Introduction
Cancer is one of the leading causes of mortality in
a number of high-income countries (1). Prostate cancer (PCa) is the second most
common non-dermatological cancer in men (2–5). Based
on the 2018 GLOBOCAN estimates, the age-standardized incidence rate
was 29.3 per 100,000 standard population, with 1,276,106 new cases
registered worldwide (3). Prostate
cancer incidence rates are highly variable, with the highest
incidence rates having been reported in Oceania (79.1 per 100,000
standard population), followed by North America (73.7) and Europe
(62.1) (3,4). PCa is categorized as an
androgen-dependent neoplasia (3).
Thus, androgen deprivation therapy (ADT) is a commonly prescribed
treatment that decreases androgens, such as testosterone to
castration levels in an attempt to slow tumor progression and
improve overall survival time in men (6). Half of all men with PCa receive ADT at
some stage following diagnosis (7).
When PCa cells acquire the capacity to proliferate without
androgens, ADT becomes ineffective and the transition is termed
castration-resistant disease (CRD) (8). Bone metastases are present in 90% of
patients with CRD and can produce significant morbidity, including
pain, pathological fractures, spinal cord compression and bone
marrow failure (9). Paraneoplastic
effects due to bone metastases in patients with CRD are also
common, such as anemia, weight loss, fatigue, hypercoagulability
and increased susceptibility to infection (9).
Radiotherapy and/or chemotherapy are treatment
options that can increase the life expectancy of patients with
CRD-PCa (10–12); however, they are aggressive
treatments that decrease physical independence and can increase
weight loss (13–18). Mortality in patients with CRD-PCa has
been reported at a median follow-up period of 41 months, generally
with a poor quality of life during the last months of life
(19). Certain patients with CRD
possess adequate knowledge of disease prognosis and the associated
consequences, and thus decline the standard treatment and adopt an
approach of only taking symptomatological treatment. In these
instances, patient autonomy prevails, defined as having the ability
to make a rational decision based on the personal understanding of
his or her future, and supported by his or her own values (20). The healthcare provider is obligated
to respect patient autonomy, if the law does not dictate
otherwise.
New treatments, such as those with abiraterone or
enzalutamide have shown therapeutic success in metastatic CRD-PCa,
although this remains limited (21).
Therefore, complementary treatments can still be researched, which
can increase the antitumor effect of the implemented therapies or
can provide an alternative for patients that are not candidates for
conventional therapies (22,23). Anti-inflammatory agents are currently
being investigated as a treatment option in different types of
neoplasia, such as lung, cervix, ovarian, colon and gastric cancer
(24). Inflammation is observed in
numerous pathologies, and the current available data demonstrate
that it is a critical component in the origin, proliferation and
dissemination of different types of cancer, including PCa (25). In PCa, there is evidence of
inflammation in the processes of DNA damage, tumor progression and
tumor expansion. Hence, sustained use of nonsteroidal
anti-inflammatory drugs (NSAIDs) have been proposed as a mechanism
that may retard PCa disease progression by decreasing the
inflammatory response in PCa cells (25). Observational studies have revealed
that NSAIDs are associated with a lower risk of developing PCa
(25) and a lower risk of
progression to high-grade PCa (26,27),
resulting in different NSAIDs being postulated for the treatment of
PCa. Clinical trials have been performed with certain NSAIDs
(celecoxib, ibuprofen and indomethacin) (5,28), with
unsatisfactory results, upon analyzing endpoints such as PSA
levels, tumor size or overall survival time (29,30).
Preclinical in vitro and in vivo
(xenograft nude mouse model) studies in PCa have demonstrated that
the fenamate NSAIDs have a more notable antineoplastic effect
compared with previously examined NSAIDs in PCa (31). Mefenamic acid and meclofenamate
demonstrate this type of antitumor effect (31). Notably, in a preclinical study,
mefenamic acid, a freely sold NSAID whose everyday use is for
dysmenorrhea, had a cytotoxic effect on PCa cells at concentrations
that can be feasibly achieved in human plasma (31). To the best of our knowledge, the
antineoplastic use of a fenamate in humans has not yet been
investigated due to advanced tumor stages of PCa, higher PSA levels
and weight loss being associated with poor quality of life in
patients (32,33). The aforementioned variables provide
the rationale for the evaluation of the usefulness of new treatment
options in PCa. In the present study the therapeutic effects of
mefenamic acid on PSA levels, weight loss and quality of life were
investigated in patients with CRD-PCa, who were either not
candidates for standard therapy or had declined it.
Patients and methods
Study design
A prospective, double-blinded, 2-arm, controlled,
randomized phase II–III clinical trial was conducted between August
2017 and March 2019. The study was performed according to the
CONSORT statement guidelines for randomized controlled trials
(34).
The National Commission on Scientific Research
(Central Ethics Committee) of the Mexican Social Security Institute
(IMSS; Colima, Mexico) approved the present study. Written informed
consent was obtained from all participants. The present clinical
trial was registered as MEFEPROST: RPCEC00000248 in the Cuban
Public Registry of Clinical Trials (RPCEC) Database (http://rpcec.sld.cu). The RPCEC trial registration
dataset is part of the International Clinical Trials Platform
Registry database, as established by the World Health Organization
and the International Committee of Medical Journal Editors.
Study subjects
A total of 46 subjects for the present clinical
trial were recruited from the General Hospital Zone 1 of the IMSS
and the Cancerology State Institute of the Health Department of the
State of Colima (Colima, Mexico).
The following inclusion criteria were used in the
present study: Male patients of any age with a histological
diagnosis of prostate cancer; patients presenting with CRD
according to the Prostate Cancer Clinical Trial Working Group 3
(35), who by their own decision or
the clinical opinion of their treating physician, were not
candidates for taxane chemotherapy or any other standard first-line
treatment for that type of patient; patients whose PSA levels were
at stages 1–3 of the D'Amico Risk Classification (1–100 ng/ml)
(36); patients undergoing ADT prior
to recruitment that was maintained under the treating physician's
judgment, during the 6 months of follow-up; patients with an
Eastern Cooperative Oncology Status functional status of 0–2
(37) and patients with no history
of hepatic impairment (any of the Child-Pugh classification stages)
(38) or renal impairment with
creatinine clearance >60 ml/min.
