Introduction
Breast cancer is one of the most common malignant
diseases and poses significant morbidity and mortality to women
worldwide. As the study of breast cancer has progressed and the
biological behaviors of tumors are starting to be elucidated,
increasing evidence has suggested that breast cancer is a systemic
disease. This is in opposition to the traditional view, which
suggested that the disease only occurred in the breast (1–4).
Treatment strategies for breast cancer have been
gradually moving toward a systemic approach. Breast-conserving
surgery together with post-operative radiotherapy and chemotherapy
do not increase the risk of recurrence, and even if there are one
or two positive sentinel lymph nodes, comprehensive treatment after
surgery can result in similar effects to those of traditional
radical surgery (5). The surgical
treatment of breast cancer has vastly improved in recent years, and
is becoming more precise and with improved cosmetic outcomes.
Endoscopic techniques for breast surgery were first reported by
Kompatscher (6), who removed breast
contracture implants under endoscopy. Since then, endoscopic
techniques have been applied to other surgeries. In 1994, Fine
et al (7) reported
endoscopic-assisted muscle flap harvest to guide abdominal visceral
surgery. The first endoscopic surgery for the treatment of breast
cancer was reported by Salvat et al (8), who performed an endoscopic axillary
lymph node dissection (ALND). In 2000, endoscopic sentinel node
detection in patients with breast cancer patients was documented by
Kühn et al (9). Therefore,
endoscopic techniques have been used in benign and malignant breast
disease treatment.
Mastoscopic axillary lymph node dissection (MALND)
has become one of the most important methods for the treatment of
breast cancer. MALND results in less trauma and blood loss, fewer
post-operative complications and faster post-operative recovery
(10). However, the breast is a
solid organ with no natural lumen. Furthermore, the abundant fat
and gland increase make this procedure challenging and have limited
the wider development of endoscopic techniques in the surgical
treatment of breast cancer. The most widely used mastoscopic
technique is the lipolysis method which has been mostly performed
on patients with limited breast tissue and mostly Asian women. In
1998, Brun et al (11)
reported fat and lymph node suction in breast cancer axillary
lymphadenectomy. Luo et al (10) also shared their abundant experience
on lipolytic endoscopic axillary surgery. Although certain reports
indicate that lipolysis does not increase the risk of recurrence of
breast cancer and does not reduce the overall survival of patients,
liposuction may destroy, not only the whole tumor, but also the
lymph nodes, thereby increasing the risk of local and distant
metastases (12). Therefore,
lipolytic therapy is not a popular treatment method for breast
cancer. Endoscopic axillary lymphadenectomy without prior
liposuction was reported in 1999 (13). This therapeutic approach has not been
comprehensively used for wounds close to the armpits and does not
result in improved outcomes compared with traditional
small-incision-axillary surgery.
In order to overcome these flaws, the present study
described a novel endoscopic axillary lymphadenectomy technique
without prior liposuction and less visible scarring after
surgery.
Patients and methods
Case selection
A total of 16 female patients with breast cancer who
underwent MALND from 2016.01.12 to 2018.06.01 at the Second
Affiliated Hospital of Fujian Medical University (Quanzhou, China)
were selected. The mean age of the patients was 44.81 years (range,
27–58 years). Patients had masses 1.1–4.0 cm in diameter that were
located in the upper outer quadrant of the breast. The patients had
a mean body mass index (BMI) of 21.21 kg/m2 (range,
17.93–24.35 kg/m2). The BMI of the 16 patients is
presented in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| No. | Height (m) | Weight (kg) | BMI
(kg/m2) |
|---|
| 1 | 1.53 | 57 | 24.35 |
| 2 | 1.59 | 59 | 23.34 |
| 3 | 1.69 | 66 | 23.11 |
| 4 | 1.59 | 57 | 22.55 |
| 5 | 1.59 | 56 | 22.15 |
| 6 | 1.6 | 55 | 21.48 |
| 7 | 1.56 | 52 | 21.37 |
| 8 | 1.61 | 55 | 21.22 |
| 9 | 1.64 | 57 | 21.19 |
| 10 | 1.64 | 56 | 20.82 |
| 11 | 1.65 | 56 | 20.57 |
| 12 | 1.56 | 50 | 20.55 |
| 13 | 1.66 | 56 | 20.32 |
| 14 | 1.62 | 51 | 19.43 |
| 15 | 1.67 | 53 | 19.00 |
| 16 | 1.67 | 50 | 17.93 |
Breast cancer was diagnosed by core needle biopsy,
and the relevant examination was completed prior to surgery. If the
preoperative ultrasound suggested an axillary lymph node (ALN),
fine needle aspiration was performed to determine whether a
sentinel lymph node biopsy (SLNB) should be performed. The
inclusion criteria for this study were as follows: Non-obese breast
cancer patients, and patients with breast tumors not in the inward
quadrant. The exclusion criteria for this study were: Obese breast
cancer patients, breast cancer patients with tumors in the inner
quadrant, and elderly patients with severe heart or lung disease.
