified several
survival factors using data from the SEER database from 1975–2016.
In the present study, the median survival of patients with PSC was
8 months, and the 1-year survival rate was 38.6%. The 2-, 3-, and
5-year survival rates were 26.3, 22.1 and 18.1%, respectively.
Compared with previous studies (6,17,18), the
present study demonstrated that the survival rate was slightly low
with a median survival range of 11–19 months, a 1-year survival
rate of 32–71% and a 5-year survival rate of 17–29%. This outcome
may be attributable to patients enrolled in previous studies that
had surgical resection (8,12,13,17). One
of these studies (13) also had a
high proportion of patients with early-stage PSC.
Morphologically, PSC is generally characterized by a
high degree of variability and heterogeneity of the tumor (19,20). The
present study also revealed that the most common histological
subtype was giant cell carcinoma, followed by spindle cell
carcinoma, pleomorphic carcinoma and carcinosarcoma. Previous
reports demonstrated that pleomorphic carcinoma was a common
histological subtype (1,3,21,22). The
WHO 2015 lung cancer classification (14) reported that the least common subtype
of PSC is pulmonary blastoma (23),
which is consistent with the findings of the present study.
Previous studies have identified that pulmonary blastoma counts
accounts for 3.02% of all PSC cases (3,6). Most
types of sarcomatoid carcinoma are poorly differentiated,
pleomorphic-like carcinoma (7,24),
spindle cell carcinoma (25), giant
cell carcinoma (26) and
carcinosarcoma (21,25). Pulmonary blastoma is a subtype of a
well-differentiated biphasic tumor and mainly composed of a
primitive epithelial component (21). The present results demonstrated that
51.8% of pulmonary blastoma cases were well differentiated or
moderately differentiated. Poorly differentiated or
undifferentiated occurred in a majority of other types of PSC
(93.6–99.7%). The present study also demonstrated that patients
with pulmonary blastoma exhibited improved survival, with a 60.2%
5-year OS rate compared with 12.2–23.7% for the other types of
sarcamatoid carcinoma. Therefore, pulmonary blastoma is a
well-differentiated malignancy and has a favorable survival
outcome.
In the present study, 776 of the 1,723 patients
underwent surgery. Of the 776 patients who underwent surgery, 67.2%
had stage I–II PSC. Meanwhile, of the 776 patients who underwent
surgery, 255 (32.8%) had stage III/IV PSC. PSC has been reported to
be associated with high rates of recurrence and low OS rates even
after complete surgical resection (6,11,17).
Aforementioned studies (13,27) also investigated the effects of
neoadjuvant chemotherapy and radiotherapy on the OS rate of
patients with PSC. In patients with high-risk stages IB, II and
IIIA NSCLC, platinum-based combination regimens are the mainstream
treatment strategies (28). A
beneficial effect of chemotherapy on the OS rate of patients with
PSC can be difficult to detect due to the low incidence of this
disease (3). Furthermore, to the
best of our knowledge, studies investigating the effects of
chemotherapy on the OS rate of patients with PSC are uncommon. The
present study considered multimodal treatment; however, could not
assess the potential survival impact of chemotherapy among patients
with PSC due to the lack of specific information regarding
chemotherapy in the SEER database.
The role of radiotherapy in patients with PSC is
poorly defined (29). Radiotherapy
has been used alone and in combination with chemotherapy in
patients with unresectable disease or in an adjuvant setting for
those with high rates of recurrence (30). Clinically, radiotherapy is always
reserved for patients with rare types of lung cancer (31,32). To
date, few studies have focused on the value of radiotherapy in PSC.
Several prospective randomized studies have failed to demonstrate
advantages in delivering radiotherapy in patients with PSC
(24). In the present study,
radiotherapy has an important impact on the survival of patients
with PSC stratified by stage, particularly stages I–III. However,
patients with PSC who had undergone surgery combined with
radiotherapy had a worse OS compared with those who only received
surgery. Propensity score matching was also used to reduce bias in
the present study. After matching the propensity scores, the
prognostic analysis did not significantly differ between the two
groups. Chaft et al (3)
conducted a prospective study on patients with PSC and demonstrated
patients that underwent surgery plus adjuvant therapy did not have
a statistically significant survival benefit compared with those
who received surgery alone. This finding is similar to the results
obtained by the current study.
The present study had several limitations. First,
the study was conducted in a retrospective manner, which always
leads to a selection bias. Second, patients with pulmonary blastoma
were few in number in the SEER database, which may be a strong
confounding factor affecting the result of the survival analysis.
Third, specific information, such as chemotherapy sequence with
surgery, dose, and agent of chemotherapy are lacking in the SEER
database. Therefore, the present study could not account for the
effect of chemotherapy on PSC prognosis. Fourth, a number of other
factors may influence the results of the present study, such as
radiation regimen and the technological infrastructure of the
equipment.