The following exclusion criteria were used in the
present study: Diagnosis of a second primary cancer; uncontrolled
diabetes or high blood pressure; leukocytes <3,000 cells/µl, or
a platelet count <10,000 cells/µl; leukocytes >100,000
cells/µl or evidence of systemic infection according to the Third
International Consensus Definitions for Sepsis and Septic Shock
(Sepsis-3) (39); blood hemoglobin
<9 g/dl; alcoholism and/or drug addiction; gastrointestinal
ulcer; inflammatory bowel disease; diagnosis of ischemic heart
disease; chronic heart failure and other pathologies at the
discretion of the researcher.
The following elimination criteria were used in the
present study: Patients that voluntarily abandoned the study;
patients that, at some point during the study, presented with
severe toxicity (grade ≥3) (40),
according to the common terminology criteria for adverse events
(CTCAE v4.0; U.S. Department of Health and Human Services)
attributable to the administration of the experimental medication
(mefenamic acid); and patients in whom the treating physician
suspended the experimental medication for >2 weeks, regardless
of the origin of the adverse event.
Following the application of all the inclusion,
exclusion and elimination criteria, 20 patients (57–81 years) were
randomized for the present clinical trial. The 6-month intervention
consisted of two delivery arms, one with patients receiving
mefenamic acid (n=10) and the other with patients receiving placebo
(n=10). All patients continued to receive ADT, through the
administration of gonadotropin-releasing hormone agonists
(leuprolide and goserelin), oral antiandrogens (flutamide and
bicalutamide) or through bilateral orchiectomy. The two study
groups consisted of one group that took a 500 mg pill of mefenamic
acid every 12 h for 6 months, and another group that took a sugar
placebo pill every 12 h for the same length of time. The pills were
recommended to be taken with meals or milk in order to decrease the
risk of adverse gastrointestinal events. All patients took one
tablet of 20 mg omeprazole daily during the study period to prevent
severe acute NSAID-associated gastroduodenal damage.
The treating physician was blinded to the study
group the patient belonged to and could prescribe additional
treatment if necessary (usual medical care), including radiotherapy
for symptom palliation (41).
Outcome measures and patient
follow-up
Outcome measures of the present clinical trial were
determined, and the primary endpoint was a clinically significant
variation in PSA levels in patient blood samples at 6 months. The
variation percentage was calculated and the number of patients that
had biochemical disease progression was determined through an
increase in PSA levels of ≥25%, in accordance with the criteria of
the Prostate Cancer Clinical Trials Working Group 3. The same was
done with respect to the number of patients that had a biochemical
therapeutic response defined as a ≥50% decrease in PSA levels
(34). Other endpoints of the
present trial were the variations in the quality of life score
(through the EQ-5D-5L questionnaire) and body mass index (BMI)
(42). The previously validated
Spanish version of the EQ-5D-5L questionnaire was used in the
present study, which evaluates 5 general domains, each one with a
score ranging from 0–4 (with a lower score indicating better
quality of life) (43). Complete
blood count (red and white blood cells), hemoglobin, hematocrit,
platelets, kidney (serum creatinine, blood urea nitrogen-BUN-, uric
acid) and liver function (albumin, bilirubin, alanine
aminotransferase, aspartate aminotransferase, gamma-glutamyl
transferase, alkaline phosphatase and lactate dehydrogenase) serum
test were monitored in all patients.
Blinding
The researchers who evaluated treatment
effectiveness and performed the statistical analyses were blinded
to the treatment that the patients received, as were the
patients.
Sample size
The sample size calculation was based on the number
of treated patients that had biochemical disease progression (a 25%
increase in PSA levels), which was stipulated at 39% and was
performed using ClinCalc online software (version 1; http://clincalc.com/stats/samplesize.aspx). This was
based on a previous study on patients with CRD-PCa treated with
docetaxel (44). As a comparison
figure, it was stipulated that 95% of the patients with no
treatment would present with biochemical disease progression. A
total of 10 patients were needed in each arm of the clinical trial
to reach the required power (0.8) when the sample size was
calculated, using the one-tailed α (0.05). At the end of the study,
the statistical power for detecting a difference between the 2 arms
of the study was calculated (α=0.05) using the number of patients
with disease progression at 6 months in the mefenamic acid group
and the placebo group, and the result was 100%.
Statistical analysis
The data are presented as percentages or mean ±
standard error or standard deviation. For inferential statistics,
normal data distribution was first determined using the
Kolmogorov-Smirnov test and the equality of variances was confirmed
using the Levene's test. A paired Student's t-test was employed to
compare the numerical variables (with normal distribution) between
the 2 groups (mefenamic acid and placebo). The categorical values
were compared using the Fisher's exact test or χ2 test.
The relative risk (RR), number needed to treat (NNT) and 95%
confidence interval (CI) were calculated to determine the
probability of not having disease progression (an increase in serum
PSA levels ≥25%), comparing the mefenamic acid group vs. the
placebo group. As the sample size was small, the Laplace/De Morgan
correction was employed for the risk analysis, in which 1 was added
to each cell of the 2×2 contingency table (45). The statistical analysis was performed
using SPSS 20.0 (IBM Corp), with the exception of the RR and NNT,
which were calculated using MedCalc v17.7.2 (MedCalc Software
bvba). Sample size and the post-hoc power analysis were calculated
using ClinCalc online software. One-tailed P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical trial flow-process
Of the 46 CRD-PCa patients screened, 20 were
randomized into two different study groups, with 10 patients in
each group: 10 patients in the mefenamic acid group and 10 patients
in the placebo control group. All 20 patients completed the trial
(Fig. 1). The clinical
characteristics and treatment procedures of the patients are
presented in Table I. The results
demonstrate that there is no significant difference between the
groups, which is the starting point for treatment.
 | Table I.Distribution of the main clinical
characteristics and treatment procedures of study subjects. |
Table I.