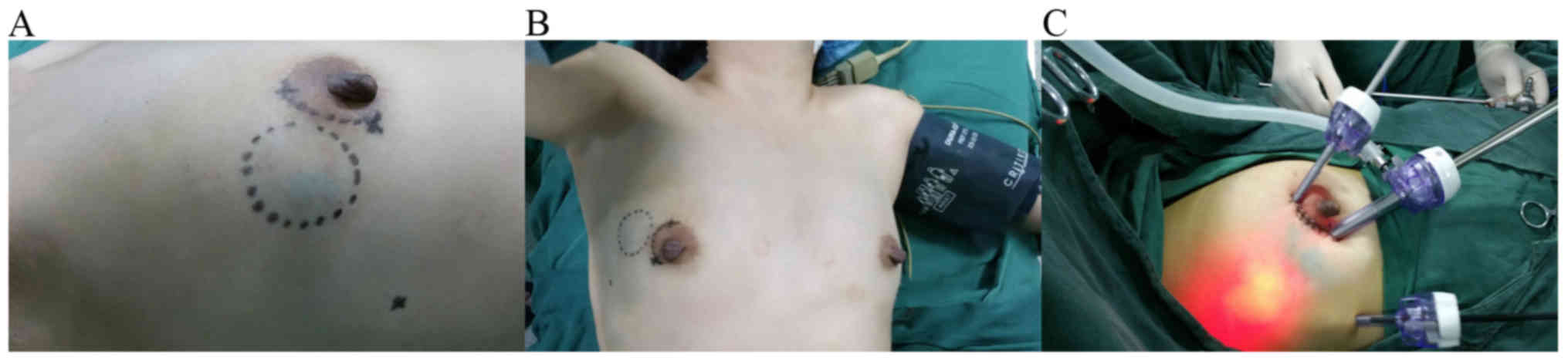
For the surgical procedure, periareolar incision on the quadrant
ring was made as the main incision and the other 0.5 cm trocar
incision was located on the anterior axillary line (Fig. 1A).
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Fujian Medical University
(Quanzhou, China). Written informed consent was obtained from all
patients.
Resection of malignant tumor and
margin of the breast
The patients were placed in a supine position with
the ipsilateral arm at a 90° abduction under general anesthesia
(Fig. 1B). An endoscopic operating
system (Storz HD Xenon Nova 300 system.) was used. The whole tumor
was excised through a periareolar incision. During the process,
safety margins were identified and a core needle biopsy puncture
was used for resection. Cavity shaving was used to confirm that the
margins were negative. A fast-frozen pathology report indicated
that the margins were negative.
Endoscopic surgery and tracer lymph
nodes
Prior to endoscopy, two trocars (5 and 10 mm,
respectively) were placed in the cavity through the periareolar
incision. The wound between the two trocars was closed to ensure
that the carbon dioxide did not leak out. A third trocar was placed
in the cavity through the 0.5 cm incision located on the anterior
axillary line. A 30° 10 mm endoscope was inserted through the 10 mm
trocar, and carbon dioxide was infused into the cavity until ~8
mmHg pressure was reached (Fig. 1C).
The sentinel lymph node-labeled tracer (methylene blue dye double
diluted with normal saline) was injected along the anterior
axillary line close to the armpit to ensure that the dye did not
leak into the cavity. The subcutaneous tissue was subsequently
separated and sentinel lymph node progress was observed.