In conclusion, PSC is a type of poorly
differentiated NSCLC composed of spindle or giant cells. Surgery is
often the first choice of treatment for patients with PSC.
Furthermore, radiotherapy can influence the long-term outcome in
stage I–III patients with PSC who only underwent radiation therapy
without surgical resection. The present population cohort study was
conducted to stratify prognoses of patients with PSC despite
incidence being low. Age, tumor size, TNM and pathological type
were independent risk factors for the prognosis of patients with
PSC. Female, married and treatment modality were independent
protective factors for patients with PSC.
In summary, for patients with stage I–III who are
unsuitable for surgery, radiotherapy has been demonstrated to
provide a better prognosis compared with no specific treatment. In
order to provide an individualized prediction method, the present
study developed a novel nomogram. Taken together, the present
results can aid clinical decision-making to improve prognosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Social
Development Project of Jiangsu Province (grant no. BE2019768), and
the Zhangjiagang City Science and Technology Support Plan (Social
Development) Medical and Health Project (grant no. ZKS1918). The
funding institutions did not have any roles in the study design,
data collection or analysis.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available in the SEER database (https://seer.cancer.gov/).
Authors' contributions
XL and YC have made substantial contributions to
data collection, data analysis and manuscript writing. ZYa, YH and
ZYu performed data analysis. QL contributed to acquisition and
interpretation of data. GF designed the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sim JK, Chung SM, Choi JH, Oh JY, Lee SH,
Kim JH, Min KH, Hur GY, Shim JJ, Kang KH, et al: Clinical and
molecular characteristics of pulmonary sarcomatoid carcinoma.
Korean J Intern Med. 33:737–744. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yendamuri S, Caty L, Pine M, Adem S,
Bogner P, Miller A, Demmy TL, Groman A and Reid M: Outcomes of
sarcomatoid carcinoma of the lung: A Surveillance, Epidemiology,
and end results database analysis. Surgery. 152:397–402. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaft JE, Sima CS, Ginsberg MS, Huang J,
Kris MG, Travis WD and Azzoli CG: Clinical outcomes with
perioperative chemotherapy in sarcomatoid carcinomas of the lung. J
Thorac Oncol. 7:1400–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yaguchi D, Ichikawa M, Ito M, Okamoto S,
Kimura H and Watanabe K: Dramatic response to nivolumab after local
radiotherapy in pulmonary pleomorphic carcinoma with rapid
progressive post-surgical recurrence. Thorac Cancer. 10:1263–1266.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antoine M, Vieira T, Fallet V, Hamard C,
Duruisseaux M, Cadranel J and Wislez M: Pulmonary sarcomatoid
carcinoma. Ann Pathol. 36:44–54. 2016.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steuer CE, Behera M, Liu Y, Fu C,
Gillespie TW, Saba NF, Shin DM, Pillai RN, Pakkala S, Owonikoko TK,
et al: Pulmonary sarcomatoid carcinoma: An analysis of the national
cancer data base. Clin Lung Cancer. 18:286–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hendriksen BS, Hollenbeak CS, Reed MF and
Taylor MD: Perioperative chemotherapy is not associated with
improved survival in stage I pleomorphic lung cancer. J Thorac
Cardiovasc Surg. 158:581–591.e11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maneenil K, Xue Z, Liu M, Boland J, Wu F,
Stoddard SM, Molina J and Yang P: Sarcomatoid carcinoma of the
lung: The Mayo clinic experience in 127 patients. Clin Lung Cancer.
19:e323–e333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schrock AB, Li SD, Frampton GM, Suh J,
Braun E, Mehra R, Buck SC, Bufill JA, Peled N, Karim NA, et al:
Pulmonary sarcomatoid carcinomas commonly harbor either potentially
targetable genomic alterations or high tumor mutational burden as
observed by comprehensive genomic profiling. J Thorac Oncol.
12:932–942. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahouma M, Kamel M, Narula N, Nasar A,
Harrison S, Lee B, Stiles B, Altorki NK and Port JL: Pulmonary
sarcomatoid carcinoma: An analysis of a rare cancer from the
Surveillance, Epidemiology, and End Results database. Eur J
Cardiothorac Surg. 53:828–834. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lewis JA, Petty WJ, Urbanic J, Bernstein
ED and Ahmed T: Cure of oligometastatic classic biphasic pulmonary
blastoma using aggressive Tri-modality treatment: Case series and
review of the literature. Cureus. 10:e35862018.PubMed/NCBI
|
|
12
|
Park JS, Lee Y, Han J, Kim HK, Choi YS,
Kim J, Shim YM and Kim K: Clinicopathologic outcomes of curative
resection for sarcomatoid carcinoma of the lung. Oncology.