Distribution of the main clinical
characteristics and treatment procedures of study subjects.
| Clinical
characteristics | Mefenamic acid
group | Placebo group | P-value |
|---|
| Number of
patients | 10 | 10 |
|
| Age, years (Mean ±
standard deviation) | 71.88±9.42 | 67.44±5.50 | 0.240 |
| Clinical stage |
|
| 0.370 |
|
IIIA | 40% | 30% |
|
|
IIIB | 20% | 0% |
|
|
IIIC | 10% | 20% |
|
|
IVB | 30% | 50% |
|
| Diabetes
mellitus | 20% | 40% | 0.437 |
| High blood
pressure | 70% | 50% | 0.335 |
| Statins | 30% | 20% | 0.563 |
| Hyperlipidemia | 20% | 30% | 0.437 |
| Cardiovascular
diseases | 50% | 40% | 0.581 |
| Antidiabetics | 20% | 40% | 0.437 |
| Antiplatelets | 0% | 20% | 0.206 |
| Anticoagulants | 10% | 20% | 0.735 |
|
Antihypertensives | 70% | 50% | 0.335 |
| Depression | 10% | 10% | 0.735 |
| Treatments |
|
|
|
| Radical
prostatectomy | 30% | 10% | 0.335 |
|
Radiotherapy | 10% | 10% | 0.735 |
| Other
NSAIDs | 0% | 30% | 0.082 |
|
Surgical castration | 10% | 0% | 0.563 |
| During the
study |
|
|
|
| Radical
prostatectomy | 0% | 0% | NA |
|
Radiotherapy | 10% | 10% | 0.735 |
|
Chemotherapy | 0% | 0% | NA |
| Other
NSAIDs | 30% | 0% | 0.175 |
|
Gastritis | 30% | 10% | 0.400 |
Comparison of the PSA levels in
patients treated with mefenamic acid compared with those treated
with placebo
Table II presents a
comparison between groups for the following variables, PSA, BMI and
quality of life. Before and after comparisons between baseline and
after 6 months for the same group, for each variable, where
evaluated, in order to determine the effects of each treatment.
While comparing the percentage change per patient, there was a
significant decrease in the PSA levels at 6 months of treatment
with mefenamic acid (mean decrease of 41.9±35.8%), whereas there
was a mean increase in PSA levels in the placebo group of
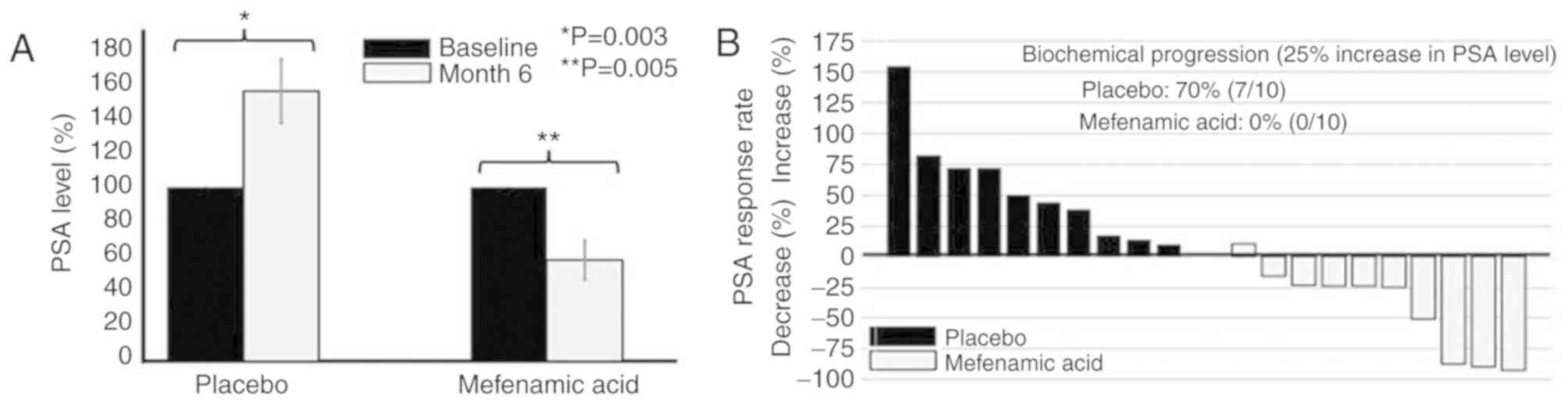
55.4±43.1% (Fig. 2A). Notably, 70%
of the patients in the placebo group exhibited biochemical disease
progression (an increase of ≥25% in PSA levels), but this did not
occur in any patients treated with mefenamic acid (Fig. 2B).
 | Table II.Comparison of body mass index,
prostate specific antigen and quality of life (EQ-5D-5L) scores
within and between patients in the placebo and mefenamic acid
groups. |
Table II.
Comparison of body mass index,
prostate specific antigen and quality of life (EQ-5D-5L) scores
within and between patients in the placebo and mefenamic acid
groups.
|
| Time |
|
|---|
|
|
|
|
|---|
| Parameters per
group | Baseline (Mean ±
standard deviation) | 6 months (Mean ±
standard deviation) | bP-value |
|---|
| Prostate-specific
antigen, ng/ml |
|
|
|
|
Placebo | 10.15±7.17
ng/ml | 17.18±13.04
ng/ml | 0.012 |
|
Mefenamic acid | 7.00±7.80
ng/ml | 5.38±7.80
ng/ml | 0.018 |
| aP-value | 0.383 | 0.024 |
|
| Body Mass
Index |
|
|
|
|
Placebo | 27.70±1.87 | 27.70±3.63 | 0.898 |
|
Mefenamic acid | 30.33±4.79 | 32.50±5.75 | 0.064 |
| aP-value | 0.112 | 0.038 |
|
| EQ-5D-5L score |
|
|
|
|
Placebo | 6.66±2.05 | 5.38±0.75 | 0.108 |
|
Mefenamic acid | 7.33±2.00 | 5.66±0.66 | 0.015 |
| aP-value | 0.513 | 0.422 |
|
BMI and quality of life changes in the
patients treated with mefenamic acid compared with those treated
with placebo
Patients receiving placebo exhibited no changes in
their BMI when the baseline and end of trial values were compared
(P=0.898; Table II). In contrast,
patients in the mefenamic acid arm of the trial had an increased
BMI; however, this result was not significant (P=0.064; Table II). A statistically significant
difference was observed between the BMI of the patients in the
mefenamic acid and placebo groups on completion of the trial
(P=0.038; Table II). Quality of
life was evaluated using the EQ-5D-5L score, in which a lower score
denotes better quality of life. The patients treated with mefenamic
acid had a significantly improved quality of life at the end of the
study (P=0.015; Table II). The
patients treated with placebo had no significant changes in their
quality of life at the end of the study (P=0.108; Table II).