SLNB
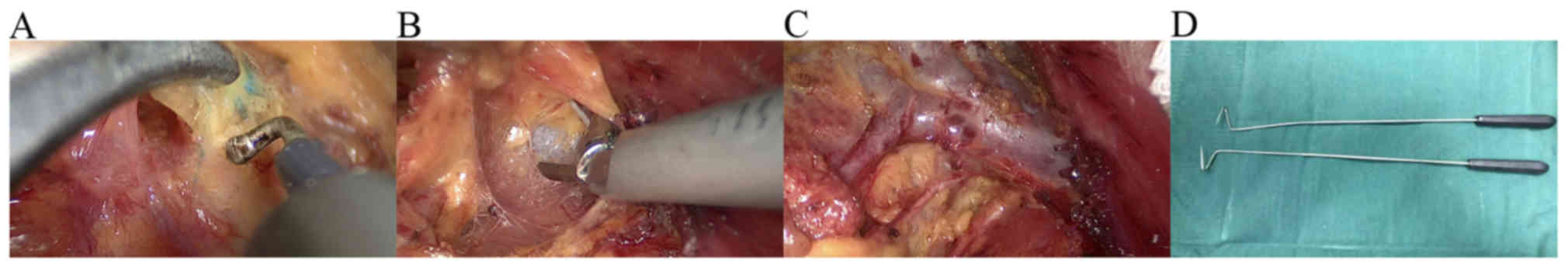
Tissues were separated along the pectoralis muscle
to the axilla. Sentinel lymph nodes are often located outside the
margin of the superficial layer of fascia coracocleidopectoralis of
the pectoralis minor muscle. During this process, attention should
be paid to finding blue-stained lymph nodes and lymphatic channels.
After locating the blue-stained areas, the tissue was separated,
but not removed, from the lymphatic tissue. A total of 2–3 lymph
nodes were found (Fig. 2A). The
lymph nodes were subsequently resected and sent for further
pathological examination. ALND should be performed when SLNB is not
successful or pathological reports indicate cancer cells in the
lymph nodes.
ALND
The axillary vein was exposed by separating the
pectoralis and the pectoralis minor muscles in the direction of the
axilla. During this process, the axillary vein was used to indicate
direction (Fig. 2B). Next, the
structure was separated as follows: Thoracodorsal vein and nerve,
axillary vein and long thoracic nerve, as observed by mastoscopy
(Fig. 2C), other important
structures and anatomical landmarks. Certain important structures,
such as the medial pectoral nerve, were preserved if possible.
Axillary level I and II lymph nodes were dissected. When visible
lymph nodes appeared between the pectoralis major and minor, the
lymph nodes between the pectoralis muscles were also resected. In
the present study, two methods were used to create a good view of
the back of the pectoral muscle. The first method was the rotation
and displacement of the endoscope lens for a better exposure of the
back of the pectoral muscle. The second method was the use a
special V-shaped hook for a better exposure of the back of the
pectoral muscle. The V-shaped hook (Fig.
2D) is often used for endoscopic surgery of thyroid tumors. The
axillary lymph tissue was removed. The axilla was washed with warm
distilled water, and drained using a suction tube placed in the
inferior trocar hole. Lastly, the chest and axilla were
bandaged.