81:206–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lococo F, Rapicetta C, Cardillo G, Stefani
A, Margaritora S, Leuzzi G, Rossi G, Petracca Ciavarella L, Morandi
U, Facciolo F, et al: Pathologic findings and long-term results
after surgical treatment for pulmonary sarcomatoid tumors: A
multicenter analysis. Ann Thorac Surg. 103:1142–1150. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World Health Organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
NCI SEER, . Number of Persons by Race and
Hispanic Ethnicity for SEER Participants (2010 Census Data 1).
https://seer.cancer.gov/registries/data.htmlMarch
3–2020
|
|
16
|
NCI SEER, . Site Recode ICD-O-3/WHO 2008
Definition*^. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/#footnotesMarch
3–2020
|
|
17
|
Ung M, Rouquette I, Filleron T, Taillandy
K, Brouchet L, Bennouna J, Delord JP, Milia J and Mazières J:
Characteristics and clinical outcomes of sarcomatoid carcinoma of
the lung. Clin Lung Cancer. 17:391–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang X, Li Q, Xu B, Hu S, Wang Q, Li Y,
Zong Y, Zhang S and Li C: Mutation landscape and tumor mutation
burden analysis of Chinese patients with pulmonary sarcomatoid
carcinomas. Int J Clin Oncol. 24:1061–1068. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boland JM, Mansfield AS and Roden AC:
Pulmonary sarcomatoid carcinoma-a new hope. Ann Oncol.
28:1417–1418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basu A, Moreira AL, Simms A and Brandler
TC: Sarcomatoid carcinoma in cytology: Report of a rare entity
presenting in pleural and pericardial fluid preparations. Diagn
Cytopathol. 47:813–816. 2019.PubMed/NCBI
|
|
21
|
Franks TJ and Galvin JR: Sarcomatoid
carcinoma of the lung: Histologic criteria and common lesions in
the differential diagnosis. Arch Pathol Lab Med. 134:49–54.
2010.PubMed/NCBI
|
|
22
|
Yokoyama S, Murakami T, Tao H, Onoda H,
Hara A, Miyazaki R, Furukawa M, Hayashi M, Inokawa H, Okabe K and
Akagi Y: Tumor spread through air spaces identifies a distinct
subgroup with poor prognosis in surgically resected lung
pleomorphic carcinoma. Chest. 154:838–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu L, Xu Y, Chen Z, Pan Y and Lu S:
Clinical analysis of 95 cases of pulmonary sarcomatoid carcinoma.
Biomed Pharmacother. 76:134–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin J, Yang Y, Ma K, Yang X, Lu T, Wang S,
Shi Y, Zhan C, Zhu Y and Wang Q: Clinicopathological
characteristics and prognosis of pulmonary pleomorphic carcinoma: A
population--based retrospective study using SEER data. J Thorac
Dis. 10:4262–4273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weissferdt A, Kalhor N, Rodriguez Canales
J, Fujimoto J, Wistuba II and Moran CA: Spindle cell and
pleomorphic (‘sarcomatoid’) carcinomas of the lung: An
immunohistochemical analysis of 86 cases. Hum Pathol. 59:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weng SS, Cao Y, Tang XJ, Zhu LZ, Tan YN,
Dong CX, Chen JQ, Shen H and Yuan Y: Epidemiological features of
lung giant cell carcinoma and therapy for patients with EGFR
mutations based on case reports and the surveillance, epidemiology,
and end results (SEER) database. Oncotarget. 8:25323–25333. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong JY, Choi MK, Uhm JE, Park MJ, Lee J,
Park YH, Ahn JS, Park K, Han JH and Ahn MJ: The role of palliative
chemotherapy for advanced pulmonary pleomorphic carcinoma. Med
Oncol. 26:287–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakayama S, Sasaki M, Morinaga S and
Minematsu N: Nonsmall cell lung carcinoma with giant cell features
expressing programmed Death-ligand 1: A report of a patient
successfully treated with pembrolizumab. Case Rep Oncol Med.
2018:58630152018.PubMed/NCBI
|
|
30
|
Wei W, Zhou J, Zhang Q, Liao DH, Liu QD,
Zhong BL, Liang ZB, Zhang YC, Jiang R, Liu GY, et al: Postoperative
intensity-modulated radiation therapy reduces local recurrence and
improves overall survival in III-N2 non-small-cell lung cancer: A
single-center, retrospective study. Cancer Med. Feb 26–2020.doi:
10.1002/cam4.2937 (Epub ahead of print). View Article : Google Scholar
|
|
31
|
Qin BD, Jiao XD, Liu K, Wu Y, He X, Liu J
and Zang YS: Clinical, pathological and treatment factors
associated with the survival of patients with primary pulmonary
salivary gland-type tumors. Lung Cancer. 126:174–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang H, Lin Y and Liang Y: Treatment of
lung carcinosarcoma and other rare histologic subtypes of non-small
cell lung cancer. Curr Treat Options Oncol. 18:542017. View Article : Google Scholar : PubMed/NCBI
|