Effects of mefenamic acid on disease
progression and therapeutic response in patients with CRD-PCa
The NNT with mefenamic acid to prevent a patient
with CRD-PCa and no chemotherapy from presenting with disease
progression was 1.71 (Table III).
In addition, mefenamic acid administration significantly decreased
the probability of biochemical disease progression at 6 months by
88% compared with the placebo group (RR=0.1250; 95% CI,
0.0183–0.8515; P=0.0337; Table
III). Even though there was a therapeutic response (a decrease
in PSA levels of ≥50%) in four patients (40%) with the
administration of mefenamic acid, the result was not statistically
significant when compared with the placebo group, which had a 0%
therapeutic response (P=0.081; Table
III).
 | Table III.Association between mefenamic acid
treatment and therapeutic effect. |
Table III.
Association between mefenamic acid
treatment and therapeutic effect.
| Parameters of
therapeutic effect | Mefenamic acid
group (%) | Placebo group
(%) | RR (95% CI) | NNT | P-value |
|---|
| Response | 40 | 0 | 0.63
(0.38–1.05) | 3.00 | 0.081 |
| Progression | 0 | 70 | 0.12
(0.01–0.85) | 1.71 | 0.033 |
Tolerance of clinical trial
Regarding the adverse effects that were potentially
associated with the experimental medication, three (30.0%) patients
presented with abdominal pain/discomfort (gastritis) corresponding
to grades 1 and 2 from the CTCAE (40), which is a clinical scale used in
cancer trials by clinicians from the National Cancer Institute's
based upon symptomatic adverse events at some point during the
follow-up, but temporary suspension of the drug (2 weeks) was
required in only one of the patients. Gastric symptoms ceased on
insistence of patients taking the medication with meals. No
pathological alterations were observed in the complete blood count
or in the kidney and liver function tests of the patients.
Experimental treatment was not definitely suspended due to adverse
effects in any of the patients.
Discussion
The present study analyzed the effects of mefenamic
acid administration for 6 months in patients with CRD-PCa by
monitoring tumor progression and quality of life markers. There was
a statistically significant 42% decrease in serum PSA level in the
group treated with mefenamic acid compared with the placebo group.
In addition, there was an adequate therapeutic response (PSA level
decrease of ≥50%) in 40% of the patients treated with mefenamic
acid. Mefenamic acid also prevented biochemical disease
progression.
The percentage of patients treated with mefenamic
acid that had a therapeutic biochemical response (40%) was similar
to that of treatment with abiraterone (46,47) or
docetaxel (48). Patients in the
present study received abiraterone and docetaxel as part of their
normal medical care. The effect of mefenamic acid on biochemical
response in the present study was not statistically significant,
which maybe attributable to the small sample size of the present
study. On the other hand, in the present study, treatment with
mefenamic acid significantly prevented biochemical disease
progression in patients with CRD-PCa. Mefenamic acid was
well-tolerated and no serious adverse effects were reported in the
current study, unlike chemotherapy and radiotherapy that can result
in considerable adverse effects (49). The abandonment or temporary
suspension of treatment with abiraterone and/or chemotherapy is
often caused by the presence of adverse effects (50,51).
Patients with CRD-PCa do not adequately tolerate conventional
treatment regimens due to their clinical condition (52,53).
Therefore, the results of the present study pose a benefit and
potential alternative therapeutic option for patients with
CRD-PCa.
Preclinical and clinical trials have demonstrated
that the administration of certain NSAIDs, such as celecoxib does
not produce a therapeutic effect (54,55).
However, there are reports stating that chronic aspirin consumption
lowered PSA levels in patients by 5–10% at the time of PCa
diagnosis, compared with patients that did not take aspirin
(25,27,29). To
the best of our knowledge, the mechanism by which aspirin decreases
PSA levels at the time of diagnosis has not yet been determined,
nor has whether that effect is associated with disease progression
(56–58). Notably, in the present clinical
trial, mefenamic acid was demonstrated to decrease PSA levels when
administered to patients with CRD-PCa. Fenamate NSAIDs have been
reported to decrease tumor size and favor apoptosis of PCa cells in
in vitro and in vivo models with Foxn1nu mouse strain
(31). Different regulatory
mechanisms for cell proliferation and their role in cancer have
been proposed for mefenamic acid. Previous studies have
demonstrated that mefenamic acid is an inhibitor of cyclooxygenase
1(COX-1) and cyclooxygenase 2 (COX-2) isoforms; COX-2 inhibition
leads to matriptase inhibition. Matriptase is an enzyme that is
responsible for the extent of extracellular matrix degradation.
According to a report by Ko et al (59), Cox-2 inhibition hinders PCa cell
migration in culture by inhibiting the action of matriptase. In
addition, the aforementioned study reported that Cox-2 inhibition
produces androgen receptor (AR) inhibition. The AR is vital in the
production of prostaglandins, such as prostaglandin e2 (PGE2). At
the same time, PGE2 is an autocrine and paracrine lipid signal
inducer that functions by binding to the rhodopsin family of
G-protein coupled receptors. PGE2 can contribute to tumor
development by promoting cell survival, angiogenesis and motility
(51).