Results
Post-surgical results
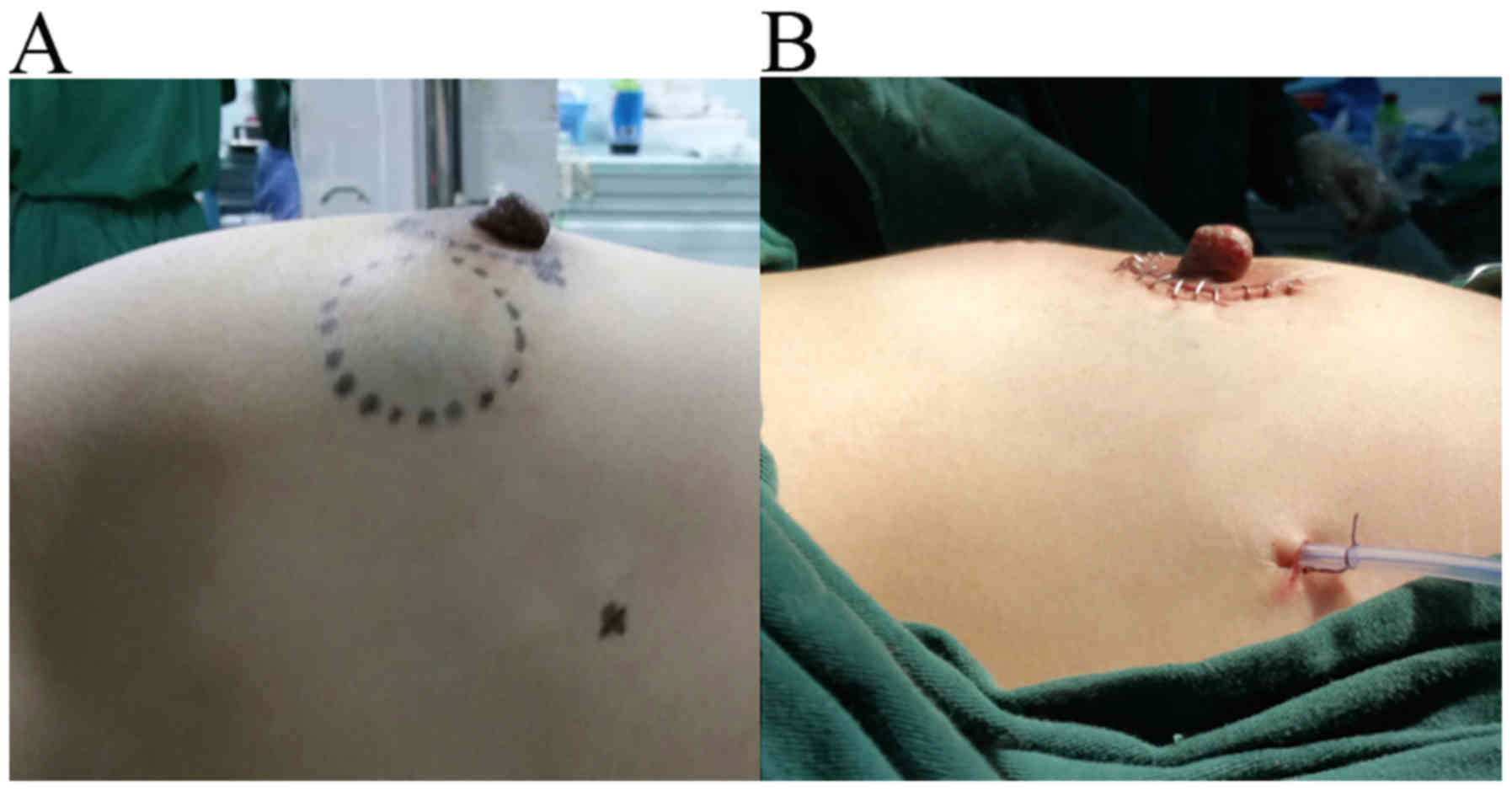
The post-surgical results are evident in Figs. 2C and 3. Fig. 2C
presents the overview following surgery. The thoracodorsal vein and
nerve, axillary vein and long thoracic nerve are clearly seen.
Fig. 3A and B show the pre- and
post-operative breast, respectively.
Patient characteristics and MALND
A total of 16 cases of MALND were examined between
2016.01.12 and 2018.06.01. The statistical results are presented in
Tables II and III. The patients' average age was
44.81±6.82 years, the average tumor diameter was 2.43±0.82 cm and
the average BMI was 21.21±1.66 kg/m2. A total of 4
patients received SLNB, 7 received ALND and 5 received both. The
mean operation time of SLNB was 35.11±3.82 min and that of ALND was
51.08±5.92 min. The mean intraoperative blood loss was 29.25±8.93
ml and the volume of drainage of SLNB and ALND was 63.25±6.36 and
148.42±21.18 ml, respectively. The average duration of drainage was
6.56±1.50 days. The mean follow-up period was ~28 months (range,
12–41 months). One of the 16 patients presented paresthesia in the
medial upper arm, which decreased or disappeared 3 months after the
operation. One patient developed mild edema in the upper arm 3
months after the operation, which decreased following treatment
with elastic bandage compression, local massage and functional
exercise to promote lymphatic reflux. The other patients exhibited
no wound effusion, upper limb edema or flap necrosis.
Post-operative B-ultrasound and molybdenum target X-ray examination
showed no tumor recurrence, and the motion of shoulder joint was
good. A total of 30 sentinel lymph nodes were harvested from 9
patients, with a mean number of 3.33±0.50 sentinel lymph nodes per
patient and a positive rate of 20%. A total of 189 ALNs were
excised from 12 patients and the mean number of ALNs per patient
was 15.75±2.67. The percentage of patients tested positive for ALNs
was 18.52%. A pathology report revealed that 14 patients were
diagnosed with invasive breast cancer, 1 with mucinous breast
cancer and 1 with ductal carcinoma in situ. Histology also revealed
62.5, 12.5 and 25% positive rates for ER/PR(+), HER2(+) and triple
negative breast cancer, respectively.
 | Table II.Patient and tumor characteristics. |
Table II.
Patient and tumor characteristics.
| Parameters | Values |
|---|
| Age (years) |
|
| Mean | 44.81±6.82 |
|
Range | 27-58 |
| Tumor diameter
(cm) |
|
| Mean |
2.43±0.82 |
|
Range | 1.1–4.0 |
| BMI
(kg/m2) |
|
| Mean | 21.21±1.66 |
|
Range | 17.93–24.35 |
| T stage [n (%)] |
|
| T1 | 6
(37.5) |
| T2 | 10
(62.5) |
| SLNB and/or ALND [n
(%)] |
|
| SLNB
alone | 4 (25) |
| ALND
alone | 7
(43.8) |
| SLNB and
ALND | 5
(31.2) |
| N stage [n (%)] |
|
| N0 | 4 (25) |
| N+ | 12 (75) |
| Quadrant [n (%)] |
|
| Upper
outer | 16 (100) |
| Upper
inter | 0 (0) |
| Lower
outer | 0 (0) |
| Lower
inter | 0 (0) |
|
Areolar | 0 (0) |
| Histological
type |
|
|
Invasive breast cancer | 14 |
|
Mucinous breast cancer | 1 |
| Ductal
carcinoma in situ | 1 |
| Estrogen receptor
status [n (%)] |
|
|
Positive | 10
(62.5) |
|
Negative | 6
(37.5) |
| Progesterone
receptor status [n (%)] |
|
|
Positive | 10
(62.5) |
|
Negative | 6
(37.5) |
| Human epidermal
growth factor receptor-2 status [n (%)] |
|
|
Positive | 2
(12.5) |
|
Negative | 14
(87.5) |
 | Table III.Clinical results of 16 patients. |
Table III.
Clinical results of 16 patients.
| Parameters | Values |
|---|
| Average operation
time (min) |
|
|
SLNB | 35.11±3.82 |
|
ALND | 51.08±5.92 |
| Operative blood
loss (ml) | 29.25±8.93 |
| Volume of drainage
(ml) |
|
|
SLNB | 63.25±6.36 |
|
ALND | 148.42±21.18 |
| Average duration of
drainage (days) |
6.56±1.50 |
| Complications |
|
|
Paresthesia (pain,
numbness) | 1 |
| Wound
effusion | 0 |
| Upper
limb edema | 1 |
| Flap
necrosis | 0 |
| Local
and distant recurrence | 0 |
|
Shoulder joint movement
disorder | 0 |
| Average number of
lymph nodes |
|
| SLNs
from 9 patients |
3.33±0.50 |
| ALNs
from 12 patients | 15.75±2.67 |
Discussion
Endoscopic techniques have been used in breast
surgery for several years. A number of mastoscopic therapeutic
methods including benign tumor resection, SLNB, ALND and breast
reconstruction have been previously reported (6,9,14,15).
Endoscopic methods are broadly divided into lipolytic and
non-lipolytic methods, of which the former are more widely used.
The majority of mastoscopic incisions are located in or next to the
axilla (9,13,16,17). A
number of innovative cases have also been reported (18–21).
However, mastoscopic surgery has several shortcomings. Lipolytic
mastoscopy remains controversial and non-lipolytic therapy is
challenging to perform. As normal ALND and SLNB can be performed
with a small incision through the armpits, endoscopic incisions in
this region offer no advantage with respect to appearance. The
present study revealed that ALND can be achieved under mastoscopy
with additional incisions, and can result in a cosmetically
pleasing result. Furthermore, the operation time, intraoperative
blood loss, drainage flow and direction of drainage have been
reported to offer no disadvantage compared with traditional radical
excision (10,12). A retrospective study suggested that
endoscopic axillary lymphadenectomy without liposuction does not
increase the risk of complications and is safe to perform (22). In the present study, only two cases
presented complications among the 16 cases of non-lipolytic
endoscopic axillary surgery. The complications were paresthesia and
upper limb edema, which were significantly improved following
treatment. Therefore, MALND requires further investigation.