In addition, mefenamic acid has been demonstrated to
induce apoptosis in human cancer cell lines through the caspase-3
pathway (60). Mefenamic acid is
also a very potent aldo-keto reductase (AKR) inhibitor (61). AKR enzymes may contribute to the
growth of certain types of cancer and their inhibition,
particularly of AKR family 1 member C3 (AKR1C3), which potentially
exhibits antineoplastic effects (62). Relatively high AKR1C3 mRNA expression
was observed in human prostate and mammary glands, where it was
involved in regulating ligand access to the androgen and estrogen
receptors. AKR1C3 is an interesting target for the development of
therapeutic agents for hormone-dependent forms of cancer, such as
prostate cancer, breast cancer and endometrial cancer. NSAIDs,
specifically indomethacin, celecoxib and fenamates, have been
reported as potent inhibitors (63,64).
Thus, the inhibitory effect of mefenamic acid on AKR1C3 is proposed
to be one of its main antineoplastic mechanisms (31). To the best of our knowledge, no
previous studies have yet evaluated the antineoplastic effect of
mefenamic acid in humans.
Furthermore, patients treated with mefenamic acid
had an increased BMI based on the results of the present study and
a significant difference was observed between the mefenamic acid
and placebo groups. To the best of our knowledge, there are no
previous reports that demonstrate that NSAIDs modify body weight.
However, this is the first time the drug has been administered for
a prolonged period of time. Mefenamic acid has been postulated to
significantly increase both carbohydrate absorption and
postprandial metabolism in the intestine, through an increase in
intestinal blood flow and oxygen consumption (65), which could have been the cause of the
weight gain observed in patients in the treatment arm of the
present study. Future studies are required in order to confirm the
aforementioned results.
There were no changes in quality of life between the
placebo and mefenamic acid groups, upon comparing the 5Q-5D-5L
scores. Nevertheless, when analyzing the intragroup quality of life
scores, only the mefenamic group showed improvement in relation to
the baseline scores. This improvement in the mefenamic group could
be explained by the treatment with NSAIDS and the consequent
decrease in baseline pain levels. According to a recent study, a
decrease in pain levels is associated with an improvement in
quality of life (66). However, in
the present study, there was no statistically significant
difference between the baseline and final pain levels measured by
the EQ-5D-5L, which is why the effect observed was perhaps not due
to a decrease in pain level. On the other hand, there is an
association between PSA levels, weight loss and quality of life in
patients with prostate cancer (67–69).
Therefore, quality of life improvement can be explained by the
decrease in PSA levels and the increase in BMI. BMI maintenance is
important in patients with cancer. Men with PCa and a BMI <22.5
are at greater risk for cancer-specific death (70), and general mortality is twice as
great with a body weight loss of more than 5% in patients with a
BMI <22.5 (19). Weight loss is
the main component of cachexia due to cancer and acts as an
indicator of negative energy balance and a pro-inflammatory state
(71). The latter decreases the
efficacy of antitumor treatment (67). Improvement in quality of life and BMI
with NSAID use has been reported in a previous clinical trial
(28), concurring with the results
of the present study.
Notably, the dose of mefenamic acid administered in
the present study (1 g oral dose/day) was lower than the maximum
daily recommended dose (1.5 g/day). This lower dose was selected
for patients who consumed the drug for a prolonged period of time.
There were no serious adverse events reported in the present study
due to drug ingestion. Even though no kidney function alterations
occurred in patients treated with mefenamic acid, prolonged NSAID
use can cause kidney damage (49).
This was one of the reasons the decision was made not to administer
the drug longer than 6 months.
Another relevant observation is that only patients
with PSA levels <100 ng/ml were evaluated in the present study
and results may be different in patients with higher PSA levels.
Future studies investigating patients with PCa in early clinical
stages or hormone-sensitive cancer would be of interest. In
preclinical trials, mefenamic acid has been reported to increase
the sensitivity of certain types of cancer to chemotherapy and
radiotherapy, including colon cancer and lung adenocarcinoma
(72). Therefore, future clinical
trials to investigate the effect of mefenamic acid in combination
with other therapies in patients with PCa are required. PSA
kinetics, which is a bone metastasis and survival predictor, was
not investigated in the present study (73). However, certain studies have
demonstrated contradictory results in PSA kinetics secondary to
antineoplastic drug mechanisms (74,75). PSA
values at baseline and at 6 months of treatment were investigated
for the mefenamic acid and placebo groups in the present study.
According to the criteria of the Prostate Cancer Clinical Trials
Working Group 3, this is a useful measurement to determine
therapeutic efficacy and tumor progression in clinical trials
(34,76).
The present study had limitations, such as small
sample size and the length of follow-up. Future studies with a
higher number of patients evaluated for a longer period of time
with strict monitoring of adverse effects are required in order to
confirm the results of the present study.
In conclusion, mefenamic acid administration
decreased biochemical progression, increased BMI and improved
quality of life in patients with CRD-PCa. Future studies with a
higher number of patients investigating the effects of mefenamic
acid in combination with other therapies and at different clinical
stages of PCa disease, are needed to evaluate its therapeutic
potential.
Acknowledgements
The authors would like to thank postgraduate
students Dr. Carlos E. Barajas-Saucedo and Dr. Mario Alberto Gaitan
Hinojosa from the Clinical Analysis Laboratory, Faculty of Chemical
Sciences, University of Colima (Coquimatlán, México), for their
assistance in inputting clinical data in different platforms for
analysis.
Funding
The present study was funded by the
2016-FOSISS-CONACYT (grant no. 272792).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
IDE, JGE, JDE and IRS designed the present study,
performed the analyses and drafted the initial manuscript. DTJ,
SZF, OAZ, MMH, JCP, ABA, LBR, LLZ and JPF participated in the
clinical evaluation of the patients. MMF, DTJ and CMR performed the
statistical analyses. JDE was the clinical trial administrative
coordinator. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the National Scientific
Commission of the Mexican Social Security Institute (Central Ethics
Committee) (R-2018-785-058), and all patients agreed and signed an
informed consent form to participate in the study. Patient
anonymity was guaranteed in the study. All procedures performed in
this protocol were in accordance with The Declaration of Helsinki.