Breast-conserving surgery is gradually becoming the
first choice for the treatment of breast cancer for several
patients with clinical stage I and II cancer. In the field of
breast surgery, safety, acceptable cosmetic outcome and minimal
invasiveness are of paramount importance. Non-lipolytic mastoscopic
SLNB is challenging to perform (9,23) and
the appearance of the affected area has not been satisfactory, as
locating lymph nodes tracts is difficult even in open surgery.
However, as sentinel lymph node dye continues to advance, locating
sentinel lymph nodes under endoscopy may become less difficult. In
the present study, methylene blue dye was used to locate sentinel
lymph nodes. Experience suggests that the application of methylene
blue dye is an examination technique worth using with mastoscopy.
The present study also suggested that non-lipolytic mastoscopy may
be performed without the need for an additional incision next to
the armpits and can result in good outcomes following surgery.
However, non-lipolytic endoscopic axillary surgery
also has shortcomings. Firstly, the operation is more difficult in
obese patients and those with large breasts, and may not be
suitable for patients with inner quadrant breast cancer. Secondly,
the number of cases evaluated in the present study was limited and
longer-term follow-up data are required. Thirdly, non-lipolytic
endoscopic cavity mirror technology increases the difficulty of the
surgery and the operation time, and requires expert surgeons. Prior
to developing this therapy, 210 cases of endoscopic thyroid
operation were performed in order to prepare for this technique
(24).
The present study introduced a novel practice for
breast-conserving surgery. SLNB and ALND may be performed without
other incisions. Non-lipolytic mastoscopic axillary surgery may be
a good choice for breast cancer therapy and may become part of the
repertoire of breast cancer treatment strategies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YC conceived the study, wrote and edited the
manuscript. SX edited the paper and made substantial contributions
to the conception of the study. JX was involved in the design,
conception, and data of the article and provided advice on its
revision. XZ and YL analyzed the data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Fujian Medical University
(Quanzhou, China). Written informed consent was obtained from all
patients.
Patient consent for publication
All participants gave their consent to the use of
personal data for scientific purposes.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guerra MR, Silva GA, Nogueira MC, Leite
IC, Oliveira RV, Cintra JR and Bustamante-Teixeira MT: Breast
cancer survival and health iniquities. Cad Saude Publica.
31:1673–1684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donninger H, Hobbing K, Schmidt ML,
Walters E, Rund L, Schook L and Clark GJ: A porcine model system of
BRCA1 driven breast cancer. Front Genet. 6:2692015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koroukian SM, Bakaki PM, Han X, Schluchter
M, Owusu C, Cooper GS and Flocke SA: Lasting Effects of the Breast
and Cervical Cancer Early Detection Program on Breast Cancer
Detection and Outcomes, Ohio, 2000-2009. Prev Chronic Dis.