The present clinical trial was registered as MEFEPROST:
RPCEC00000248 in the Cuban Public Registry of Clinical Trials
Database.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hassanipour-Azgomi S,
Mohammadian-Hafshejani A, Ghoncheh M, Towhidi F, Jamehshorani S and
Salehiniya H: Incidence and mortality of prostate cancer and their
relationship with the Human Development Index worldwide. Prostate
Int. 4:118–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Afriansyah A, Hamid ARAH, Mochtar CA and
Umbas R: Prostate specific antigen (PSA) kinetic as a prognostic
factor in metastatic prostate cancer receiving androgen deprivation
therapy: Systematic review and meta-analysis. F1000Res. 7:2462018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rawla P: Epidemiology of prostate cancer.
World J Oncol. 10:63–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taitt HE: Global Trends and Prostate
cancer: A review of incidence, detection, and mortality as
influenced by race, ethnicity, and geographic location. Am J Men's
Health. 12:1807–1823. 2018. View Article : Google Scholar
|
|
5
|
Adhyam M and Gupta AK: A review on the
clinical utility of PSA in cancer prostate. Indian J Surg Oncol.
3:120–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mundell NL, Daly RM, Macpherson H and
Fraser SF: Cognitive decline in prostate cancer patients undergoing
ADT: A potential role for exercise training. Endocr Relat Cancer.
24:R145–R155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McHugh DJ, Root JC, Nelson CJ and Morris
MJ: Androgen-deprivation therapy, dementia, and cognitive
dysfunction in men with prostate cancer: How much smoke and how
much fire? Cancer. 124:1326–1334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maughan BL, Antonarakis ES and Hopkins
Sidney Kimmel J: Androgen pathway resistance in prostate cancer and
therapeutic implications HHS Public Access. Expert Opin
Pharmacother. 16:1521–1537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hotte SJ and Saad F: Current management of
castrate-resistant prostate cancer. Curr Oncol. 17 (Suppl
2):S72–S79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nader R, El Amm J and Aragon-Ching JB:
Role of chemotherapy in prostate cancer. Asian J Androl.
20:221–229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sichero L and Villa LL: Epidemiological
and functional implications of molecular variants of human
papillomavirus. Brazilian J Med Biol Res. 39:707–717. 2006.
View Article : Google Scholar
|
|
12
|
Handy CE and Antonarakis ES: Sequencing
treatment for castration-resistant prostate cancer. Curr Treat
Options Oncol. 17:642016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vanagas G, Mickevičienė A and Ulys A: Does
quality of life of prostate cancer patients differ by stage and
treatment? Scand J Public Health. 41:58–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farris MS, Kopciuk KA, Courneya KS,
McGregor SE, Wang Q and Friedenreich CM: Identification and
prediction of health-related quality of life trajectories after a
prostate cancer diagnosis. Int J Cancer. 140:1517–1527. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Charalambous A and Kouta C: Cancer related
fatigue and quality of life in patients with advanced prostate
cancer undergoing chemotherapy. Biomed Res Int. 2016:39892862016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dun YJ, Liu HX, Yu LP, Li Q, Zhang XW,
Tang X, Qin CP and Xu T: Development and initial validation of the
novel scale for assessing quality of life of prostate cancer
patients receiving androgen deprivation therapy. Chin Med J (Engl).
130:2082–2087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chambers SK, Ng SK, Baade P, Aitken JF,
Hyde MK, Wittert G, Frydenberg M and Dunn J: Trajectories of
quality of life, life satisfaction, and psychological adjustment
after prostate cancer. Psychooncology. 26:1576–1585. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang JW, Drumm MR, Efstathiou JA, Paly JJ,
Niemierko A, Ancukiewicz M, Talcott JA, Clark JA and Zietman AL:
Long-term quality of life after definitive treatment for prostate
cancer: Patient-reported outcomes in the second posttreatment
decade. Cancer Med. 6:1827–1836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Griffin K, Csizmadi I, Howard LE, Pomann
GM, Aronson WJ, Kane CJ, Amling CL, Cooperberg MR, Terris MK,
Beebe-Dimmer J and Freedland SJ: First-year weight loss with
androgen-deprivation therapy increases risks of prostate cancer
progression and prostate cancer-specific mortality: Results from
SEARCH. Cancer Causes Control. 30:259–269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valente SM: End-of-life challenges:
Honoring autonomy. Cancer Nurs. 27:314–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Attard G, Borre M, Gurney H, Loriot Y,
Andresen-Daniil C, Kalleda R, Pham T and Taplin ME; PLATO
collaborators, : Abiraterone alone or in combination with
enzalutamide in metastatic castration-resistant prostate cancer
with rising prostate-specific antigen during enzalutamide
treatment. J Clin Oncol. 36:2639–2646. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haberkorn U, Eder M, Kopka K, Babich JW
and Eisenhut M: New strategies in prostate cancer:
Prostate-specific membrane antigen (PSMA) ligands for diagnosis and
therapy. Clin Cancer Res. 22:9–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sumanasuriya S and De Bono J: Treatment of
advanced prostate cancer-a review of current therapies and future
promise. Cold Spring Harb Perspect Med. 8:a0306352018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yiannakopoulou E: Targeting epigenetic
mechanisms and microRNAs by aspirin and other non steroidal
anti-inflammatory agents-Implications for cancer treatment and
chemoprevention. Cell Oncol (Dordr). 37:167–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Y and Brusselaers N: Maintenance use of
aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) and
prostate cancer risk. Prostate Cancer Prostatic Dis. 21:147–152.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Algotar AM, Behnejad R, Stratton MS and
Stratton SP: Chronic use of NSAIDs and/or statins does not affect
PSA or PSA velocity in men at high risk for prostate cancer. Cancer
Epidemiol Biomarkers Prev. 23:2196–2198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manley G: Aspirin, NSAID and risk of
prostate cancer: Results from the REDUCE study. Clin Cancer Res.
71:233–236. 2013.
|
|
28
|
Solheim TS, Fearon KC, Blum D and Kaasa S:
Non-steroidal anti-inflammatory treatment in cancer cachexia: A
systematic literature review. Acta Oncol. 52:6–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Veitonmäki T, Murtola TJ, Talala K, Taari
K, Tammela T and Auvinen A: Non-steroidal anti-inflammatory drugs
and cancer death in the finnish prostate cancer screening trial.
PLoS One. 11:e01534132016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flamiatos JF, Beer TM, Graff JN, Eilers
KM, Tian W, Sekhon HS and Garzotto M: Cyclooxygenase-2 (COX-2)
inhibition for prostate cancer chemoprevention: double-blind
randomised study of pre-prostatectomy celecoxib or placebo. BJU
Int. 119:709–716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soriano-Hernández AD, Galvan-Salazar HR,
Montes-Galindo DA, Rodriguez-Hernandez A, Martinez-Martinez R,
Guzman-Esquivel J, Valdez-Velazquez LL, Baltazar-Rodriguez LM,
Espinoza-Gómez F, Rojas-Martinez A, et al: Antitumor effect of
meclofenamic acid on human androgen-independent prostate cancer: A
preclinical evaluation. Int Urol Nephrol. 44:471–477. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonn SE, Wiklund F, Sjölander A, Szulkin
R, Stattin P, Holmberg E, Grönberg H and Bälter K: Body mass index
and weight change in men with prostate cancer: Progression and
mortality. Cancer Causes Control. 25:933–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jackson SE, Heinrich M, Beeken RJ and
Wardle J: Weight loss and mortality in overweight and obese cancer
survivors: A systematic review. PLoS One. 12:1–21. 2017. View Article : Google Scholar
|
|
34
|
Pandis N, Chung B, Scherer RW, Elbourne D
and Altman DG: CONSORT 2010 statement: Extension checklist for
reporting within person randomised trials. BMJ. 357:j28352017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scher HI, Morris MJ, Stadler WM, Higano C,
Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, et
al: Trial design and objectives for castration-resistant prostate
cancer: Updated recommendations from the prostate cancer clinical
trials working Group 3. J Clin Oncol. 34:1402–1418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hernandez DJ, Nielsen ME, Han M and Partin
AW: Contemporary evaluation of the D'amico risk classification of
prostate cancer. Urology. 70:931–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kok B and Abraldes J: Child-pugh
classification: Time to abandon? Semin Liver Dis. 39:96–103. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). Jama.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dueck AC, Mendoza TR, Mitchell SA, Reeve
BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM,
O'Mara AM, et al: Validity and reliability of the US national
cancer institute's patient-reported outcomes version of the common
terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol.
1:1051–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lutz S, Berk L, Chang E, Chow E, Hahn C,
Hoskin P, Howell D, Konski A, Kachnic L, Lo S, et al: Palliative
radiotherapy for bone metastases: An ASTRO evidence-based
guideline. Int J Radiat Oncol Biol Phys. 79:965–976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blanchard CM, Stein K and Courneya KS:
Body mass index, physical activity, and health-related quality of
life in cancer survivors. Med Sci Sports Exerc. 42:665–671. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hernandez G, Garin O, Pardo Y, Vilagut G,
Pont À, Suárez M, Neira M, Rajmil L, Gorostiza I, Ramallo-Fariña Y,
et al: Validity of the EQ-5D-5L and reference norms for the Spanish
population. Qual Life Res. 27:2337–2348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhao J, Shen P, Sun G, Chen N, Liu J, Tang
X, Huang R, Cai D, Gong J, Zhang X, et al: The prognostic
implication of intraductal carcinoma of the prostate in metastatic
castration-resistant prostate cancer and its potential predictive
value in those treated with docetaxel or abiraterone as first-line
therapy. Oncotarget. 8:55374–83. 2017.PubMed/NCBI
|
|
45
|
Greenland S: Small-sample bias and
corrections for conditional maximum-likelihood odds-ratio
estimators. Biostatistics. 1:113–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang LW, Hung SC, Wang SS, Li JR, Yang
CK, Chen CS, Ho HC, Cheng CL and Ou YC CK: Abiraterone acetate and
enzalutamide: Similar efficacy in treating post docetaxel
metastatic castration-resistant prostate cancer: Single center
experience. Anticancer Res. 39:3901–3908. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yamashita S, Kohjimoto Y, Iguchi T, Koike
H, Kusumoto H, Iba A, Kikkawa K, Kodama Y, Matsumura N and Hara I:
Prognostic factors and risk stratification in patients with
castration-resistant prostate cancer receiving docetaxel-based
chemotherapy. BMC Urol. 16:132016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Harirforoosh S, Asghar W and Jamali F:
Adverse effects of nonsteroidal antiinflammatory drugs: An update
of gastrointestinal, cardiovascular and renal complications. J
Pharm Pharm Sci. 16:821–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mimeault M, Johansson SL, Henichart JP,
Depreux P and Batra SK: Cytotoxic effects induced by docetaxel,
gefitinib, and cyclopamine on side population and nonside
population cell fractions from human invasive prostate cancer
cells. Mol Cancer Ther. 9:617–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baker J, Ajani J, Scotté F, Winther D,
Martin M, Aapro MS and von Minckwitz G: Docetaxel-related side
effects and their management. Eur J Oncol Nurs. 13:49–59. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Climent MÁ, Torregrosa MD, Vázquez S,
Gironés R and Arranz JA: Aged patients with metastatic castration
resistant prostate cancer: Should we treat with chemotherapy?
Cancer Treat Rev. 55:173–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Benidir T, Hersey K, Finelli A, Hamilton
R, Joshua AM, Kulkarni G, Zlotta A and Fleshner N: Understanding
how prostate cancer patients value the current treatment options
for metastatic castration resistant prostate cancer. Urol Oncol.
36:240.e13–240.e20. 2018. View Article : Google Scholar
|
|
54
|
Gupta S, Adhami VM, Subbarayan M,
MacLennan GT, Lewin JS, Hafeli UO, Fu P and Mukhtar H: Suppression
of prostate carcinogenesis by dietary supplementation of celecoxib
in transgenic adenocarcinoma of the mouse prostate model. Cancer
Res. 64:3334–3343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
James ND, Sydes MR, Mason MD, Clarke NW,
Anderson J, Dearnaley DP, Dwyer J, Jovic G, Ritchie AW, Russell JM,
et al: Celecoxib plus hormone therapy versus hormone therapy alone
for hormone-sensitive prostate cancer: first results from the
STAMPEDE multiarm, multistage, randomised controlled trial. Lancet
Oncol. 13:549–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Algotar AM, Thompson PA, Ranger-Moore J,
Stratton MS, Hsu CH, Ahmann FR, Nagle RB and Stratton SP: Effect of
aspirin, other NSAIDs, and statins on PSA and PSA velocity.