12:E1162015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeichner SB, Herna S, Mani A, Ambros T,
Montero AJ, Mahtani RL, Ahn ER and Vogel CL: Survival of patients
with de-novo metastatic breast cancer: Analysis of data from a
large breast cancer-specific private practice, a university-based
cancer center and review of the literature. Breast Cancer Res
Treat. 153:617–624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giuliano AE, McCall L, Beitsch P,
Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M
and Ballman K: Locoregional recurrence after sentinel lymph node
dissection with or without axillary dissection in patients with
sentinel lymph node metastases: The American College of Surgeons
Oncology Group Z0011 randomized trial. Ann Surg. 252:426–432;
discussion 432-433. 2010.PubMed/NCBI
|
|
6
|
Kompatscher P: Endoscopic capsulotomy of
capsular contracture after breast augmentation: A very challenging
therapeutic approach. Plast Reconstr Surg. 90:1125–1126. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fine NA, Orgill DP and Pribaz JJ: Early
clinical experience in endoscopic-assisted muscle flap harvest. Ann
Plast Surg. 33:465–469; discussion 469-472. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salvat J, Knopf JF, Ayoubi JM, Slamani L,
Vincent-Genod A, Guilbert M and Walker D: Endoscopic exploration
and lymph node sampling of the axilla. Preliminary findings of a
randomized pilot study comparing clinical and anatomo-pathologic
results of endoscopic axillary lymph node sampling with traditional
surgical treatment. Eur J Obstet Gynecol Reprod Biol. 70:165–173.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kühn T, Santjohanser C, Koretz K, Böhm W
and Kreienberg R: Axilloscopy and endoscopic sentinel node
detection in breast cancer patients. Surg Endosc. 14:573–577. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo C, Guo W, Yang J, Sun Q, Wei W, Wu S,
Fang S, Zeng Q, Zhao Z, Meng F, et al: Comparison of mastoscopic
and conventional axillary lymph node dissection in breast cancer:
Long-term results from a randomized, multicenter trial. Mayo Clin
Proc. 87:1153–1161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brun JL, Rousseau E, Belleannée G, de
Mascarel A and Brun G: Axillary lymphadenectomy prepared by fat and
lymph node suction in breast cancer. Eur J Surg Oncol. 24:17–20.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Langer I, Kocher T, Guller U, Torhorst J,
Oertli D, Harder F and Zuber M: Long-term outcomes of breast cancer
patients after endoscopic axillary lymph node dissection: A
prospective analysis of 52 patients. Breast Cancer Res Treat.
90:85–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamprath S, Bechler J, Kühne-Heid R,
Krause N and Schneider A: Endoscopic axillary lymphadenectomy
without prior liposuction. Development of a technique and initial
experience. Surg Endosc. 13:1226–1229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mátrai Z, Kunos C, Pukancsik D, Sávolt A,
Gulyás G and Kásler M: Modern breast reconstruction with
endoscopically assisted latissimus dorsi flap harvesting. Orv
Hetil. 155:106–113. 2014.(In Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Veir Z, Dujmović A, Duduković M, Mijatović
D, Cvjeticanin B and Veir M: Endoscopically assisted latissimus
dorsi flap harvesting and breast reconstruction in young female
with Poland syndrome. Coll Antropol. 35:1303–1305. 2011.PubMed/NCBI
|
|
16
|
Chengyu L, Jian Z, Xiaoxin J, Hua L, Qi Y
and Chen G: Experience of a large series of mastoscopic axillary
lymph node dissection. J Surg Oncol. 98:89–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto D, Tsubota Y, Yoshida H,
Kanematsu S, Sueoka N, Uemura Y, Tanaka K and Kwon AH: Endoscopic
appearance and clinicopathological character of breast cancer.
Anticancer Res. 31:3517–3520. 2011.PubMed/NCBI
|
|
18
|
Tukenmez M, Ozden BC, Agcaoglu O, Kecer M,
Ozmen V, Muslumanoglu M and Igci A: Videoendoscopic single-port
nipple-sparing mastectomy and immediate reconstruction. J
Laparoendosc Adv Surg Tech A. 24:77–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu SD, Fan Y, Kong J and Yu H: Single
incision for quadrantectomy and laparoscopic axillary lymph node
dissection in the treatment of early breast cancer: Initial
experience of 5 cases. J Laparoendosc Adv Surg Tech A. 24:791–794.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gomatos IP, Filippakis G, Albanopoulos K,
Zografos G, Leandros E, Bramis J and Konstadoulakis MM: Complete
endoscopic axillary lymph node dissection without liposuction for
breast cancer: Initial experience and mid-term outcome. Surg
Laparosc Endosc Percutan Tech. 16:232–236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakamoto N, Fukuma E, Higa K, Ozaki S,
Sakamoto M, Abe S, Kurihara T and Tozaki M: Early results of an
endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg
Oncol. 16:3406–3413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malur S, Bechler J and Schneider A:
Endoscopic axillary lymphadenectomy without prior liposuction in
100 patients with invasive breast cancer. Surg Laparosc Endosc
Percutan Tech. 11:38–41; discussion 42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avisar E, Ikramuddin S and Edington H:
Thoracoscopic internal mammary sentinel node biopsy: An animal
model of a new technique. J Surg Res. 106:254–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu ST: Present situation and prospect of
endoscopic thyroid surgery. Chin J Endoscopic Surg. 9:80–82.
2016.
|