Prostate. 70:883–888. 2010.PubMed/NCBI
|
|
57
|
Derry S, Wiffen PJ, Moore R, McNicol ED,
Bell RF, Carr DB, McIntyre M and Wee B: Oral nonsteroidal
anti-inflammatory drugs (NSAIDs) for cancer pain in adults.
Cochrane Database Syst Rev. 7:CD0126382017.PubMed/NCBI
|
|
58
|
Singer EA, Palapattu GS and Van
Wijngaarden E: Prostate-specific antigen levels in relation to
consumption of nonsteroidal anti-inflammatory drugs and
acetaminophen: Results from the 2001–2002 National Health and
Nutrition Examination Survey. Cancer. 113:2053–2057. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ko CJ, Lan SW, Lu YC, Cheng TS, Lai PF,
Tsai CH, Hsu TW, Lin HY, Shyu HY, Wu SR, et al: Inhibition of
cyclooxygenase-2-mediated matriptase activation contributes to the
suppression of prostate cancer cell motility and metastasis.
Oncogene. 36:4597–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Woo DH, Han IS and Jung G: Mefenamic
acid-induced apoptosis in human liver cancer cell-lines through
caspase-3 pathway. Life Sci. 75:2439–2449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Penning TM, Steckelbroeck S, Bauman DR,
Miller MW, Jin Y, Peehl DM, Fung KM and Lin HK: Aldo-keto reductase
(AKR) 1C3: Role in prostate disease and the development of specific
inhibitors. Mol Cell Endocrinol. 248:182–191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Škarydová L, Živná L, Xiong G, Maser E and
Wsól V: AKR1C3 as a potential target for the inhibitory effect of
dietary flavonoids. Chem Biol Interact. 178:138–144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bauman DR, Rudnick SI, Szewczuk LM, Jin Y,
Gopishetty S and Penning TM: Development of nonsteroidal
anti-inflammatory drug analogs and steroid carboxylates selective
for human aldo-keto reductase isoforms: Potential antineoplastic
agents that work independently of cyclooxygenase isozymes. Mol
Pharmacol. 67:60–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Soriano-Hernández AD, Madrigal-Pérez D,
Galván-Salazar HR, Martínez-Fierro ML, Valdez-Velazquez LL,
Espinoza-Gómez F, Vazquez-Vuelvas OF, Olmedo-Buenrostro BA,
Guzman-Esquivel J, Rodriguez-Sanchez IP, et al: Anti-inflammatory
drugs and uterine cervical cancer cells: Antineoplastic effect of
meclofenamic acid. Oncol Lett. 10:2574–2578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gallavan RH Jr and Chou CC: The effects of
mefenamic acid on postprandial intestinal carbohydrate metabolism.
Prostaglandins. 31:1069–1076. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gaertner J, Stamer UM, Remi C, Voltz R,
Bausewein C, Sabatowski R, Wirz S, Müller-Mundt G, Simon ST,
Pralong A, et al: Metamizole/dipyrone for the relief of cancer
pain: A systematic review and evidence-based recommendations for
clinical practice. Palliat Med. 31:26–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chen SS, Cheng TC, Chiu LP, Tasi LY, Huang
SS and Tsay SL: Predictors for lower urinary tract symptoms and the
urinary specific quality of life in prostate cancer patients:
One-year follow-up. J Chinese Med Assoc. 82:482–487. 2019.
View Article : Google Scholar
|
|
68
|
Vagnildhaug OM, Blum D, Wilcock A, Fayers
P, Strasser F, Baracos VE, Hjermstad MJ, Kaasa S, Laird B, Solheim
TS, et al: The applicability of a weight loss grading system in
cancer cachexia: A longitudinal analysis. J Cachexia Sarcopenia
Muscle. 8:789–797. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kelly SP, Graubard BI, Andreotti G, Younes
N, Cleary SD and Cook MB: Prediagnostic body mass index
trajectories in relation to prostate cancer incidence and mortality
in the PLCO cancer screening trial. J Natl Cancer Inst.
109:2016.
|
|
70
|
Cantarutti A, Bonn SE, Adami HO, Grönberg
H, Bellocco R and Bälter K: Body mass index and mortality in men
with prostate cancer. Prostate. 75:1129–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Reid J, Hughes CM, Murray LJ, Parsons C
and Cantwell MM: Non-steroidal anti-inflammatory drugs for the
treatment of cancer cachexia: A systematic review. Palliat Med.
7:295–303. 2013. View Article : Google Scholar
|
|
72
|
Kobayashi S, Okada S, Yoshida H and
Fujimura S: Indomethacin Enhances the Cytotoxicity of VCR and ADR
in human pulmonary adenocarcinoma cells. Tohoku J Exp Med.
181:361–370. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kim JK, Jeong CW, Ku JH, Kim HH and Kwak
C: Prostate specific antigen (PSA) persistence 6 weeks after
radical prostatectomy and pelvic lymph node dissection as
predictive factor of radiographic progression in node-positive
prostate cancer patients. J Cancer. 10:2237–2242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kanzaki H, Kataoka M, Nishikawa A, Uwatsu
K, Nagasaki K, Nishijima N and Hashine K: Kinetics differences
between PSA bounce and biochemical failure in patients treated with
125I prostate brachytherapy. Jpn J Clin Oncol. 45:688–694. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fenner A: Prostate cancer: PSA kinetics
predict survival in patients treated with abiraterone. Nat Rev
Urol. 12:2402015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Takeuchi H, Ohori M and Tachibana M:
Clinical significance of the prostate-specific antigen doubling
time prior to and following radical prostatectomy to predict the
outcome of prostate cancer. Mol Clin Oncol. 6:249–254. 2017.
View Article : Google Scholar : PubMed/NCBI
|